Hepatocellular Carcinoma (HCC) Metastasis to the Diaphragm Muscle: A Systematic Review and Meta-Analysis of Case Reports
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Criteria
2.3. Data Evaluation
2.4. Data Extraction
2.5. Clinical Definitions
2.5.1. Tumor Diameter
2.5.2. HCC Metastasis to the Diaphragm
2.5.3. Primary and Recurrent HCC
2.6. Data Synthesis
2.7. Data Processing
2.8. Data Analysis
3. Results
3.1. Study Selection
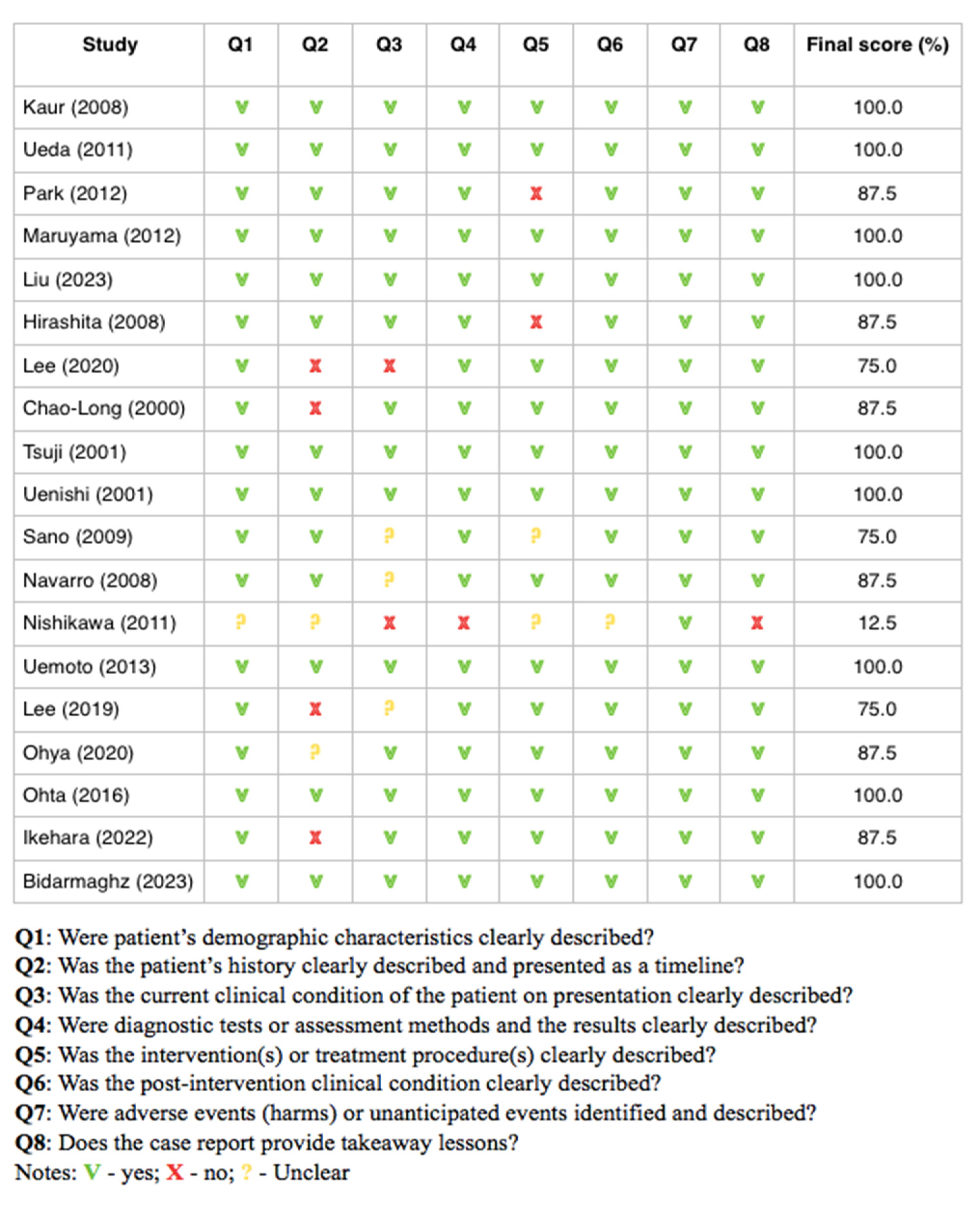
3.2. Results of Risk of Bias Analysis
3.3. General Patients Characteristics
3.4. Clinical Characteristics of Patients
3.5. Symptoms Presentation
3.6. Liver Condition Laboratory Blood Tests
3.7. Diaphragm Muscle Metastasis
3.8. Other Factors
3.9. Survival Prognosis
4. Discussion
5. Conclusions
- Among diaphragm involvement by HCC cells, the diaphragm invasion is more frequently observed than diaphragm adhesion.
