Orally Ingested Micro- and Nano-Plastics: A Hidden Driver of Inflammatory Bowel Disease and Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Micro- and Nanoplastics (MNPLs): An Overview
2.1. Definition and Composition of MNPLs
- (a)
- There are two classes of MNPLs: the primary and the secondary. The first class includes all the MNPLs that are directly produced at micro- and nanoscale for industrial purposes (such as cosmetics, textiles, and medicine) [52,53,54]. The second category includes MNPLs created when larger plastic fragments break down through various processes, including mechanical and photochemical degradation and/or microbial actions [55,56];
- (b)
- Four groups of MNPLs can be defined starting from their sizes: macroplastics (>25 mm), mesoplastics (5–25 mm), MPs (0.001–5 mm), and NPs (1 nm–1000 nm) [57].
2.2. Detection Techniques of MNPLs
3. Oral Exposure to MNLPs: Implications for Human Health
3.1. The Role of MNPLs in Cell Membrane Disruption
3.2. MNPL Activation of Oxidative Stress and Apoptosis Pathways
| Particle Type | Particle Size | Experimental Models | Mechanisms of Membrane Interaction/Internalization | Findings | Ref. |
|---|---|---|---|---|---|
| PS | 20 nm 200 nm |
|
| In in vitro model, the exposure to PS particles was associated with the following:
| [126] |
| PS | 800 nm |
|
| In in vitro model, the exposure to PS particles was associated with the following:
| [127] |
| PS | 50 nm 500 nm 5 μm |
|
| In in vitro model, the exposure to PS particles was associated with the following:
| [128] |
| PS | 50 nm 250 nm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [131] |
| PS | 50 nm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [132] |
| PS | 50 nm 500 nm 5000 nm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [133] |
| PS | 0.5 μm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [134] |
| PS | 0.5 µm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [135] |
| PS | 0.5 µm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [136] |
| PS | 100 nm |
|
| In in vitro models, the exposure to PS particles was associated with the following:
| [137] |
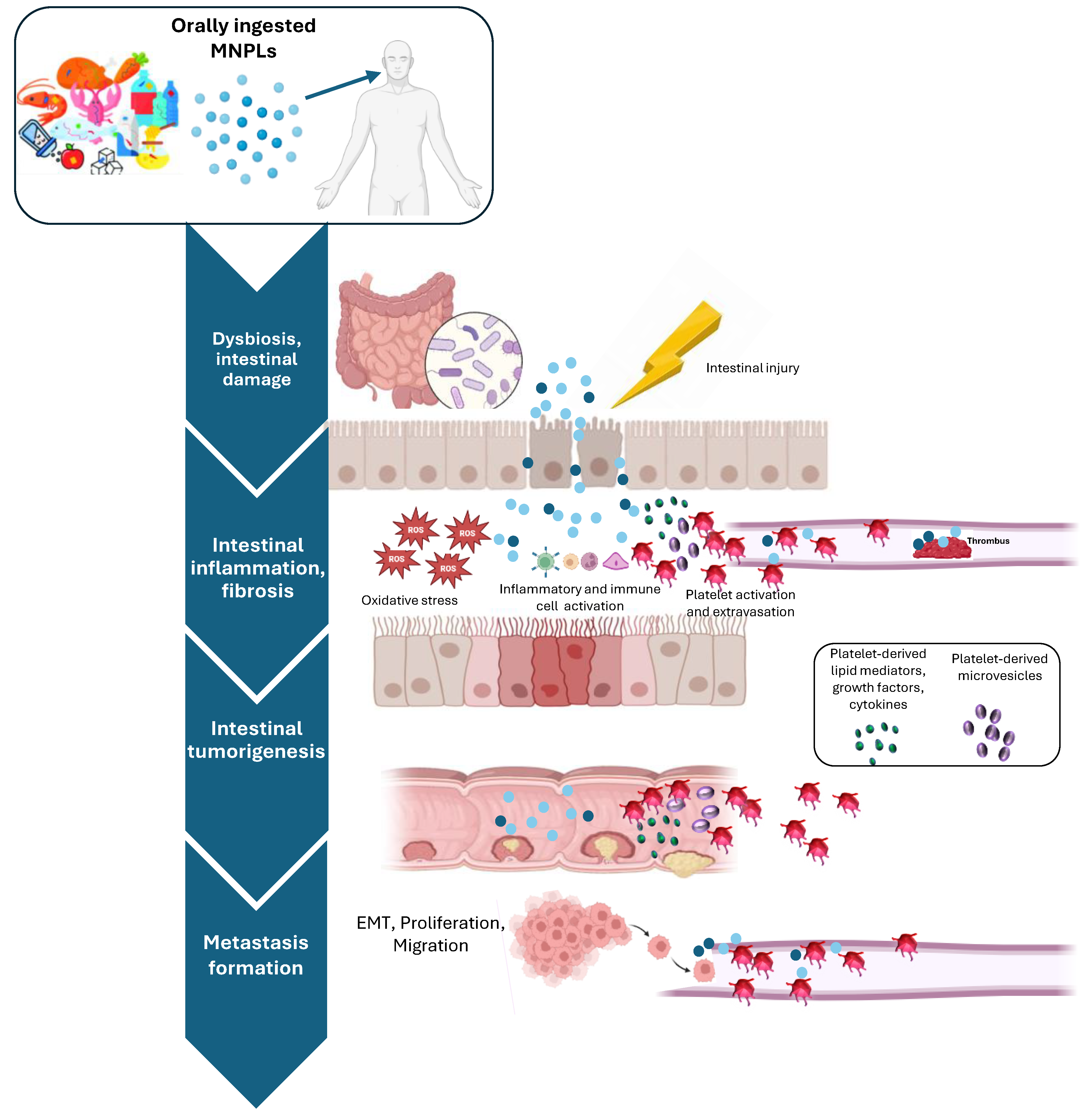
4. Intestinal Effects of Oral Exposure to MNPLs
4.1. Impact of MNPLs on Healthy Intestine
| Mouse Model | MNPLs | MNPLs Dose | Routes of Exposure | Time of Exposure | Key Findings | Ref. |
|---|---|---|---|---|---|---|
| ICR | PS 0.5 and 50 µm | 100 and 1000 μg/L | Drinking water | 5 weeks |
| [173] |
| ICR | PS 5 µm | 100 and 1000 μg/L | Drinking water | 6 weeks |
| [176] |
| C57BL/6NTac | PS 1, 4, and 10 µm | Mixture of 1 µm (1.25 mg/kg), 4 µm (25 mg/kg), and 10 µm (34 mg/kg) | Oral gavage | 28 days |
| [166] |
| C57BL/6 | PE 10–150 μm | 2–20–200 μg/g Food (6, 60, and 600 μg/d) | Food | 5 weeks | In mice treated with 600 μg/day:
| [20] |
| CD-1 | PE 45–53 μm | 100 mg/kg/d | Oral gavage | 30 days |
| [66] |
| ICR | PS 5 μm | 100 and 1000 μg/L | Drinking water | 6 weeks |
| [181] |
| ICR | PE 1–10 µm | 0, 0.002, and 0.2 μg/g/d | Oral gavage | 30 days |
| [182] |
| C57BL/6 | PVC 2 µm | 100 mg/kg | Oral gavage | 60 days |
| [183] |
| ICR | PS 0.5 μm | 10 μg/mL, 50 μg/mL, and 100 μg/mL | Oral gavage | 2 weeks |
| [184] |
| C57BL/6J | PS 1 µm | 80 μg/kg/d | Drinking water | 8 weeks |
| [185] |
| C57BL/6J | PS 5 µm | 18 and 180 μg/kg/d | Drinking water | 90 days |
| [186] |
| KM | PE, PS, PP, PVC, and PET 150–300 µm | 20 mg/mL | Oral gavage | 1 week |
| [187] |
| C57BL/6 | PP 8, 70 µm | 1, 10, and 100 mg/kg/d | Oral gavage | 28 days |
| [188] |
| C57BL/6 J | PS 50, 500, and 5000 nm | 2.5–500 mg/kg | Oral gavage | 28 days |
| [133] |
| C57BL/6 | PS 200 and 800 nm | 109 MPLs | Oral gavage | 4 weeks |
| [189] |
| C57BL/6 | PE 5 μm | 1 and 10 mg/L | Oral administration | 21 days |
| [190] |
| C57BL/6N | PS 100 nm | 5 mg/kg bw/d | Oral gavage | 28 days |
| [191] |
| KM healthy-aged | PS 1 μm | 4.67 × 10−15, 4.67 × 10−12, 4.67 × 10−9, 4.67 × 10−6 mg/kg/d | Oral gavage | 10 days |
| [192] |
| C57BL/6 J | PS 500 nm | 5 mg/kg/d | Oral gavage | 30 days |
| [132] |
| C57BL/6 J | PS 0.5, 5 μm | 0.5 mg/mice in 100 µL sterile deionized water | Oral gavage | 8 weeks |
| [193] |
| C57BL/6 J | PS 0.2, 1, 5 μm | 1 mg/kg/d | Oral gavage | 28 days |
| [194] |
| C57BL/6 J | PS 50 nm | 0.1, 1, 10 mg/L | Drinking water | 32 weeks |
| [132] |
| C57BL/6 | PS 2 μm | 0.5, 2 mg/kg/d | Oral gavage | 8 weeks |
| [195] |
4.2. MNPLs and Inflammatory Bowel Diseases
| Mouse Model | MNPLs | MNPLs Dose | Routes of Exposure | Time of Exposure | Key Findings | Ref. |
|---|---|---|---|---|---|---|
| C57BL/6 and DSS-induced colitis | PS 5 µm | 100 μg/L, ~1.456 × 106 MPLs/L | Drinking water | 42 days |
| [213] |
| C57 and DSS-induced colitis | PS 5 µm | 500 μg/L | Oral gavage | 28 days |
| [214] |
| C57BL/6 J and DSS-induced colitis | PS 5 µm | 7280/72,800 MPLs/day | Oral gavage | 14 days |
| [215] |
| Lewis rats TNBS induced acute colitis | PS 0.1, 1, and 10 µm | 12.5 mg particles/kg bw | Oral administration | 3 days |
| [210] |
| C57-BL/6 mice and DSS-induced colitis | PS 5 µm | 500 μg/L | Drinking water | 28 days |
| [36] |
| C57BL/6 mice and LPS-induced inflammation | PS~102 nm | 5 μg/g | Intraperitoneal injection | 14 days |
| [210] |
4.3. MNPLs in Colorectal Cancer Development and Progression
5. Platelet Activation in Intestinal Inflammation and Cancer: The Unexplored Contribution of MNPL Ingestion
5.1. Role of Platelets in Intestinal Inflammation and Cancer
5.2. Effects of MNLPs on Platelet Function
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A.J.; Hartmann, N.B.; Drobne, D.; Potthoff, A.; Kühnel, D. Quality of nanoplastics and microplastics ecotoxicity studies: Refining quality criteria for nanomaterial studies. J. Hazard. Mater. 2021, 415, 125751. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Statement on the presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, 4501. [Google Scholar] [CrossRef]
