Immune Cell Molecular Pharmacodynamics of Lanreotide in Relation to Treatment Response in Patients with Gastroenteropancreatic Neuroendocrine Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Assay
2.1.1. Specimens
2.1.2. SSTR Expression in Peripheral Blood
2.1.3. Intracellular IL-2 and IFNg Expression
2.1.4. Apoptosis Assay
2.1.5. Transcription Factor Assays
2.2. In Vivo Analyses
2.2.1. Patient Selection Criteria
2.2.2. Treatment Response
2.2.3. Treatment Schedule
2.2.4. Sorting of T Cell Subpopulations for NanoString Analysis
2.2.5. NanoString Assay
2.2.6. Statistical Analysis of NanoString Data
3. Results
3.1. In Vitro Results
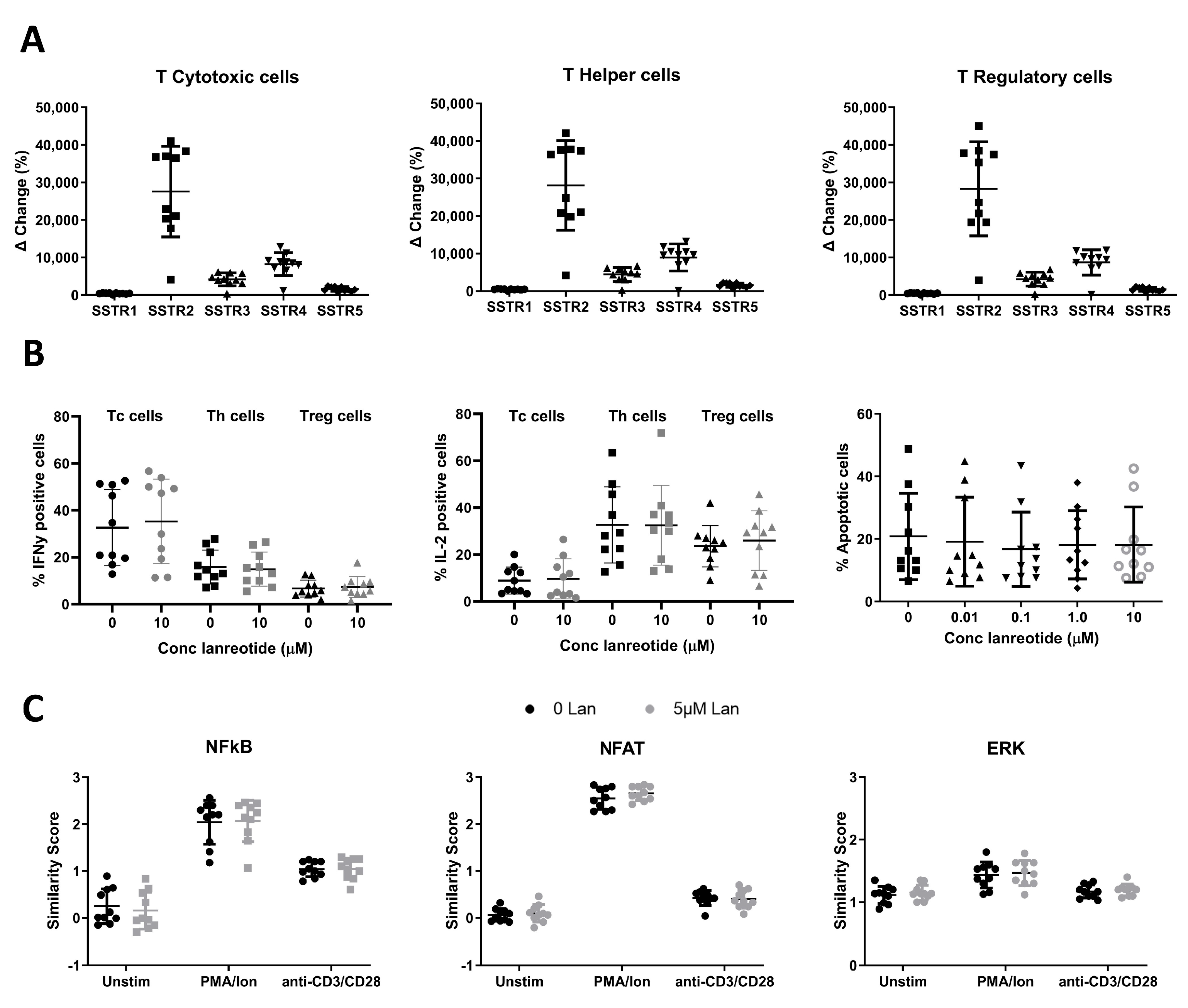
3.1.1. SSTR Expression on T Cells
3.1.2. In Vitro Effects of Lanreotide on T Cell Function and Survival
3.2. In Vivo Results
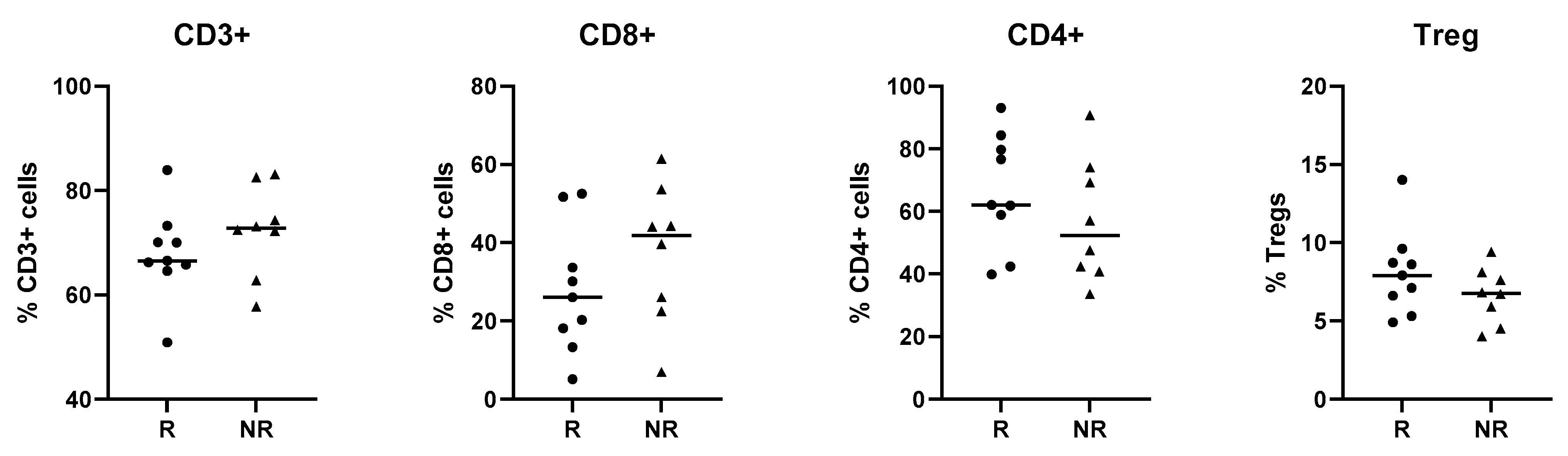
3.2.1. Patient Characteristics
3.2.2. Proteomic and Transcriptional Analysis of T Cells of NET Patients in Relation to Lanreotide Response
3.2.3. Treatment Response Effect
3.2.4. Treatment Effect
3.2.5. Interaction Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Healthy Donor T Cell Phenotyping | ||||
|---|---|---|---|---|
| Antigen | Fluor | Clone | Vendor | Cat # |
| CD3 | APCH7 | SK7 | BD Pharmingen | 560176 |
| CD4 | PECy7 | SK3 | BD Pharmingen | 557852 |
| CD8 | PerCP | SK1 | BD Biosciences | 347314 |
| CD25 | PE | 2A3 | BD Biosciences | 341009 |
| CD127 | BV421 | HIL-7R-M21 | BD Horizon | 562436 |
| SSTR expression | ||||
| Rb anti-SSTR1 | N/A | UMB7 | Abcam | ab137083 |
| Ms anti-SSTR2 | N/A | 402038 | Biotechne | MAB4224 |
| Rb anti-SSTR3 | N/A | UMB5 | Abcam | ab137026 |
| Rb anti-SSTR4 | N/A | Polyclonal | Abcam | ab28578 |
| Ms anti-SSTR5 | N/A | 394401 | Biotechne | MAB4448 |
| Dky anti Rb IgG | AF647 | N/A | Jackson ImmunoResearch | 711-605-152 |
| Gt anti Ms. IgG | AF647 | N/A | Jackson ImmunoResearch | 115-605-003 |
| Cytokine expression | ||||
| IL-2 | APC | 5344.111 | BD FastImmune | 341116 |
| IFN-γ | BV510 | B27 | BD Horizon | 563287 |
| Transcription Factor signaling | ||||
| NFAT1 | N/A | D43B1 | Cell Signaling | 5861 |
| NF-κB p65 | N/A | D14E12 | Cell Signaling | 8242 |
| ERK1/2 | N/A | 137F5 | Cell Signaling | 4695 |
| NanoString antibodies | ||||
| CD3 | FITC | UCHT1 | Biolegend | 300406 |
| CD8 | PerCPCy5.5 | HIT8a | Biolegend | 300924 |
