Apoptosis of Pancreatic Cancer Cells after Co-Treatment with Eugenol and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagent and Antibodies
2.3. WST-8 Assay
2.4. Crystal Violet Assay
2.5. Colony Formation Assay
2.6. Wound Healing Assay
2.7. Flow Cytometry Analysis
2.8. Western Blotting
2.9. Caspase 3/7 Assay
2.10. ROS Measurement
2.11. Transfection (siRNA)
2.12. Statistical Analysis
3. Results
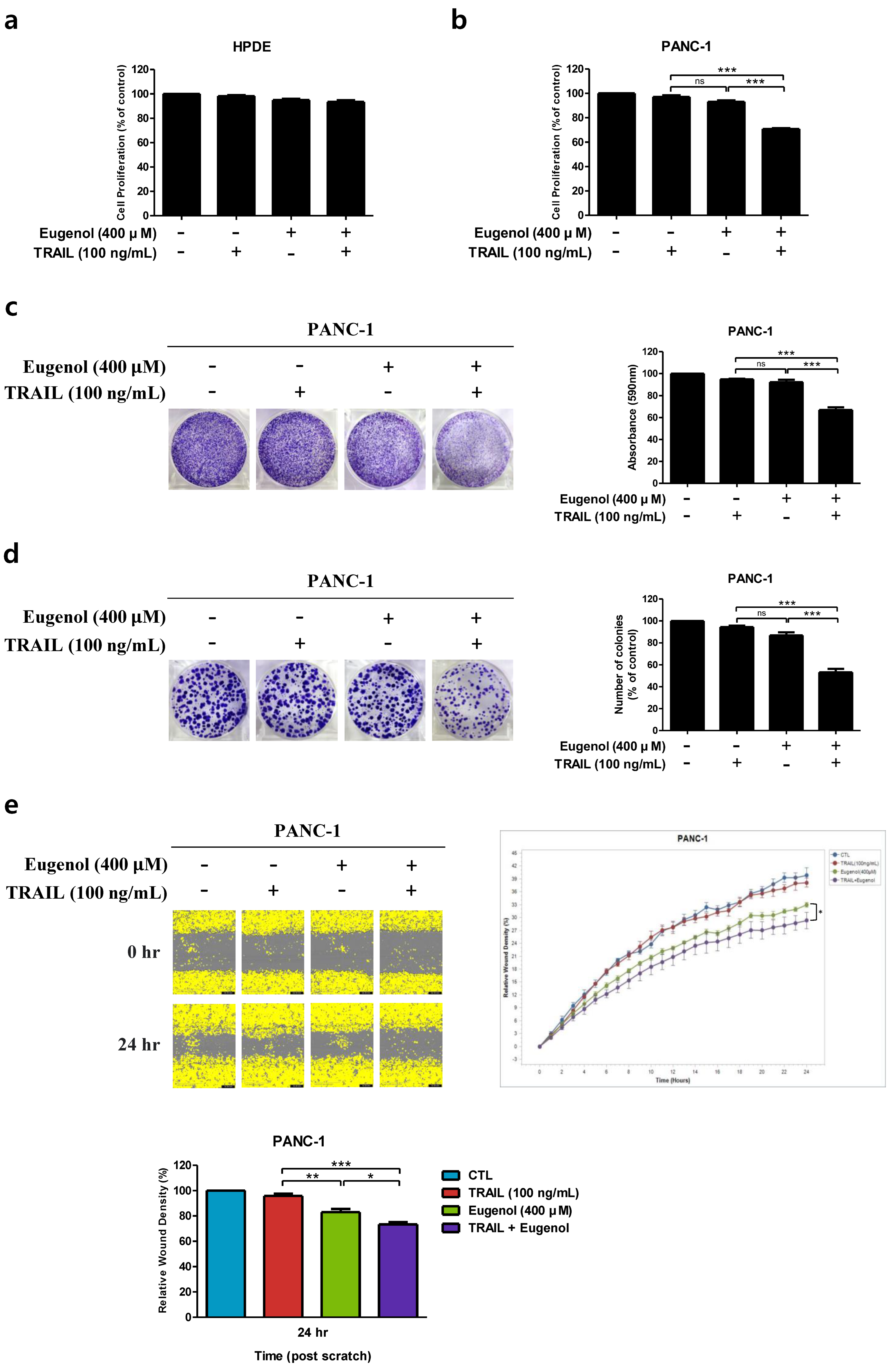
3.1. Effects of TRAIL and Eugenol on Human Pancreatic Cancer Cell Lines
3.2. Eugenol at Subtoxic Concentrations Enhances TRAIL-Induced Cell Death in Pancreatic Cancer Cells
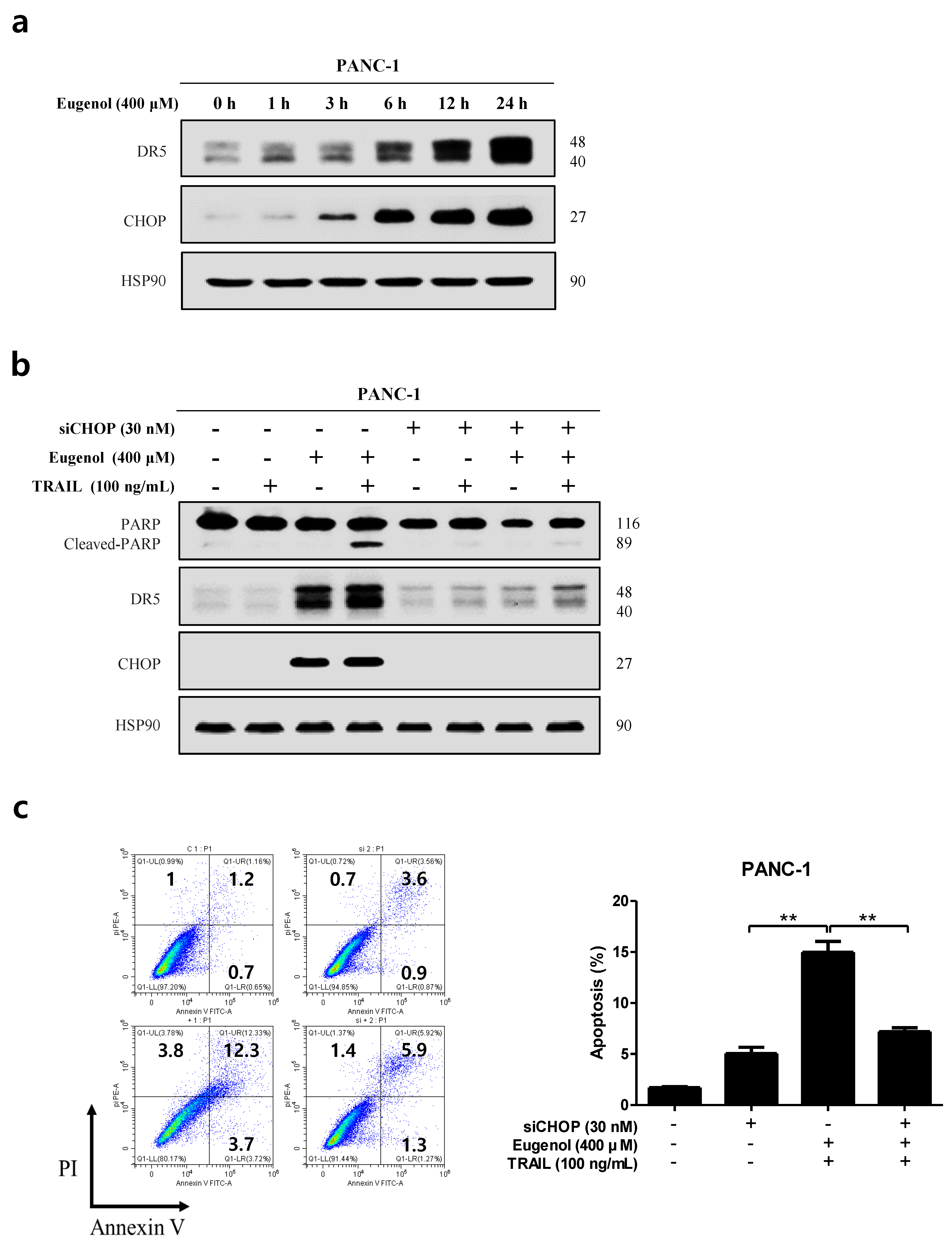
3.3. Co-Treatment with Eugenol and TRAIL Induces Apoptosis in Pancreatic Cancer Cells
3.4. Eugenol Increases TRAIL Sensitivity by Upregulating DR5
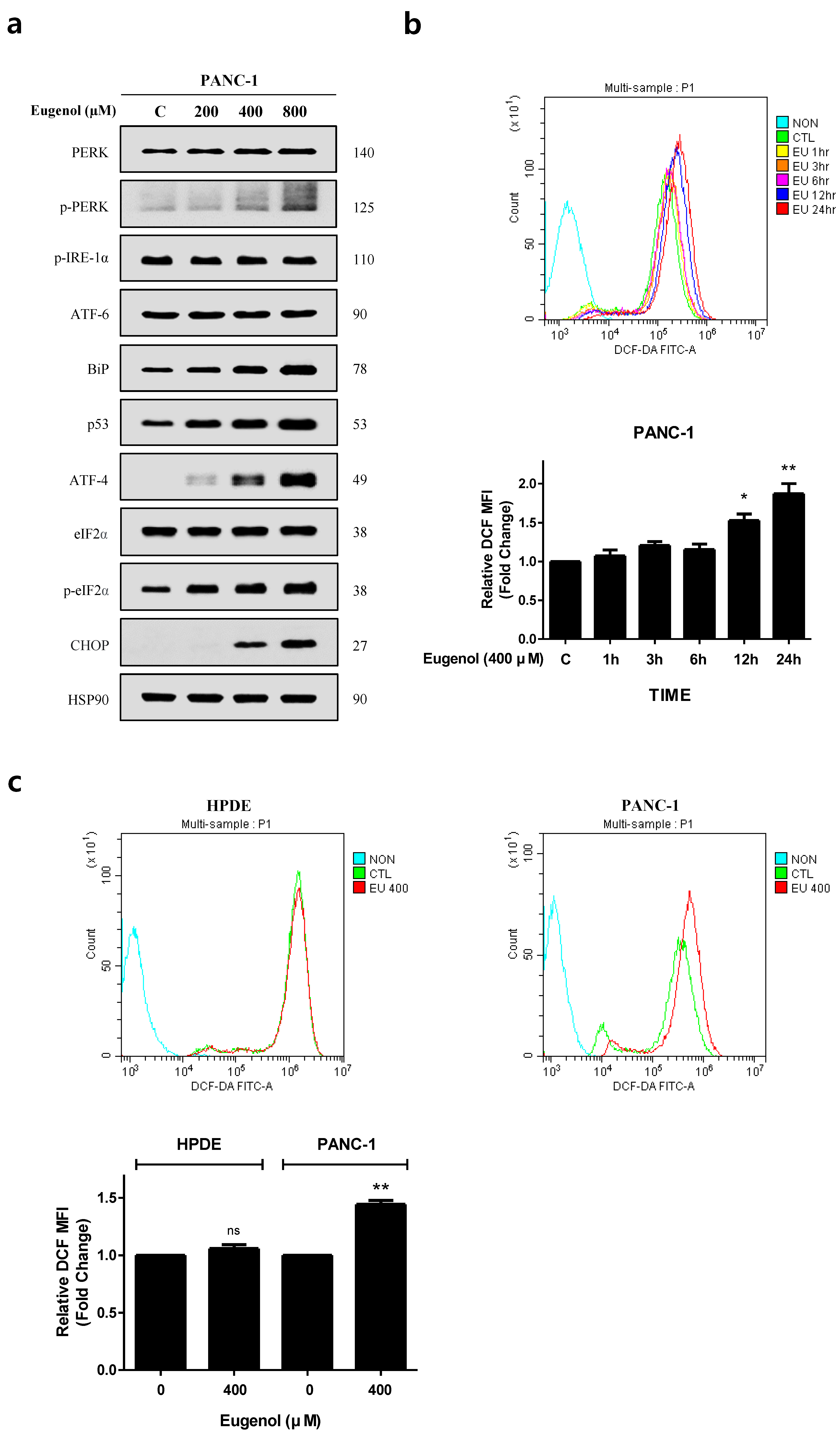
3.5. Eugenol Induces ROS and Activates the ER Stress Pathway
3.6. Enhanced Eugenol-Induced Apoptosis by DR5 and CHOP Upregulation Is ROS-Dependent
3.7. Effects of Eugenol on Anti-Apoptotic Proteins and DR Levels of Two Human Pancreatic Cancer Cell Lines
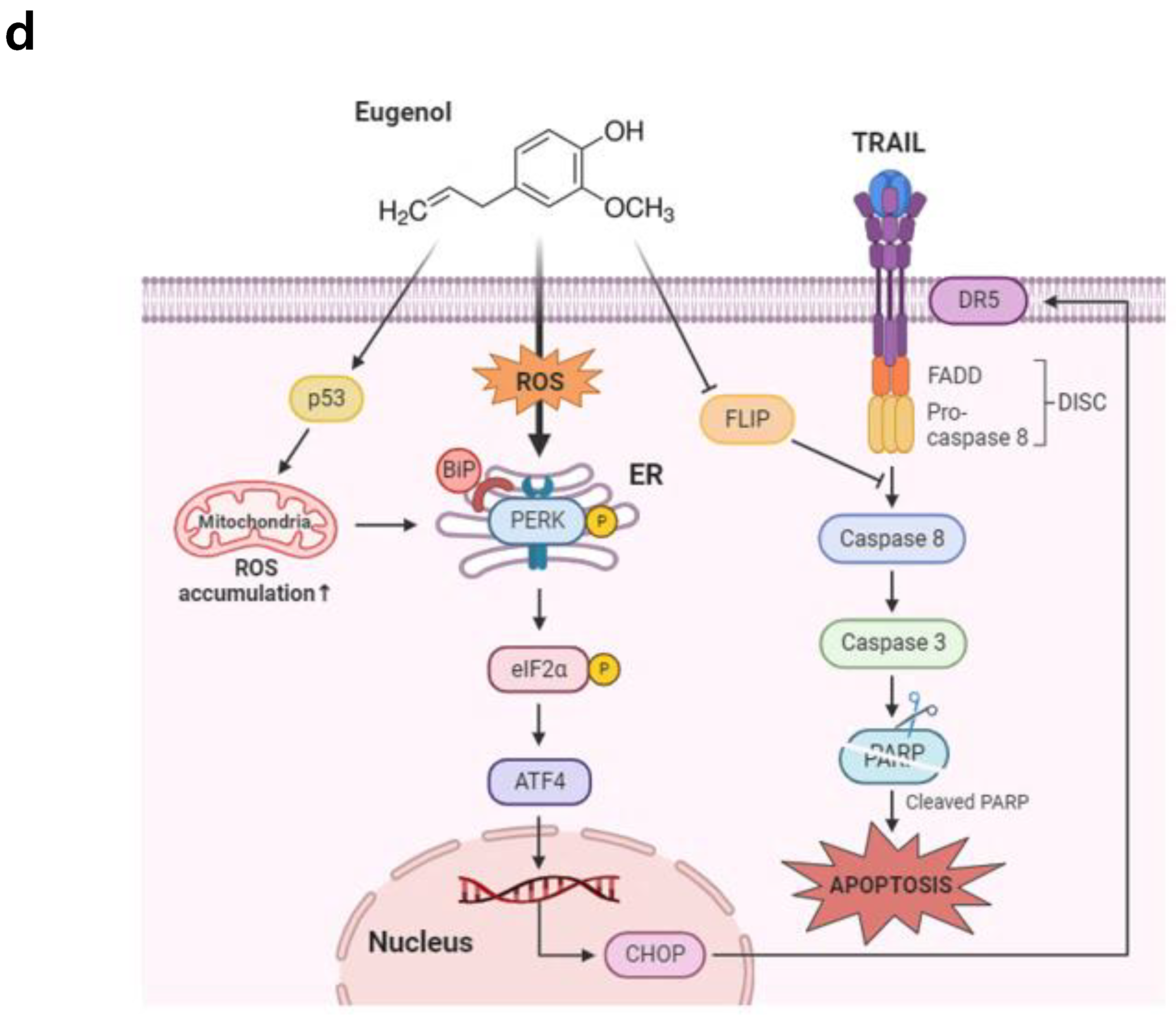
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, B.Y.; Woo, S.M. Treatment of metastatic pancreatic cancer. J. Dig. Cancer Rep. 2018, 6, 64–68. [Google Scholar]