- Kihara, S.; Köper, I.; Mata, J.P.; McGillivray, D.J. Reviewing nanoplastic toxicology: It’s an interface problem. Adv. Colloid. Interface Sci. 2021, 288, 102337. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Science Assessment of Plastic Pollution. 2020. Available online: https://www.canada.ca/content/dam/eccc/documents/pdf/pded/plastic-pollution/Science-assessment-plastic-pollution.pdf (accessed on 17 June 2024).
- Parks Canada. Microplastics: More Than a Drop in the Ocean. 2018. Available online: https://parks.canada.ca/nature/science/conservation/plastique-plastic/microplastique-microplastic (accessed on 17 June 2024).
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Woods, M.N.; Stack, M.E.; Fields, D.M.; Shaw, S.D.; Matrai, P.A. Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. Pollut. Bull. 2018, 137, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Martins Breyner, N.; Houdeau, E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Part. Fibre Toxicol. 2020, 17, 19. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Chen, L.; Lv, S.; Liu, S.; Nie, P.; Aguilar, Z.P.; Xu, H. Restraining the TiO2 nanoparticles-induced intestinal inflammation mediated by gut microbiota in juvenile rats via ingestion of Lactobacillus rhamnosus GG. Ecotoxicol. Environ. Saf. 2020, 206, 111393. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chen, C.Y.; Lu, T.H.; Liao, C.M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2019, 366, 703–713. [Google Scholar] [CrossRef]
- Guilloteau, E.; Djouina, M.; Caboche, S.; Waxin, C.; Deboudt, K.; Beury, D.; Hot, D.; Pichavant, M.; Dubuquoy, L.; Launay, D.; et al. Exposure to atmospheric Ag, TiO2, Ti and SiO2 engineered nanoparticles modulates gut inflammatory response and microbiota in mice. Ecotoxicol. Environ. Saf. 2022, 236, 113442. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Xu, H. Effects of microplastic and engineered nanomaterials on inflammatory bowel disease: A review. Chemosphere 2023, 326, 138486. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, L.; Liu, Z.; Zhou, Q.; Zhu, Y.; Zhao, Y.; Yang, X. Long-term exposure to titanium dioxide nanoparticles promotes diet-induced obesity through exacerbating intestinal mucus layer damage and microbiota dysbiosis. Nano Res. 2021, 14, 1512–1522. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Liu, Y.; Tang, Y.; Xu, X.; Wang, M.; Tao, X.; Xu, H. Pre-Exposure to TiO2-NPs Aggravates Alcohol-Related Liver Injury by Inducing Intestinal Barrier Damage in Mice. Toxicol. Sci. 2021, 185, 28–37. [Google Scholar] [CrossRef]
- Remmelts, M. Effect of Nano-And Microplastics on the Human Immune System and Their Influence on Inflammatory Bowel Disease. Bachelor’s Thesis, University of Groningen, Groningen, The Netherlands, 2021. [Google Scholar]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, P.; Ballerini, P.; Buccella, S.; Ciccarelli, R.; Rathbone, M.P.; Romano, S.; D’Alimonte, I.; Caciagli, F.; Di Iorio, P.; Pokorski, M. Guanosine protects glial cells against 6-hydroxydopamine toxicity. Neurotransm. Interact. Cogn. Funct. 2015, 837, 23–33. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Meyer, B.; Are, C. Current Status and Future Directions in Colorectal Cancer. Indian. J. Surg. Oncol. 2018, 9, 440–441. [Google Scholar] [CrossRef]
- Tenailleau, Q.M.; Lanier, C.; Gower-Rousseau, C.; Cuny, D.; Deram, A.; Occelli, F. Crohn’s disease and environmental contamination: Current challenges and perspectives in exposure evaluation. Environ. Pollut. 2020, 263, 114599. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Wang, J.; Wu, B.; Guo, X. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance. Sci. Total Environ. 2022, 833, 155198. [Google Scholar] [CrossRef]
- Cetin, M.; Demirkaya Miloglu, F.; Kilic Baygutalp, N.; Ceylan, O.; Yildirim, S.; Eser, G.; İnci Gul, H. Higher number of microplastics in tumoral colon tissues from patients with colorectal adenocarcinoma. Environ. Chem. Lett. 2023, 21, 639–646. [Google Scholar] [CrossRef]
- Eng, C.; Jácome, A.A.; Agarwal, R.; Hayat, M.H.; Byndloss, M.X.; Holowatyj, A.N.; Bailey, C.; Lieu, C.H. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. 2022, 23, e116–e128. [Google Scholar] [CrossRef]
- REACCT Collaborative; Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; d’Allens,, L.; Allmer, C.; Álvarez, A.; Anula, R.; Andric, M.; et al. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865–874. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C.; Duan, X.; Liang, B.; Genbo Xu, E.; Huang, Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ. Int. 2023, 171, 107662. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Wilmot, K.A.; O’Flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef]
- Ballerini, P.; Contursi, A.; Bruno, A.; Mucci, M.; Tacconelli, S.; Patrignani, P. Inflammation and Cancer: From the Development of Personalized Indicators to Novel Therapeutic Strategies. Front. Pharmacol. 2022, 13, 838079. [Google Scholar] [CrossRef]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Böhmert, L. Micro- and nanoplastics–current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv. 2020, 2, 4350–4367. [Google Scholar] [CrossRef]
- REACCT Collaborative. Microsatellite instability in young patients with rectal cancer: Molecular findings and treatment response. Br. J. Surg. 2022, 109, 251–255. [Google Scholar] [CrossRef]
- Bibi, A.; Can, A.; Pant, U.; Hardiman, G.; Hill, D.; Elliott, C.; Cao, C. A review on state-of-the-art detection techniques for micro- and nano-plastics with prospective use in point-of-site detection. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; Volume 101, pp. 143–196. [Google Scholar] [CrossRef]