- The presence of HCC nodules in segment 6 is a risk factor for diaphragm adhesion.
- A greater number of affected liver segments in the course of HCC contributes more to diaphragm adhesion than to diaphragm infiltration.
- Metastases to the diaphragm muscle occurred much more often when the superior segments of the liver were involved by HCC nodules than the lower ones. The presence of HCC nodules in the upper segments also seems to be a risk factor for diaphragmatic metastases. This indicates the need to monitor patients at a later stage.
- There are no specific symptoms reported by patients that could indicate HCC metastases to the diaphragm muscle. Comparing our data with other available scientific works, the presence of respiratory problems may indicate more serious problems such as a diaphragmatic hernia.
- Tumor size is not associated with HCC spread to diaphragm muscle, while hepatitis B is the risk factor for diaphragmatic metastases in patients diagnosed with HCC for the second time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Samant, H.; Amiri, H.S.; Zibari, G.B. Addressing the worldwide hepatocellular carcinoma: Epidemiology, prevention and management. J. Gastrointest. Oncol. 2021, 12 (Suppl. S2), S361–S373. [Google Scholar] [CrossRef]
- Kinsey, E.; Lee, H.M. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers 2024, 16, 666. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.Y.; Du, S.L.; Wang, Q.L.; Zhang, Y.F.; Song, H.Y. Traditional Chinese medicine targeting cancer stem cells as an alternative treatment for hepatocellular carcinoma. J. Integr. Med. 2020, 18, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Zou, B.; Kam, L.; Lee, K.; Huang, D.Q.; Henry, L.; Cheung, R.; Nguyen, M.H. Updates in Characteristics and Survival Rates of Hepatocellular Carcinoma in a Nationwide Cohort of Real-World US Patients, 2003–2021. J. Hepatocell. Carcinoma. 2023, 10, 2147–2158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarveazad, A.; Agah, S.; Babahajian, A.; Amini, N.; Bahardoust, M. Predictors of 5 year survival rate in hepatocellular carcinoma patients. J. Res. Med. Sci. 2019, 24, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Wang, J.; Chen, J.; Wu, S.; Zeng, X.; Xiong, Q.; Guo, Y.; Sun, J.; Song, F.; Xu, J.; et al. Upregulation of miR-520c-3p via hepatitis B virus drives hepatocellular migration and invasion by the PTEN/AKT/NF-κB axis. Mol. Ther. Nucleic Acids 2022, 29, 47–63. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Qiu, X.; Li, J.; Song, K.; Shen, S.; Huo, L.; Chen, L.; Xu, M.; Wang, H.; et al. A Noninvasive Approach to Evaluate Tumor Immune Microenvironment and Predict Outcomes in Hepatocellular Carcinoma. Phenomics 2023, 3, 549–564. [Google Scholar] [CrossRef]
- Natsuizaka, M.; Omura, T.; Akaike, T.; Kuwata, Y.; Yamazaki, K.; Sato, T.; Karino, Y.; Toyota, J.; Suga, T.; Asaka, M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J. Gastroenterol. Hepatol. 2005, 20, 1781–1787. [Google Scholar] [CrossRef]
- Katyal, S.; Oliver, J.H., 3rd; Peterson, M.S.; Ferris, J.V.; Carr, B.S.; Baron, R.L. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000, 216, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Hirohashi, S.; Sakamoto, M.; Kanai, T.; Shimosato, Y. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer 1990, 66, 2174–2179. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.; Ginsberg, R.J. Tumors of the diaphragm. Chest Surg. Clin. N. Am. 1998, 8, 441–447. [Google Scholar] [PubMed]