| CD4 | BV510 | A161A1 | Biolegend | 357420 |
| CD25 | PE | BC96 | Biolegend | 302606 |
| CD127 | PECy7 | A019D5 | Biolegend | 351320 |
| LiveDead | Violet | N/A | ThermoFisher | L23105 |
| NFAT1 | Lanreotide (µM) | NFAT1 Baseline Mean SS (SE) | NFAT1 PMA/Ion Mean SS (SE) | NFAT1 CD3/CD28 Mean SS (SE) |
|---|---|---|---|---|
| T-helper | 0 | 0.06 (0.04) | 2.54 (0.07) | 0.42 (0.05) |
| 0.5 | 0.04 (0.04) | 2.69 (0.05) | 0.42 (0.05) | |
| 5 | 0.09 (0.06) | 2.66 (0.05) | 0.40 (0.06) | |
| T-cytotoxic | 0 | −0.04 (0.05) | 2.58 (0.08) | 0.36 (0.06) |
| 0.5 | −0.06 (0.05) | 2.68 (0.06) | 0.33 (0.05) | |
| 5 | −0.03 (0.05) | 2.68 (0.05) | 0.34 (0.06) | |
| Treg | 0 | 0.26 (0.04) | 2.52 (0.07) | 0.60 (0.05) |
| 0.5 | 0.23 (0.05) | 2.67 (0.05) | 0.56 (0.06) | |
| 5 | 0.25 (0.05) | 2.60 (0.05) | 0.56 (0.07) | |
| NF-κB/p65 | Lanreotide (µM) | NF-κB/p65 Baseline Mean SS (SE) | NF-κB/p65 PMA/Ion Mean SS (SE) | NF-κB/p65 CD3/CD28 Mean SS (SE) |
| T-helper | 0 | 0.25 (0.12) | 2.04 (0.15) | 1.04 (0.05) |
| 0.5 | 0.25 (0.11) | 2.04 (0.13) | 0.98 (0.07) | |
| 5 | 0.16 (0.12) | 2.07 (0.14) | 1.05 (0.07) | |
| T-cytotoxic | 0 | 0.15 (0.12) | 1.94 (0.12) | 0.86 (0.07) |
| 0.5 | 0.14 (0.10) | 1.92 (0.13) | 0.83 (0.08) | |
| 5 | 0.05 (0.12) | 1.97 (0.13) | 0.86 (0.09) | |
| Treg | 0 | 0.50 (0.14) | 2.13 (0.13) | 1.26 (0.07) |
| 0.5 | 0.53 (0.10) | 2.09 (0.12) | 1.18 (0.10) | |
| 5 | 0.41 (0.14) | 2.12 (0.14) | 1.24 (0.09) | |
| ERK1/2 | Lanreotide (µM) | ERK1/2 Baseline Mean SS (SE) | ERK1/2 PMA/Ion Mean SS (SE) | ERK1/2 CD3/CD28 Mean SS (SE) |
| T-helper | 0 | 1.12 (0.04) | 1.44 (0.07) | 1.17 (0.03) |
| 0.5 | 1.10 (0.05) | 1.51 (0.07) | 1.22 (0.03) | |
| 5 | 1.15 (0.04) | 1.47 (0.06) | 1.20 (0.03) | |
| T-cytotoxic | 0 | 1.02 (0.05) | 1.39 (0.09) | 1.10 (0.07) |
| 0.5 | 0.98 (0.05) | 1.47 (0.08) | 1.09 (0.05) | |
| 5 | 1.05 (0.05) | 1.42 (0.09) | 1.06 (0.05) | |
| Treg | 0 | 1.33 (0.05) | 1.53 (0.06) | 1.37 (0.04) |
| 0.5 | 1.32 (0.05) | 1.61 (0.07) | 1.40 (0.04) | |
| 5 | 1.38 (0.06) | 1.60 (0.07) | 1.37 (0.03) |

References