- Pico, Y.; Alfarhan, A.; Barcelo, D. Nano- and Microplastic Analysis: Focus on Their Occurrence in Freshwater Ecosystems and Remediation Technologies. TrAC Trends Anal. Chem. 2019, 113, 409–425. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Dehaut, A.; Hermabessiere, L.; Duflos, G. Current frontiers and recommendations for the study of microplastics in seafood. TrAC Trends Anal. Chem. 2019, 116, 346–359. [Google Scholar] [CrossRef]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s something in the air: A review of sources, prevalence and behaviour of microplastics in the atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are there nanoplastics in your personal care products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Yang, T.; Luo, J.; Nowack, B. Characterization of Nanoplastics, Fibrils, and Microplastics Released during Washing and Abrasion of Polyester Textiles. Environ. Sci. Technol. 2021, 55, 15873–15881. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Martins, M.A.; Soares, A.M.V.; Cuesta, A.; Oliveira, M. Polystyrene nanoplastics alter the cytotoxicity of human pharmaceuticals on marine fish cell lines. Environ. Toxicol. Pharmacol. 2019, 69, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Efimova, I.; Bagaeva, M.; Bagaev, A.; Kileso, A.; Chubarenko, I. Secondary Microplastics Generation in the Sea Swash Zone with Coarse Bottom Sediments: Laboratory Experiments. Front. Mar. Sci. 2018, 5, 313. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B..; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Rødland, E.S.; Samanipour, S.; Rauert, C.; Okoffo, E.D.; Reid, M.J.; Heier, L.S.; Lind, O.C.; Thomas, K.V.; Meland, S. A novel method for the quantification of tire and polymer-modified bitumen particles in environmental samples by pyrolysis gas chromatography mass spectroscopy. J. Hazard. Mater. 2022, 423, 127092. [Google Scholar] [CrossRef] [PubMed]
- Karak, N. 15—Biopolymers for paints and surface coatings. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 333–368. [Google Scholar]
- Singh, B.; Kumar, A. Advances in microplastics detection: A comprehensive review of methodologies and their effectiveness. TrAC Trends Anal. Chem. 2024, 170, 117440. [Google Scholar] [CrossRef]
- Mapping Exercise—Plastic Additives Initiative—ECHA. Available online: https://european-union.europa.eu/index_en (accessed on 9 August 2024).
- Gao, D.; Junaid, M.; Chen, X.; Liao, H.; Chen, G.; Wang, J. Interaction of micro(nano)plastics and bisphenols in the environment: A recent perspective on adsorption mechanisms, influencing factors and ecotoxic impacts. TrAC Trends Anal. Chem. 2023, 165, 117132. [Google Scholar] [CrossRef]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Xiang, L.; Gu, C.; Redmile-Gordon, M.; Sheng, H.; Wang, Z.; Fu, Y.; Bian, Y.; Jiang, X. Risk Assessment of Agricultural Plastic Films Based on Release Kinetics of Phthalate Acid Esters. Environ. Sci. Technol. 2021, 16, 3676–3685. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Fang, C.; Luo, Y.; Naidu, R. Advancements in Raman imaging for nanoplastic analysis: Challenges, algorithms and future Perspectives. Anal. Chim. Acta 2024, 1290, 342069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Eriksen, M.; Liboiron, M.; Kiessling, T.; Charron, L.; Alling, A.; Lebreton, L.; Richards, H.; Roth, B.; Ory, N.C.; Hidalgo-Ruz, V.; et al. Microplastic sampling with the AVANI trawl compared to two neuston trawls in the Bay of Bengal and South Pacific. Environ. Pollut. 2018, 232, 430–439. [Google Scholar] [CrossRef]
- Schönlau, C.; Karlsson, T.M.; Rotander, A.; Nilsson, H.; Engwall, M.; van Bavel, B.; Kärrman, A. Microplastics in sea-surface waters surrounding Sweden sampled by manta trawl and in-situ pump. Mar. Pollut. Bull. 2020, 153, 111019. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.; Taladriz-Blanco, P.; Lehner, R.; Lubskyy, A.; Ortuso, R.D.; Rothen-Rutishauser, B.; Petri-Fink, A. The micro-, submicron-, and nanoplastic hunt: A review of detection methods for plastic particles. Chemosphere 2022, 293, 133514. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Delshab, H.; Soltani, N.; Sorooshian, A. Investigation of microrubbers, microplastics and heavy metals in street dust: A study in Bushehr city, Iran. Environ. Earth Sci. 2017, 76, 798. [Google Scholar] [CrossRef]
- Fang, C.; Luo, Y.; Naidu, R. Microplastics and nanoplastics analysis: Options, imaging, advancements and challenges. TrAC Trends Anal. Chem. 2023, 166, 117158. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Dehghani, S.; Moore, F.; Akhbarizadeh, R. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ. Sci. Pollut. Res. Int. 2017, 24, 20360–20371. [Google Scholar] [CrossRef] [PubMed]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Z.; Yang, H.; Wang, J. An overview of analytical methods for detecting microplastics in the atmosphere. TrAC Trends Anal. Chem. 2020, 130, 115981. [Google Scholar] [CrossRef]
- Peñalver, R.; Costa-Gómez, I.; Arroyo-Manzanares, N.; Moreno, J.M.; López-García, I.; Moreno-Grau, S.; Córdoba, M.H. Assessing the level of airborne polystyrene microplastics using thermogravimetry-mass spectrometry: Results for an agricultural area. Sci. Total Environ. 2021, 787, 147656. [Google Scholar] [CrossRef]
- Jarosz, K.; Janus, R.; Wądrzyk, M.; Wilczyńska-Michalik, W.; Natkański, P.; Michalik, M. Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study. Int. J. Environ. Res. Public Health 2022, 19, 12252. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. Int. 2018, 25, 11319–11332. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int. 2019, 132, 105127. [Google Scholar] [CrossRef] [PubMed]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Overview on the occurrence of microplastics in air and implications from the use of face masks during the COVID-19 pandemic. Sci. Total Environ. 2021, 800, 149555. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, M.; Lee, Y.; Park, J.; Lee, H.; Song, Y.C.; Ro, C.U. Novel Single-Particle Analytical Technique for Inhalable Airborne Microplastic Particles by the Combined Use of Fluorescence Microscopy, Raman Microspectrometry, and SEM/EDX. Anal. Chem. 2023, 95, 8552–8559. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef]