- Lee, T.J.; Kwon, T.K. TNF-Related Apoptosis-Inducing Ligands (TRAIL) as anticancer agents. Cancer Prev. Res. 2006, 11, 9–21. [Google Scholar]
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G. Cannabidiol enhances the therapeutic effects of TRAIL by upregulating DR5 in colorectal cancer. Cancers 2019, 11, 642. [Google Scholar] [CrossRef]
- Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2002, 2, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Liu, L.-C.; Ho, C.-T.; Lin, Y.-C.; Way, T.-D. Pterostilbene enhances TRAIL-induced apoptosis through the induction of death receptors and downregulation of cell survival proteins in TRAIL-resistance triple negative breast cancer cells. J. Agric. Food Chem. 2017, 65, 11179–11191. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 1999, 11, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.; Abd-Rabou, A.; Reda, A. TRAIL combinations: The new ‘trail’ for cancer therapy. Oncol. Lett. 2014, 7, 1327–1332. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Sun, Q.; Zhang, Z.; Guo, X.; Sheng, X.; Tai, G.; Cheng, H.; Zhou, Y. Ginsenoside compound K sensitizes human colon cancer cells to TRAIL-induced apoptosis via autophagy-dependent and-independent DR5 upregulation. Cell Death Dis. 2016, 7, e2334. [Google Scholar] [CrossRef]