- Sonbol, M.B.; Mazza, G.L.; Mi, L.; Oliver, T.; Starr, J.; Gudmundsdottir, H.; Cleary, S.P.; Hobday, T.; Halfdanarson, T.R. Survival and Incidence Patterns of Pancreatic Neuroendocrine Tumors over the Last 2 Decades: A SEER Database Analysis. Oncologist 2022, 27, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Calomino, N.; Poto, G.E.; Carbone, L.; Bagnacci, G.; Piccioni, S.; Andreucci, E.; Nenci, L.; Marano, L.; Verre, L.; Petrioli, R.; et al. Neuroendocrine tumors’ patients treated with somatostatin analogue could complicate with emergency cholecystectomy. Ann. Ital. Chir. 2023, 94, 518–522. [Google Scholar]
- Bruns, C.; Weckbecker, G.; Raulf, F.; Kaupmann, K.; Schoeffter, P.; Hoyer, D.; Lubbert, H. Molecular pharmacology of somatostatin-receptor subtypes. Ann. N. Y. Acad. Sci. 1994, 733, 138–146. [Google Scholar] [CrossRef]
- Godara, A.; Siddiqui, N.S.; Byrne, M.M.; Saif, M.W. The safety of lanreotide for neuroendocrine tumor. Expert Opin. Drug Safety 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Hankus, J.; Tomaszewska, R. Neuroendocrine neoplasms and somatostatin receptor subtypes expression. Nucl. Med. Rev. Cent. East. Eur. 2016, 19, 111–117. [Google Scholar] [CrossRef]
- Volante, M.; Rosas, R.; Allia, E.; Granata, R.; Baragli, A.; Muccioli, G.; Papotti, M. Somatostatin, cortistatin and their receptors in tumours. Mol. Cell Endocrinol. 2008, 286, 219–229. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open-label extension study. Endocr. Relat. Cancer 2016, 23, 191–199. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Cwikla, J.B.; Sedlackova, E.; Thanh, X.T.; Wolin, E.M.; Ruszniewski, P.; Investigators, C. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study. Endocrine 2021, 71, 502–513. [Google Scholar] [CrossRef]
- Cardoso, A.; el Ghamrawy, C.; Gautron, J.P.; Horvat, B.; Gautier, N.; Enjalbert, A.; Krantic, S. Somatostatin increases mitogen-induced IL-2 secretion and proliferation of human Jurkat T cells via sst3 receptor isotype. J. Cell Biochem. 1998, 68, 62–73. [Google Scholar] [CrossRef]
- Dalm, V.A.; Hofland, L.J.; Ferone, D.; Croxen, R.; Lamberts, S.W.; van Hagen, P.M. The role of somatostatin and somatostatin analogs in the pathophysiology of the human immune system. J. Endocrinol. Investig. 2003, 26, 94–102. [Google Scholar]
- Ferone, D.; van Hagen, P.M.; Semino, C.; Dalm, V.A.; Barreca, A.; Colao, A.; Lamberts, S.W.; Minuto, F.; Hofland, L.J. Somatostatin receptor distribution and function in immune system. Dig. Liver Dis. 2004, 36 (Suppl. S1), S68–S77. [Google Scholar] [CrossRef]