- Dümichen, E.; Barthel, A.K.; Braun, U.; Bannick, C.G.; Brand, K.; Jekel, M.; Senz, R. Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res. 2015, 85, 451–457. [Google Scholar] [CrossRef]
- Niu, S.; Liu, R.; Zhao, Q.; Gagan, S.; Dodero, A.; Ying, Q.; Ma, X.; Cheng, Z.; China, S.; Canagaratna, M. Quantifying the Chemical Composition and Real-Time Mass Loading of Nanoplastic Particles in the Atmosphere Using Aerosol Mass Spectrometry. Environ. Sci. Technol. 2024, 58, 3363–3374. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 15, 202–209. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process Impacts. 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Dini, L.; Panzarini, E.; Mariano, S.; Passeri, D.; Reggente, M.; Rossi, M.; Vergallo, C. Microscopies at the Nanoscale for Nano-Scale Drug Delivery Systems. Curr. Drug Targets 2015, 16, 1512–1530. [Google Scholar] [CrossRef] [PubMed]
- Damaj, S.; Trad, F.; Goevert, D.; Wilkesmann, J. Bridging the Gaps between Microplastics and Human Health. Microplastics 2024, 3, 46–66. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tu, C.; Li, R.; Wu, D.; Yang, J.; Xia, Y.; Peijnenburg, W.J.G.M.; Luo, Y. A systematic review of the impacts of exposure to micro- and nano-plastics on human tissue accumulation and health. Eco Environ. Health 2023, 2, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Dorsch, A.; Förschner, F.; Ravandeh, M.; da Silva Brito, W.A.; Saadati, F.; Delcea, M.; Wende, K.; Bekeschus, S. Nanoplastic Size and Surface Chemistry Dictate Decoration by Human Saliva Proteins. ACS Appl. Mater. Interfaces 2024, 16, 25977–25993. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Techol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, S.; Bittar, T.B.; Stubbins, A.; Li, D. Photochemical dissolution of buoyant microplastics to dissolved organic carbon: Rates and microbial impacts. J. Hazard. Mater. 2020, 383, 121065. [Google Scholar] [CrossRef]
- Hüffer, T.; Praetorius, A.; Wagner, S.; von der Kammer, F.; Hofmann, T. Microplastic Exposure Assessment in Aquatic Environments: Learning from Similarities and Differences to Engineered Nanoparticles. Environ. Sci. Technol. 2017, 51, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jia, Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. iScience 2023, 26, 106061. [Google Scholar] [CrossRef]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood-Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, W.; Choi, D.; Heo, J.; Han, U.; Jung, S.Y.; Park, H.H.; Hong, S.-T.; Park, J.H.; Hong, J. Potential threats of nanoplastic accumulation in human induced pluripotent stem cells. Chem. Eng. J. 2022, 427, 131841. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Zhou, C.S.; Wu, J.W.; Liu, B.F.; Ma, W.L.; Yang, S.S.; Cao, G.L. Micro nanoplastics promote the risk of antibiotic resistance gene propagation in biological phosphorus removal system. J. Hazard. Mater. 2022, 431, 128547. [Google Scholar] [CrossRef]
- Poma, A.M.G.; Morciano, P.; Aloisi, M. Beyond genetics: Can micro and nanoplastics induce epigenetic and gene-expression modifications? Front. Epigenet. Epigenom. 2023, 1, 1241583. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Garcia, M.M.; Romero, A.S.; Merkley, S.D.; Meyer-Hagen, J.L.; Forbes, C.; Hayek, E.E.; Sciezka, D.P.; Templeton, R.; Gonzalez-Estrella, J.; Jin, Y.; et al. In Vivo Tissue Distribution of Microplastics and Systemic Metabolomic Alterations After Gastrointestinal Exposure. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Junaid, M.; Liu, S.; Yue, Q.; Wei, M.; Wang, J. Trophic transfer and interfacial impacts of micro(nano)plastics and per-and polyfluoroalkyl substances in the environment. J. Hazard. Mater. 2024, 465, 133243. [Google Scholar] [CrossRef]
- Kleinteich, J.; Seidensticker, S.; Marggrander, N.; Zarfl, C. Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. Int. J. Environ. Res. Public Health 2018, 15, 287. [Google Scholar] [CrossRef]
- Huang, B.; Tan, Z.; Bohinc, K.; Zhang, S. Interaction between nanoparticles and charged phospholipid membranes. Phys. Chem. Chem. Phys. 2018, 20, 29249–29263. [Google Scholar] [CrossRef]
- Kihara, S.; Ashenden, A.; Kaur, M.; Glasson, J.; Ghosh, S.; van der Heijden, N.; Brooks, A.E.S.; Mata, J.P.; Holt, S.; Domigan, L.J.; et al. Cellular interactions with polystyrene nanoplastics-The role of particle size and protein corona. Biointerphases 2021, 16, 041001. [Google Scholar] [CrossRef]
- Milillo, C.; Aruffo, E.; Di Carlo, P.; Patruno, A.; Gatta, M.; Bruno, A.; Dovizio, M.; Marinelli, L.; Dimmito, M.P.; Di Giacomo, V.; et al. Polystyrene nanoplastics mediate oxidative stress, senescence, and apoptosis in a human alveolar epithelial cell line. Front. Public Health 2024, 12, 1385387. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef]
- Pulvirenti, E.; Ferrante, M.; Barbera, N.; Favara, C.; Aquilia, E.; Palella, M.; Cristaldi, A.; Conti, G.O.; Fiore, M. Effects of Nano and Microplastics on the Inflammatory Process: In Vitro and In Vivo Studies Systematic Review. Front. Biosci. 2022, 27, 287. [Google Scholar] [CrossRef]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Xiong, F.; Xu, K.; Huang, J.; Liu, J.; Wang, D.; Pu, Y. Polystyrene micro- and nanoplastics induce gastric toxicity through ROS mediated oxidative stress and P62/Keap1/Nrf2 pathway. Sci. Total Environ. 2024, 912, 169228. [Google Scholar] [CrossRef]
- Li, L.; Lv, X.; He, J.; Zhang, L.; Li, B.; Zhang, X.; Liu, S.; Zhang, Y. Chronic exposure to polystyrene nanoplastics induces intestinal mechanical and immune barrier dysfunction in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115749. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ. Pollut. 2020, 265, 115025. [Google Scholar] [CrossRef]