- Pauli, B.U.; Schwartz, D.E.; Thonar, E.J.; Kuettner, K.E. Tumor invasion and host extracellular matrix. Cancer Metastasis Rev. 1983, 2, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Herring, C.L., Jr.; Harrelson, J.M.; Scully, S.P. Metastatic carcinoma to skeletal muscle. A report of 15 patients. Clin. Orthop. Relat. Res. 1998, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Mulsow, F. Metastatic carcinoma of skeletal muscles. Arch. Pathol. 1943, 35, 112–114. [Google Scholar]
- Kocjan, J.; Adamek, M.; Gzik-Zroska, B.; Czyżewski, D.; Rydel, M. Network of Breathing. Multifunct. Role Diaphragm Rev. Adv. Respir. Med. 2017, 85, 224–232. [Google Scholar]
- Arenas, A.P.; Sanchez, L.V.; Albillos, J.M.; Borruel, S.N.; Roldán, J.R.; Lozano, F.O. Direct dissemination of pathologic abdominal processes through perihepatic ligaments: Identification with CT. Radiographics 1994, 14, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Miyayama, S.; Choi, J.W.; Kim, G.M.; Chung, J.W. Hepatocellular Carcinoma Supplied by the Inferior Phrenic Artery or Cystic Artery: Anatomic and Technical Considerations. Radiographics 2023, 43, e220076. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int. J. Evid. Based Healthc. 2015, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Watanabe, K.; Hisamatsu, Y.; Shirao, K. Ectopic Hepatocellular Carcinoma of the Left Sub-diaphragm with Metastasis. Intern. Med. 2011, 50, 1505–1506. [Google Scholar] [CrossRef]
- Kaur, R.; Abdullah, B.; Rajasingam, V. Hepatocellular carcinoma with extension to the diaphragm, falciform ligament, rectus abdominis and paraumbilical vein. Biomed. Imaging Interv. J. 2008, 4, e37. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Yoshida, H.; Mamada, Y.; Taniai, N.; Mineta, S.; Yoshioka, M.; Hirakata, A.; Kawano, Y.; Kanda, T.; Uchida, E. Resection of hepatocellular carcinoma recurring in the diaphragm after right hepatic lobectomy. J. Nippon. Med. Sch. 2011, 78, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; Yang, S.I.; Yoon, M.H. One stage resection of spontaneous rupture of hepatocellular carcinoma in the triangular ligament with diaphragm invasion: Case report and review of the literature. World J. Emerg. Surg. 2012, 7, 30. [Google Scholar] [CrossRef]
- Maruyama, H.; Yoshida, H.; Hirakata, A.; Matsutani, T.; Yokoyama, T.; Suzuki, S.; Matsushita, A.; Sasajima, K.; Kikuchi, Y.; Uchida, E. Surgical treatment of a patient with diaphragmatic invasion by a ruptured hepatocellular carcinoma with biliary and portal venous tumor thrombi. J. Nippon. Med. Sch. 2012, 79, 147–152. [Google Scholar] [CrossRef]
- Hirashita, T.; Ohta, M.; Iwaki, K.; Kai, S.; Shibata, K.; Sasaki, A.; Nakashima, K.; Kitano, S. Direct invasion to the colon by hepatocellular carcinoma: Report of two cases. World J. Gastroenterol. 2008, 14, 4583–4585. [Google Scholar] [CrossRef]
- Liu, H.B.; Zhao, L.H.; Zhang, Y.J.; Li, Z.F.; Li, L.; Huang, Q.P. Left epigastric isolated tumor fed by the inferior phrenic artery diagnosed as ectopic hepatocellular carcinoma: A case report. World J. Clin. Cases 2023, 11, 6231–6239. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-F.; Wong, R.H.L.; Leung, H.H.W.; Lo, E.Y.J.; Chong, C.C.N.; Chan, A.W.H.; Lai, P.B.S. En bloc transdiaphragmatic lung resection for locally advanced hepatocellular carcinoma: A case report. J. Surg. Case Rep. 2020, 2020, rjaa084. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, Y.S.; Goto, S.; Jawan, B.; Cheng, Y.-F.; Eng, H.-L. Images in surgery. Front. Surg. 2000, 127, 229. [Google Scholar]