- Stuckey, D.W.; Shah, K. TRAIL on trial: Preclinical advances in cancer therapy. Trends Mol. Med. 2013, 19, 685–694. [Google Scholar] [CrossRef]
- Fulda, S. Safety and tolerability of TRAIL receptor agonists in cancer treatment. Eur. J. Clin. Pharmacol. 2015, 71, 525–527. [Google Scholar] [CrossRef]
- Borja-Cacho, D.; Yokoyama, Y.; Chugh, R.K.; Mujumdar, N.R.; Dudeja, V.; Clawson, K.A.; Dawra, R.K.; Saluja, A.K.; Vickers, S.M. TRAIL and triptolide: An effective combination that induces apoptosis in pancreatic cancer cells. J. Gastrointest. Surg. 2010, 14, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Shirley, S.; Morizot, A.; Micheau, O. Regulating TRAIL receptor-induced cell death at the membrane: A deadly discussion. Recent Pat. Anti-Cancer Drug Discov. 2011, 6, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Mishra, D.P. Trailing TRAIL resistance: Novel targets for TRAIL sensitization in cancer cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef]
- Voss, O.H.; Arango, D.; Tossey, J.C.; Villalona Calero, M.A.; Doseff, A.I. Splicing reprogramming of TRAIL/DISC-components sensitizes lung cancer cells to TRAIL-mediated apoptosis. Cell Death Dis. 2021, 12, 287. [Google Scholar] [CrossRef]
- Ganten, T.; Haas, T.L.; Sykora, J.; Stahl, H.; Sprick, M.; Fas, S.; Krueger, A.; Weigand, M.; Grosse-Wilde, A.; Stremmel, W. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004, 11 (Suppl. S1), S86–S96. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Shin, S.J.; Kim, H.-S. ERK1/2 activation mediated by the nutlin-3-induced mitochondrial translocation of p53. Int. J. Oncol. 2013, 42, 1027–1035. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Molagoda, I.M.N.; Jayasooriya, R.G.P.T.; Choi, Y.H.; Park, C.; Lee, K.T.; Lee, S.; Kim, G.-Y. p53-Mediated Oxidative Stress Enhances Indirubin-3′-Monoxime-Induced Apoptosis in HCT116 Colon Cancer Cells by Upregulating Death Receptor 5 and TNF-Related Apoptosis-Inducing Ligand Expression. Antioxidants 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, K.G. Endoplasmic reticulum stress and diabetes. J. Korean Endocr. Soc. 2008, 23, 1–8. [Google Scholar] [CrossRef][Green Version]