- Jung, B.K.; Han, S.W.; Park, S.H.; Bae, J.S.; Choi, J.; Ryu, K.Y. Neurotoxic potential of polystyrene nanoplastics in primary cells originating from mouse brain. Neurotoxicology 2020, 81, 189–196. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- German Federal Institute for Risk Assessment (BfR), Department of Food Safety, Unit Effect-based Analytics and Toxicogenomics Unit and Nanotoxicology Junior Research Group, Berlin, Germany; Shopova, S.; Sieg, H.; Braeuning, A. Risk assessment and toxicological research on micro- and nanoplastics after oral exposure via food products. EFSA J. 2020, 18, e181102. [Google Scholar] [CrossRef]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.E.; Smyth, S.H.; McCullough, M.T.; Morris, J.F.; Moyes, S.M. Morphological aspects of interactions between microparticles and mammalian cells: Intestinal uptake and onward movement. Prog. Histochem. Cytochem. 2012, 46, 185–252. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Smith, J.R.H.; Etherington, G.; Shutt, A.L.; Youngman, M.J. A study of aerosol deposition and clearance from the human nasal passage. Ann. Occup. Hyg. 2002, 46, 309–313. [Google Scholar] [CrossRef]
- Asgharian, B.; Hofmann, W.; Miller, F.J. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J. Aerosol Sci. 2001, 32, 817–832. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, S.; Ray, S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. Int. 2013, 20, 4339–4355. [Google Scholar] [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.; Helsdingen, R.; van der Zande, M.; Rietjens, I.M.; Bouwmeester, H. In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model. Nanotoxicology 2015, 9, 886–894. [Google Scholar] [CrossRef]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Hendriksen, P.J.; Woutersen, R.A.; van der Zande, M.; Undas, A.K.; Helsdingen, R.; van den Berg, H.H.; Rietjens, I.M.; Bouwmeester, H. Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J. Nanopart. Res. 2015, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Sarlo, K.; Blackburn, K.L.; Clark, E.D.; Grothaus, J.; Chaney, J.; Neu, S.; Flood, J.; Abbott, D.; Bohne, C.; Casey, K.; et al. Tissue distribution of 20 nm, 100 nm and 1000 nm fluorescent polystyrene latex nanospheres following acute systemic or acute and repeat airway exposure in the rat. Toxicology 2009, 263, 117–126. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hirota, K.; Tomoda, K.; Ito, F.; Inagawa, H.; Kochi, C.; Soma, G.; Makino, K.; Terada, H. Phagocytic activity of alveolar macrophages toward polystyrene latex microspheres and PLGA microspheres loaded with anti-tuberculosis agent. Colloids Surf. B Biointerfaces. 2007, 60, 221–228. [Google Scholar] [CrossRef]
- Tomazic-Jezic, V.J.; Merritt, K.; Umbreit, T.H. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J. Biomed. Mater. Res. 2001, 55, 523–529. [Google Scholar] [CrossRef]
- Volkheimer, G. Passage of particles through the wall of the gastrointestinal tract. Environ. Health Perspect. 1974, 9, 215–225. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One Hundred and Twenty-Seven Cultured Human Tumor Cell Lines Producing Tumores in Nude Mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Pinto, M.; Robine-Leon, S.; Appay, M.D. Enterocyte-like differentiation and polarization of the human colon Carcinoma Cell Line Caco-2 in culture. Biol. Cell. 1983, 47, 323–330. [Google Scholar]
- Hilgers, A.R.; Conradi, R.A.; Burton, P.S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharmaceut. Res. 1990, 7, 902–910. [Google Scholar] [CrossRef]
- Buhrke, T.; Lengler, I.; Lampen, A. Analysis of proteomic changes induced upon cellular differentiation of the human intestinal cell line Caco-2. Dev. Growth Differ. 2011, 53, 411–426. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Guo, J.; Gu, A.Z.; Li, D.; Chen, J. Toxicity Assessment of Nano-ZnO Exposure on the Human Intestinal Microbiome, Metabolic Functions, and Resistome Using an In Vitro Colon Simulator. Environ. Sci. Techol. 2021, 55, 6884–6896. [Google Scholar] [CrossRef]
- Li, X.; Song, L.; Hu, X.; Liu, C.; Shi, J.; Wang, H.; Zhan, L.; Song, H. Inhibition of Epithelial-Mesenchymal Transition and Tissue Regeneration by Waterborne Titanium Dioxide Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Covello, C.; Di Vincenzo, F.; Cammarota, G.; Pizzoferrato, M. Micro(nano)plastics and Their Potential Impact on Human Gut Health: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 2658–2677. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, X.; Wang, Y.; Su, J.; Li, G.; Wang, X.; Yang, Y.; Zhang, Y.; Li, B.; Zhang, G.; et al. Biological interactions of polystyrene nanoplastics: Their cytotoxic and immunotoxic effects on the hepatic and enteric systems. Ecotoxicol. Environ. Saf. 2023, 264, 115447. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, L.; Gu, W.; Li, X.; Han, Z.; Fu, X.; Wang, X.; Li, X.; Su, Z. Continuous oral exposure to micro- and nanoplastics induced gut microbiota dysbiosis, intestinal barrier and immune dysfunction in adult mice. Environ. Int. 2023, 182, 108353. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ba, T.; Pu, J.; Chen, T.; Song, Y.; Gu, Y.; Qian, Q.; Xu, Y.; Xiang, K.; et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci. Total Environ. 2021, 785, 147365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Makarova, O.; Fokichev, N. Harmful Effects of the Microplastic Pollution on Animal Health: A Literature Review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef]