- Ohya, Y.; Hayashida, S.; Tsuji, A.; Kuramoto, K.; Shibata, H.; Setoyama, H.; Hayashi, H.; Kuriwaki, K.; Sasaki, M.; Iizaka, M.; et al. Conversion hepatectomy for advanced hepatocellular carcinoma after right portal vein transection and lenvatinib therapy. Surg. Case Rep. 2020, 6, 318. [Google Scholar] [CrossRef]
- Sano, T.; Izuishi, K.; Takebayashi, R.; Kushida, Y.; Masaki, T.; Suzuki, Y. Hepatobiliary and pancreatic: Isolated diaphragmatic metastasis from hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2009, 24, 1475. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, T.; Shimizu, T.; Hirano, S.; Fukushima, K.; Yoshizawa, J.-I.; Nakamura, T.; Nakayama, A. Primary spindle cell tumor originating from the liver that was difficult to diagnose. Surg. Case Rep. 2022, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, J.; Hoshi, N.; Hirabayashi, K.; Hoshi, S.; Onodera, K.; Nishi, T.; Tomikawa, M.; Igarashi, S. Collision tumors of hepatocellular carcinoma and malignant peritoneal mesothelioma. Med. Mol. Morphol. 2013, 46, 177–183. [Google Scholar] [CrossRef]
- Ohta, M.; Nakanishi, C.; Kawagishi, N.; Hara, Y.; Maida, K.; Kashiwadate, T.; Miyazawa, K.; Yoshida, S.; Miyagi, S.; Hayatsu, Y.; et al. Surgical resection of recurrent extrahepatic hepatocellular carcinoma with tumor thrombus extending into the right atrium under cardiopulmonary bypass: A case report and review of the literature. Surg. Case Rep. 2016, 2, 110. [Google Scholar] [CrossRef] [PubMed]
- Bidarmaghz, B.; Idrees, M.; Lee, Y.Y.; Hodgkinson, P. Large hepatocellular carcinoma treated with sequential SBRT and immunotherapy with anti-VEGF (Vascular Endothelial Growth Factor) therapy. BMJ Case Rep. 2023, 16, e256931. [Google Scholar] [CrossRef]
- Uenishi, T.; Kubo, S.; Hirohashi, K.; Tanaka, H.; Ohba, K.; Kinoshita, H. Successful Treatment of Dissemination of Hepatocellular Carcinoma to the Pleura and Diaphragm after Percutaneous Liver Biopsy. Dig. Surg. 2001, 18, 225–227. [Google Scholar] [CrossRef]
- Navarro, F.; Taourel, P.; Michel, J.; Perney, P.; Fabre, J.; Blanc, F.; Domergue, J. Diaphragmatic and subcutaneous seeding of hepatocellular carcinoma following fine-needle aspiration biopsy. Liver 1998, 18, 251–254. [Google Scholar] [CrossRef]
- Lee, K.F.; Lok, H.T.; Fung, A.K.Y.; Chong, C.C.N.; Cheung, Y.S.; Wong, J.; Lai, P.B.S. Successful robotic extirpation of diaphragmatic seeding of hepatocellular carcinoma after previous rupture. J. Robot. Surg. 2019, 13, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Okada, K.; Fukuoka, M.; Watanabe, Y.; Ataka, K.; Minami, R.; Hanioka, K.; Tachibana, S.; Saito, H.; Sasada, A.; et al. Hepatocellular Carcinoma with a Sarcomatous Appearance: Report of a Case. Surg. Today 2001, 31, 735–739. [Google Scholar] [CrossRef]
- Kim, C.; Song, K.D.; Woo, J.H. Infiltrative invasion of the diaphragm: An uncommon manifestation of recurrent hepatocellular carcinoma A retrospective cohort study. Precis. Future Med. 2022, 6, 78–84. [Google Scholar] [CrossRef]
- Sun, V.C.; Sarna, L. Symptom management in hepatocellular carcinoma. Clin. J. Oncol. Nurs. 2008, 12, 759–766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ushijima, H.; Hida, J.I.; Yane, Y.; Kato, H.; Ueda, K.; Kawamura, J. Laparoscopic repair of diaphragmatic hernia after radiofrequency ablation for hepatocellular carcinoma: Case report. Int. J. Surg. Case Rep. 2021, 81, 105728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shibuya, A.; Nakazawa, T.; Saigenji, K.; Furuta, K.; Matsunaga, K. Diaphragmatic hernia after radiofrequency ablation therapy for hepatocellular carcinoma. AJR Am. J. Roentgenol. 