- Glab, J.A.; Doerflinger, M.; Nedeva, C.; Jose, I.; Mbogo, G.W.; Paton, J.C.; Paton, A.W.; Kueh, A.J.; Herold, M.J.; Huang, D. DR5 and caspase-8 are dispensable in ER stress-induced apoptosis. Cell Death Differ. 2017, 24, 944–950. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wang, H.-G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004, 279, 45495–45502. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A natural compound with versatile pharmacological actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Z.; Lin, M.; Shang, Y.; Wang, F.; Zhou, J.; Wang, F.; Zhang, X.; Luo, X.; Huang, W. In vitro and in vivo antitumor activity of cucurbitacin C, a novel natural product from cucumber. Front. Pharmacol. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, L.; Schulte, B.A.; Yang, A.; Tang, S.; Wang, G.Y. Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells. Int. J. Oncol. 2013, 43, 1999–2006. [Google Scholar] [CrossRef]
- Mérino, D.; Lalaoui, N.; Morizot, A.; Solary, E.; Micheau, O. TRAIL in cancer therapy: Present and future challenges. Expert. Opin. Ther. Targets 2007, 11, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef]
- Lee, D.H.; Sung, K.S.; Guo, Z.S.; Kwon, W.T.; Bartlett, D.L.; Oh, S.C.; Kwon, Y.T.; Lee, Y.J. TRAIL-Induced Caspase Activation Is a Prerequisite for Activation of the Endoplasmic Reticulum Stress-Induced Signal Transduction Pathways. J. Cell. Biochem. 2016, 117, 1078–1091. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, A.S. Stress induction of GRP78/BiP and its role in cancer. Curr. Mol. Med. 2006, 6, 45–54. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.H.; Lee, S.-Y.; Lee, D.-H. Apoptosis of Pancreatic Cancer Cells after Co-Treatment with Eugenol and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. Cancers 2024, 16, 3092. https://doi.org/10.3390/cancers16173092
Kim HH, Lee S-Y, Lee D-H. Apoptosis of Pancreatic Cancer Cells after Co-Treatment with Eugenol and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. Cancers. 2024; 16(17):3092. https://doi.org/10.3390/cancers16173092
Chicago/Turabian StyleKim, Hyun Hee, Suk-Young Lee, and Dae-Hee Lee. 2024. "Apoptosis of Pancreatic Cancer Cells after Co-Treatment with Eugenol and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand" Cancers 16, no. 17: 3092. https://doi.org/10.3390/cancers16173092
APA StyleKim, H. H., Lee, S.-Y., & Lee, D.-H. (2024). Apoptosis of Pancreatic Cancer Cells after Co-Treatment with Eugenol and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. Cancers, 16(17), 3092. https://doi.org/10.3390/cancers16173092