- Almeida, A.S.; de Souza, C.B. The Impact of Microplastic on Human Health. Curr. Biotechnol. 2021, 10, 158–167. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Y.; Long, J.; Yang, X.; Bao, L.; Yang, Z.; Wu, B.; Si, R.; Zhao, W.; Peng, C.; et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci. Total Environ. 2022, 838, 155937. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo Hasselerharm, P.E.; Mohamed Nor, N.H.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Welle, F.; Franz, R. Microplastic in bottled natural mineral water–literature review and considerations on exposure and risk assessment. Food Addit. Contam. Part A 2018, 35, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Techol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, J.E.; Lee, S.J.; Gong, J.E.; Jin, Y.J.; Seo, S.; Lee, J.H.; Hwang, D.Y. Inflammatory response in the mid colon of ICR mice treated with polystyrene microplastics for two weeks. Lab. Anim. Res. 2021, 37, 31. [Google Scholar] [CrossRef] [PubMed]
- Rawle, D.J.; Dumenil, T.; Tang, B.; Bishop, C.R.; Yan, K.; Le, T.T.; Suhrbier, A. Microplastic consumption induces inflammatory signatures in the colon and prolongs a viral arthritis. Sci. Total Environ. 2022, 809, 152212. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, Y.; Liu, S.; Chen, Y.; Yuan, H.; Xu, H. Polystyrene microplastics exacerbated liver injury from cyclophosphamide in mice: Insight into gut microbiota. Sci. Total Environ. 2022, 840, 156668. [Google Scholar] [CrossRef]
- Xie, L.; Chen, T.; Liu, J.; Hou, Y.; Tan, Q.; Zhang, X.; Li, Z.; Farooq, T.H.; Yan, W.; Li, Y. Intestinal flora variation reflects the short-term damage of microplastic to the intestinal tract in mice. Ecotoxicol. Environ. Saf. 2022, 246, 114194. [Google Scholar] [CrossRef]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to Polypropylene Microplastics via Oral Ingestion Induces Colonic Apoptosis and Intestinal Barrier Damage through Oxidative Stress and Inflammation in Mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lin, W.Y.; Cheng, T.J. Microbiota-mediated metabolic perturbations in the gut and brain of mice after microplastic exposure. Chemosphere 2024, 350, 141026. [Google Scholar] [CrossRef]
- Xu, R.; Cao, J.W.; Lv, H.L.; Geng, Y.; Guo, M.Y. Polyethylene microplastics induced gut microbiota dysbiosis leading to liver injury via the TLR2/NF-κB/NLRP3 pathway in mice. Sci. Total Environ. 2024, 917, 170518. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, Y.; Wei, Y.; Guo, Y.; Wang, Y.; Song, P.; Yu, J. Gut microbiota and liver metabolomics reveal the potential mechanism of Lactobacillus rhamnosus GG modulating the liver toxicity caused by polystyrene microplastics in mice. Environ. Sci. Pollut. Res. Int. 2024, 31, 6527–6542. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Li, P.; Zhu, X.; Chen, D.; Ommati, M.M.; Wang, H.; Han, L.; Xu, S.; Sun, P. Hepatotoxic of polystyrene microplastics in aged mice: Focus on the role of gastrointestinal transformation and AMPK/FoxO pathway. Sci. Total Environ. 2024, 917, 170471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.; Chan, H.; Peng, J.; Zhu, P.; Li, J.; Jiang, X.; Zhang, Z.; Wang, Y.; Tan, Z.; et al. Polystyrene microplastics induce size-dependent multi-organ damage in mice: Insights into gut microbiota and fecal metabolites. J. Hazard. Mater. 2024, 461, 132503. [Google Scholar] [CrossRef]
- Zeng, G.; Li, J.; Wang, Y.; Su, J.; Lu, Z.; Zhang, F.; Ding, W. Polystyrene microplastic-induced oxidative stress triggers intestinal barrier dysfunction via the NF-κB/NLRP3/IL-1β/MCLK pathway. Environ. Pollut. 2024, 345, 123473. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, D.; Zhan, J.; Liu, X.; Li, P.; Ma, X.; Hou, H.; Wang, P. Polystyrene microplastics induce kidney injury via gut barrier dysfunction and C5a/C5aR pathway activation. Environ. Pollut. 2024, 342, 122909. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Meng, Z.; Fu, B.; Yang, Z.; Xu, Y.; Huang, H.; Bai, Y.; Fang, X.; Shen, S.; Yang, J.; Yong, J.; et al. Polydopamine-coated thalidomide nanocrystals promote DSS-induced murine colitis recovery through Macrophage M2 polarization together with the synergistic anti-inflammatory and anti-angiogenic effects. Int. J. Pharm. 2023, 630, 122376. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Ng, S.C. Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 2016, 1, 307–316. [Google Scholar] [CrossRef]
- Duan, R.; Wu, Y.; Wang, M.; Wu, J.; Wang, X.; Wang, Z.; Hu, Y.; Duan, L. Association between short-term exposure to fine particulate pollution and outpatient visits for ulcerative colitis in Beijing, China: A time-series study. Ecotoxicol. Environ. Saf. 2021, 214, 112116. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Curtis, V.F.; Campbell, E.L. The inflammatory tissue microenvironment in IBD. Inflamm. Bowel Dis. 2013, 19, 2238–2244. [Google Scholar] [CrossRef]
- Strugala, V.; Dettmar, P.W.; Pearson, J.P. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int. J. Clin. Pract. 2008, 62, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic. Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Ogino, T.; Nishimura, J.; Barman, S.; Kayama, H.; Uematsu, S.; Okuzaki, D.; Osawa, H.; Haraguchi, N.; Uemura, M.; Hata, T.; et al. Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology 2013, 145, 1380–1391.e1. [Google Scholar] [CrossRef]
- Makita, S.; Kanai, T.; Oshima, S.; Uraushihara, K.; Totsuka, T.; Sawada, T.; Nakamura, T.; Koganei, K.; Fukushima, T.; Watanabe, M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J. Immunol. 2004, 173, 3119–3130. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef]
- Yao, J.; Wei, C.; Wang, J.Y.; Zhang, R.; Li, Y.X.; Wang, L.S. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J. Gastroenterol. 2015, 21, 6572–6581. [Google Scholar] [CrossRef]
- Sheehan, D.; Moran, C.; Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Techol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Ma, J.; Wan, Y.; Song, L.; Wang, L.; Wang, H.; Li, Y.; Huang, D. Polystyrene nanobeads exacerbate chronic colitis in mice involving in oxidative stress and hepatic lipid metabolism. Part. Fibre Toxicol. 2023, 20, 49. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.; Lehr, C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307, 135662. [Google Scholar] [CrossRef]