2006, 186 (Suppl. S5), S241–S243. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.I.; Khan, S.A.; Toledano, M.B.; Waked, I.; Taylor-Robinson, S.D. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008, 14, 4300–4308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arizumi, T.; Minami, T.; Chishina, H.; Kono, M.; Takita, M.; Yada, N.; Hagiwara, S.; Minami, Y.; Ida, H.; Ueshima, K.; et al. Impact of Tumor Factors on Survival in Patients with Hepatocellular Carcinoma Classified Based on Kinki Criteria Stage B2. Dig. Dis. 2017, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, M.; Miyanaga, Y.; Yasuda, Y.; Atsuji, K.; Tanaka, H.; Amaike, H.; Minami, M.; Ueda, K.; Ito, Y. The significance of extrahepatic feeding arteries in patients with extrahepatically growing hepatocellular carcinoma infiltrating into other organs. Kanzo 2013, 54, 257–265. [Google Scholar] [CrossRef]
- Hayano, K.; Desai, G.S.; Kambadakone, A.R.; Fuentes, J.M.; Tanabe, K.K.; Sahani, D.V. Quantitative characterization of hepatocellular carcinoma and metastatic liver tumor by CT perfusion. Cancer Imaging 2013, 13, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Mise, Y.; Satou, S.; Shindoh, J.; Conrad, C.; Aoki, T.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Three-dimensional volumetry in 107 normal livers reveals clinically relevant inter-segment variation in size. HPB 2014, 16, 439–447. [Google Scholar] [CrossRef]
- Shroff, N.; Choi, W.; Elshikh, M.; Wong, B.; Bhargava, P. Multimodality imaging approach in identifying invasive hepatocellular carcinoma. Clin. Imaging 2023, 97, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Angeli-Pahim, I.; Chambers, A.; Duarte, S.; Zarrinpar, A. Current Trends in Surgical Management of Hepatocellular Carcinoma. Cancers 2023, 15, 5378. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Liu, Y.-C.; Mao, Y.-Z.; Wang, J.-C.; Lao, X.-M.; Chen, M.-S.; Li, S.-P. Hepatocellular carcinoma with en bloc diaphragmatic resection: A single-center experience over 14 years. Int. J. Surg. 2018, 53, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shen, S.; Jiang, L.; Yan, L.; Yang, J.; Li, B.; Wen, T.; Wang, W.; Xu, M. Outcomes of anterior approach major hepatectomy with diaphragmatic resection for single huge right lobe HCC with diaphragmatic invasion. Medicine 2018, 97, e12194. [Google Scholar] [CrossRef]
- Lau, W.Y.; Leung, K.L.; Leung, T.W.; Liew, C.T.; Chan, M.; Li, A.K. Resection of hepatocellular carcinoma with diaphragmatic invasion. Br. J. Surg. 1995, 82, 264–266. [Google Scholar] [CrossRef]
- Lin, M.C.; Wu, C.C.; Chen, J.T.; Lin, C.C.; Liu, T.J. Surgical results of hepatic resection for hepatocellular carcinoma with gross diaphragmatic invasion. Hepatogastroenterology 2005, 52, 1497–1501. [Google Scholar] [PubMed]
- Invenizzi, F.; Iavarone, M.; Donato, M.F.; Mazzucco, A.; Torre, M.; Conforti, S.; Rimessi, A.; Zavaglia, C.; Schiavon, M.; Comacchio, G.; et al. Pulmonary Resection for Metastasis of Hepatocellular Carcinoma Recurring After Liver Transplant: An Italian Multicenter Experience. Front. Oncol. 2020, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Yun, J.K.; Jung, H.S.; Moon, D.H.; Lee, G.D.; Choi, S.; Kim, Y.-H.; Kim, D.K.; Park, S.I.; Kim, H.R. Surgical outcomes of pulmonary metastasectomy in hepatocellular carcinoma patients according to approach method: Thoracoscopic versus open approach. World J. Surg. Oncol. 2021, 19, 33. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Zeng, Z.-C.; Tang, Z.-Y.; Sun, J.; Cheng, J.-M.; Liu, R.; Wang, P.; Zhang, B.-H. Prognostic analysis of pulmonary metastases from hepatocellular carcinoma. Hepatol. Int. 2008, 2, 237–243. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Liang, Y.; Lu, X.; Huang, Y.; Zhu, J.; Li, J. Short-term prognosis for hepatocellular carcinoma patients with lung metastasis: A retrospective cohort study based on the SEER database. Medicine 2022, 101, e31399. [Google Scholar] [CrossRef]
- Ozer, M.; Goksu, S.Y.; Lin, R.Y.; Ayasun, R.; Kahramangil, D.; Rogers, S.C.; Fabregas, J.C.; Ramnaraign, B.H.; George, T.J.; Feely, M.; et al. Effects of Clinical and Tumor Characteristics on Survival in Patients with Hepatocellular Carcinoma with Bone Metastasis. J. Hepatocell. Carcinoma 2023, 10, 1129–1141. [Google Scholar] [CrossRef]
- Kim, S.; Choi, Y.; Kwak, D.-W.; Lee, H.S.; Hur, W.-J.; Baek, Y.H.; Lee, S.W. Prognostic factors in hepatocellular carcinoma patients with bone metastases. Radiat. Oncol. J. 2019, 37, 207–214. [Google Scholar] [CrossRef]
- Yuan, X.; Zhuang, M.; Zhu, X.; Cheng, D.; Liu, J.; Sun, D.; Qiu, X.; Lu, Y.; Sartorius, K. Emerging Perspectives of Bone Metastasis in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 943866. [Google Scholar] [CrossRef] [PubMed]


| Variables | M ± SD (Range: Min–Max)/Number of Subjects (Percentage of All) |
|---|---|
| Largest tumor diameter: | |
| mean | 6.88 ± 3.92 (1.7–15.0) |
| <2 cm | 2 (10.0%) |
| 2–5 cm | 6 (30.0%) |
| 5–10 cm | 8 (40.0%) |
| >10 cm | 4 (20.0%) |
| HCC nodule localization (liver lobe): | |
| Right | 18 (75.0%) |
| Left | 6 (25.0%) |
| HCC nodule localization (liver sections): | |
| right posterior | 6 (25.0%) |
| left lateral | 6 (25.0%) |
| left medial | 4 (16.6%) |
| right anterior | 1 (4.2%) |
| mixed | 7 (29.2%) |
| HCC nodule localization according to Coinaud classification: | |
| S1 | 1 (4.55%) |
| S2 | 4 (18.2%) |
| S3 | 3 (13.6%) |
| S4 | 7 (31.8%) |
| S5 | 3 (13.6%) |
| S6 | 5 (22.7%) |
| S7 | 7 (31.8%) |
| S8 | 7 (31.8%) |
| Number of liver segments involved by HCC: | |
| 1 | 15 (68.2%) |
| 2 | 4 (18.2%) |
| 3 | 1 (4.55%) |
| 4 | 1 (4.55%) |
| 5 | 2 (9.1%) |
| mean | 1.81 ± 1.28 (1–5) |
| Edmondson-Steiner grading system: | |
| Grade I (well-differentiated) | 1 (5.9%) |
| Grade II (moderately differentiated) | 11 (64.7%) |
| Grade III (poorly differentiated) | 5 (29.4%) |
| Grade IV (undifferentiated) | 0 (0.0%) |
| Variables | Primary | Recurrent | |
|---|---|---|---|
| AlAT (n = 8) | Normal | 3 | 0 |
| Elevated | 4 | 1 | |
| AspAT (n = 8) | Normal | 3 | 0 |
| Elevated | 4 | 1 | |
| ALP (n = 9) | Normal | 3 | 0 |
| Elevated | 4 | 2 | |
| Billirubin total (n = 7) | Normal | 3 | 0 |
| Elevated | 3 | 1 | |
| GGTP (n = 5) | Normal | 2 | 0 |
| Elevated | 3 | 0 | |
| AFP (n = 24) | Normal | 2 | 5 |
| Elevated | 7 | 10 | |
| PIVKA II (n = 15) | Normal | 1 | 4 |
| Elevated | 2 | 8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocjan, J.; Rydel, M.; Adamek, M. Hepatocellular Carcinoma (HCC) Metastasis to the Diaphragm Muscle: A Systematic Review and Meta-Analysis of Case Reports. Cancers 2024, 16, 3076. https://doi.org/10.3390/cancers16173076
Kocjan J, Rydel M, Adamek M. Hepatocellular Carcinoma (HCC) Metastasis to the Diaphragm Muscle: A Systematic Review and Meta-Analysis of Case Reports. Cancers. 2024; 16(17):3076. https://doi.org/10.3390/cancers16173076
Chicago/Turabian StyleKocjan, Janusz, Mateusz Rydel, and Mariusz Adamek. 2024. "Hepatocellular Carcinoma (HCC) Metastasis to the Diaphragm Muscle: A Systematic Review and Meta-Analysis of Case Reports" Cancers 16, no. 17: 3076. https://doi.org/10.3390/cancers16173076