- Merkley, S.D.; Moss, H.C.; Goodfellow, S.M.; Ling, C.L.; Meyer-Hagen, J.L.; Weaver, J.; Campen, M.J.; Castillo, E.F. Polystyrene microplastics induce an immunometabolic active state in macrophages. Cell Biol. Toxicol. 2022, 38, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, R.; Li, Z.; Liu, C.; Chen, Y.; Yu, Q. Microplastics perturb colonic epithelial homeostasis associated with intestinal overproliferation, exacerbating the severity of colitis. Environ. Res. 2023, 217, 114861. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef]
- Luo, T.; Wang, D.; Zhao, Y.; Li, X.; Yang, G.; Jin, Y. Polystyrene microplastics exacerbate experimental colitis in mice tightly associated with the occurrence of hepatic inflammation. Sci. Total Environ. 2022, 844, 156884. [Google Scholar] [CrossRef]
- Chlittleborough, T.J.; Gutlic, I.; Pearson, J.F.; Watson, A.; Bhatti, L.A.; Buchwald, P.; Potter, J.D.; Wakeman, C. Increasing Incidence of Young-Onset Colorectal Carcinoma A 3-Country Population Analysis. Dis. Colon. Rectum 2020, 63, 903–910. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Giovannucci, E.L.; Colditz, G.A.; Hunter, D.J.; Speizer, F.E.; Willett, W.C. A prospective study of family history and the risk of colorectal cancer. N. Engl. J. Med. 1994, 331, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.V.; Dollin, J. Half a century of the oral contraceptive pill: Historical review and view to the future. Can. Fam. Physician 2012, 58, e757–e760. [Google Scholar] [PubMed]
- Murphy, C.C.; Singal, A.G. Establishing a research agenda for early-onset colorectal cancer. PLoS Med. 2018, 15, e1002577. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef]
- Coleman, O.I.; Haller, D. Microbe-Mucus Interface in the Pathogenesis of Colorectal Cancer. Cancers 2021, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Korpela, K. Diet, Microbiota, and Metabolic Health: Trade-Off Between Saccharolytic and Proteolytic Fermentation. Annu. Rev. Food Sci. Technol. 2018, 9, 65–84. [Google Scholar] [CrossRef]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef]
- Baj, J.; Dring, J.C.; Czeczelewski, M.; Kozyra, P.; Forma, A.; Flieger, J.; Kowalska, B.; Buszewicz, G. Derivatives of Plastics as Potential Carcinogenic Factors: The Current State of Knowledge. Cancers 2022, 14, 4637. [Google Scholar] [CrossRef]
- Domenech, J.; Annangi, B.; Marcos, R.; Hernandez, A.; Catalan, J. Insights into the potential carcinogenicity of micro- and nano-plastics. Mutat. Res. Rev. Mutat. Res. 2023, 791, 108453. [Google Scholar] [CrossRef]
- Shaw, I.; Jones, H. Shaw and Jones reply: The multifactorial nature of carcinogenesis. Trends Pharmacol. Sci. 1994, 15, 323. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Iyadorai, T.; Mariappan, V.; Vellasamy, K.M.; Wanyiri, J.W.; Roslani, A.C.; Lee, G.K.; Sears, C.; Vadivelu, J. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS ONE 2020, 15, e0228217. [Google Scholar] [CrossRef] [PubMed]
- Nougayrede, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Reuter, C.; Alzheimer, M.; Walles, H.; Oelschlaeger, T.A. An adherent mucus layer attenuates the genotoxic effect of colibactin. Cell. Microbiol. 2018, 20, e12812. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Shaw, I.C.; Ye, H.; Shaw, I.C. Dietary isoflavone-induced, estrogen receptor-β-mediated proliferation of Caco-2 cells is modulated by gallic acid. Food Chem. Toxicol. 2020, 145, 111743. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Shaw, I. Does the oestrogen receptor encourage oestrogenicity in environmental pollutants? The case of 4-nonylphenol. SAR QSAR Environ. Res. 2011, 22, 329–350. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell. Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Jeong, C.B.; Kang, H.M.; Lee, M.C.; Kim, D.H.; Han, J.; Hwang, D.S.; Souissi, S.; Lee, S.J. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017, 7, 41323. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Enss, M.L.; Cornberg, M.; Wagner, S.; Gebert, A.; Henrichs, M.; Eisenblatter, R.; Beil, W.; Kownatzki, R. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm. Res. 2000, 49, 162–169. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Brynzak-Schreiber, E.; Schögl, E.; Bapp, C.; Cseh, K.; Kopatz, V.; Jakupec, M.A.; Weber, A.; Lange, T.; Toca-Herrera, J.L.; Del Favero, G.; et al. Microplastics role in cell migration and distribution during cancer cell division. Chemosphere 2024, 353, 141463. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, S.; Kim, O.H.; Kim, C.H.; Kim, J.; Kim, J.W.; Hong, S.; Lee, H.J. Polypropylene microplastics promote metastatic features in human breast cancer. Sci. Rep. 2023, 13, 6252. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Tacconelli, S.; Contursi, A.; Ballerini, P.; Patrignani, P. Cyclooxygenases and platelet functions. Adv. Pharmacol. 2023, 97, 133–165. [Google Scholar] [CrossRef] [PubMed]
- Dovizio, M.; Ballerini, P.; Fullone, R.; Tacconelli, S.; Contursi, A.; Patrignani, P. Multifaceted Functions of Platelets in Cancer: From Tumorigenesis to Liquid Biopsy Tool and Drug Delivery System. Int. J. Mol. Sci. 2020, 21, 9585. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dovizio, M.; Tacconelli, S.; Contursi, A.; Ballerini, P.; Patrignani, P. Antithrombotic Agents and Cancer. Cancers 2018, 10, 253. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef]
- Contursi, A.; Sacco, A.; Grande, R.; Dovizio, M.; Patrignani, P. Platelets as crucial partners for tumor metastasis: From mechanistic aspects to pharmacological targeting. Cell Mol. Life Sci. 2017, 74, 3491–3507. [Google Scholar] [CrossRef]
- Sacco, A.; Bruno, A.; Contursi, A.; Dovizio, M.; Tacconelli, S.; Ricciotti, E.; Guillem-Llobat, P.; Salvatore, T.; Di Francesco, L.; Fullone, R.; et al. Platelet-Specific Deletion of Cyclooxygenase-1 Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice. J. Pharmacol. Exp. Ther. 2019, 370, 416–426. [Google Scholar] [CrossRef]
- Bruno, A.; Contursi, A.; Tacconelli, S.; Sacco, A.; Hofling, U.; Mucci, M.; Lamolinara, A.; Del Pizzo, F.; Ballerini, P.; Di Gregorio, P.; et al. The specific deletion of cyclooxygenase-1 in megakaryocytes/platelets reduces intestinal polyposis in ApcMin/+ mice. Pharmacol. Res. 2022, 185, 106506. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Dovizio, M.; Maier, T.J.; Alberti, S.; Di Francesco, L.; Marcantoni, E.; Münch, G.; John, C.M.; Suess, B.; Sgambato, A.; Steinhilber, D.; et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol. Pharmacol. 2013, 84, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Llobat, P.; Dovizio, M.; Bruno, A.; Ricciotti, E.; Cufino, V.; Sacco, A.; Grande, R.; Alberti, S.; Arena, V.; Cirillo, M.; et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget 2016, 7, 32462–32477. [Google Scholar] [CrossRef]
- Dovizio, M.; Bruno, A.; Contursi, A.; Grande, R.; Patrignani, P. Platelets and extracellular vesicles in cancer: Diagnostic and therapeutic implications. Cancer Metastasis Rev. 2018, 37, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Contursi, A.; Fullone, R.; Szklanna-Koszalinska, P.; Marcone, S.; Lanuti, P.; Taus, F.; Meneguzzi, A.; Turri, G.; Dovizio, M.; Bruno, A.; et al. Tumor-Educated Platelet Extracellular Vesicles: Proteomic Profiling and Crosstalk with Colorectal Cancer Cells. Cancers 2023, 15, 350. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; et al. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 1577–1584. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schürch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef]
- Li, H.; Kilgallen, A.B.; Münzel, T.; Wolf, E.; Lecour, S.; Schulz, R.; Daiber, A.; Van Laake, L.W. Influence of mental stress and environmental toxins on circadian clocks: Implications for redox regulation of the heart and cardioprotection. Br. J. Pharmacol. 2020, 177, 5393–5412. [Google Scholar] [CrossRef] [PubMed]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.; Dinsdale, D.; Smith, T.; Xu, H.; Vermylen, J.; Nemery, B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am. J. Respir. Crit. Care Med. 2002, 166, 998–1004. [Google Scholar] [CrossRef]
- Oslakovic, C.; Cedervall, T.; Linse, S.; Dahlbäck, B. Polystyrene nanoparticles affecting blood coagulation. Nanomedicine 2012, 8, 981–986. [Google Scholar] [CrossRef]
- Bihari, P.; Holzer, M.; Praetner, M.; Fent, J.; Lerchenberger, M.; Reichel, C.A.; Rehberg, M.; Lakatos, S.; Krombach, F. Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation. Toxicology 2010, 269, 148–154. [Google Scholar] [CrossRef] [PubMed]
- McGuinnes, C.; Duffin, R.; Brown, S.L.; Mills, N.; Megson, I.L.; Macnee, W.; Johnston, S.; Lu, S.L.; Tran, L.; Li, R.; et al. Surface derivatization state of polystyrene latex nanoparticles determines both their potency and their mechanism of causing human platelet aggregation in vitro. Toxicol. Sci. 2011, 119, 359–368. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; De Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, Z.; Liu, X.; Cai, Y.; Huang, X.; Fang, J.; Shen, R.; Lu, W.; Xiao, Y.; Zhuang, W.; et al. Multimodal detection and analysis of microplastics in human thrombi from multiple anatomically distinct sites. EBioMedicine 2024, 103, 105118. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef]
- Li, S.; Keenan, J.I.; Shaw, I.C.; Frizelle, F.A. Could Microplastics Be a Driver for Early Onset Colorectal Cancer? Cancers 2023, 15, 3323. [Google Scholar] [CrossRef] [PubMed]
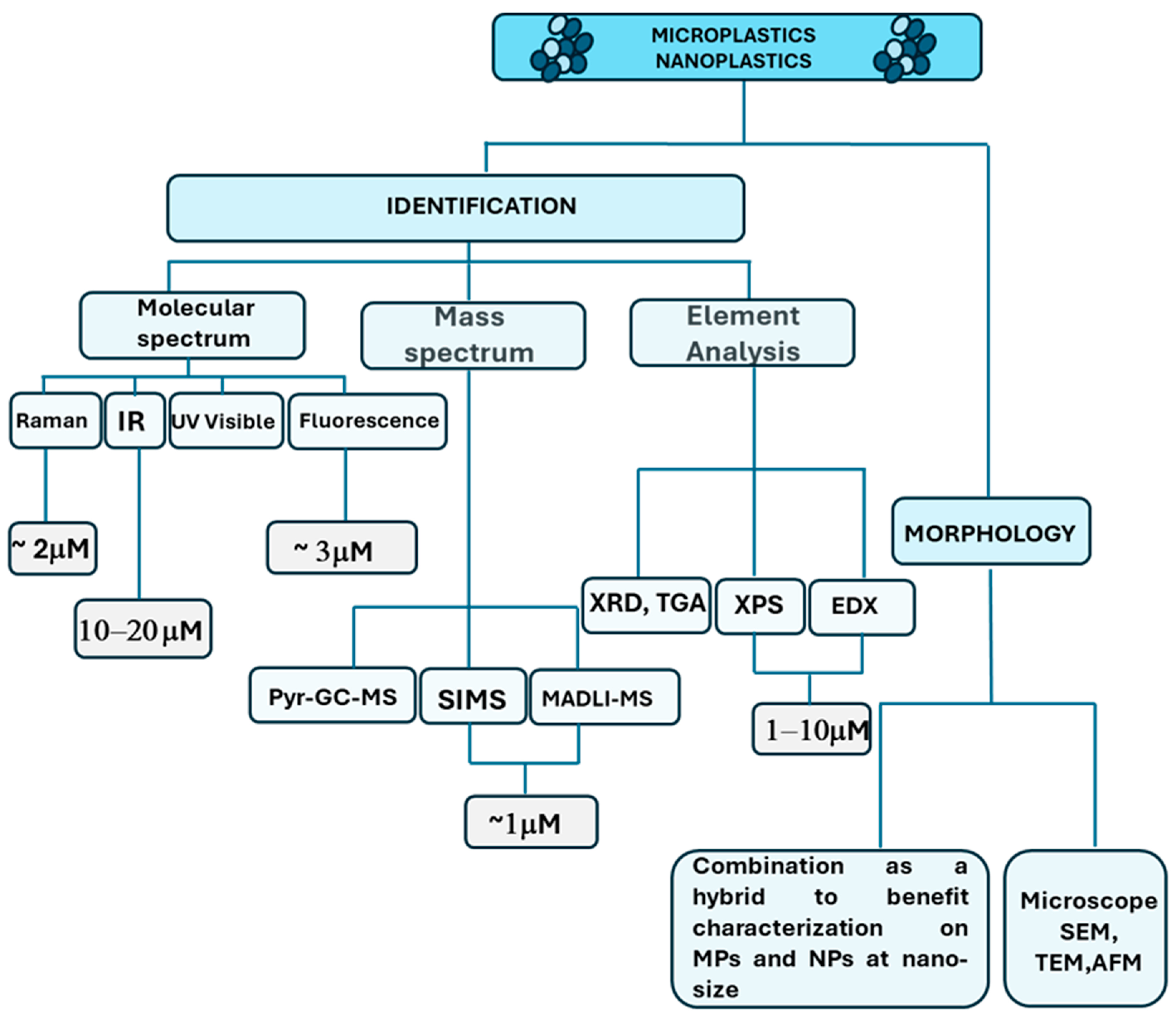


| Method | Principle | Advantages | Drawbacks | Refs. |
|---|---|---|---|---|
| Stereo microscopy | Samples identification directly under optical microscopes |
|
| [83,95] |
| Scanning electron microscopy (SEM) | Images are generated through the interaction of an electron beam with the sample |
|
| [73,83,96] |
| Transmission electron Microscopy (TEM) | Sample observation (also of infinitesimal sizes) is possible due to the wave properties of the electrons, emitted by a thin filament of thermoionic material |
|
| [8,97] |
| Fluorescence microscopy | It collects fluorescent emission from the samples excited by a specific wavelength |
|
| [8] |
| Fourier-transform infrared spectroscopy (FTIR) | Samples are exposed to infrared radiation, and the spectra are analyzed by comparison with known spectra in libraries |
|
| [8,83] |
| Raman apectroscopy | The excited light can be detected after laser irradiation of the sample. The frequency shift between two lights allows the identification of molecular structure and chemical components of samples. |
|
| [8,83,98] |
| Pyrolysis–gas chromatography–mass spectroscopy | Thermal degradation products of MPs are identified and compared to the database for determining polymer types. |
|
| [8,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, A.; Dovizio, M.; Milillo, C.; Aruffo, E.; Pesce, M.; Gatta, M.; Chiacchiaretta, P.; Di Carlo, P.; Ballerini, P. Orally Ingested Micro- and Nano-Plastics: A Hidden Driver of Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 3079. https://doi.org/10.3390/cancers16173079
Bruno A, Dovizio M, Milillo C, Aruffo E, Pesce M, Gatta M, Chiacchiaretta P, Di Carlo P, Ballerini P. Orally Ingested Micro- and Nano-Plastics: A Hidden Driver of Inflammatory Bowel Disease and Colorectal Cancer. Cancers. 2024; 16(17):3079. https://doi.org/10.3390/cancers16173079
Chicago/Turabian StyleBruno, Annalisa, Melania Dovizio, Cristina Milillo, Eleonora Aruffo, Mirko Pesce, Marco Gatta, Piero Chiacchiaretta, Piero Di Carlo, and Patrizia Ballerini. 2024. "Orally Ingested Micro- and Nano-Plastics: A Hidden Driver of Inflammatory Bowel Disease and Colorectal Cancer" Cancers 16, no. 17: 3079. https://doi.org/10.3390/cancers16173079