- Lichtenauer-Kaligis, E.G.; Dalm, V.A.; Oomen, S.P.; Mooij, D.M.; van Hagen, P.M.; Lamberts, S.W.; Hofland, L.J. Differential expression of somatostatin receptor subtypes in human peripheral blood mononuclear cell subsets. Eur. J. Endocrinol. 2004, 150, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Talme, T.; Ivanoff, J.; Hagglund, M.; Van Neerven, R.J.; Ivanoff, A.; Sundqvist, K.G. Somatostatin receptor (SSTR) expression and function in normal and leukaemic T-cells. Evidence for selective effects on adhesion to extracellular matrix components via SSTR2 and/or 3. Clin. Exp. Immunol. 2001, 125, 71–79. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Takano, H.; Ichikawa, K.; Kobayashi, S.; Koike, T. Expression of somatostatin receptor subtype 2 mRNA in human lymphoid cells. Cell. Immunol. 1997, 181, 44–49. [Google Scholar] [CrossRef]
- van Hagen, P.M.; Krenning, E.P.; Kwekkeboom, D.J.; Reubi, J.C.; Anker-Lugtenburg, P.J.; Lowenberg, B.; Lamberts, S.W. Somatostatin and the immune and haematopoetic system; a review. Eur. J. Clin. Investig. 1994, 24, 91–99. [Google Scholar] [CrossRef]
- Yu, S.; Zhai, S.; Gong, Q.; Xiang, C.; Gong, J.; Wu, L.; Pu, X. Neoadjuvant Immunotherapy and Non–Small Cell Lung Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Clin. Oncol. 2023, 46, 517–528. [Google Scholar] [CrossRef]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M.; Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef] [PubMed]
- Maguire, O.; Collins, C.; O’Loughlin, K.; Miecznikowski, J.; Minderman, H. Quantifying nuclear p65 as a parameter for NF-kappaB activation: Correlation between ImageStream cytometry, microscopy, and Western blot. Cytom. A 2011, 79, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Merino-Casabiel, X.; Aller, J.; Arbizu, J.; Garcia-Figueiras, R.; Gonzalez, C.; Grande, E.; Jimenez-Fonseca, P.; Sevilla, M.I.; Capdevila, J. Consensus document on the progression and treatment response criteria in gastroenteropancreatic neuroendocrine tumors. Clin. Transl. Oncol. 2018, 20, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Vilo, J.; Peterson, H. gprofiler2 -- an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Res 2020, 9, 709. [Google Scholar] [CrossRef]
- Zou, Y.; Tan, H.; Zhao, Y.; Zhou, Y.; Cao, L. Expression and selective activation of somatostatin receptor subtypes induces cell cycle arrest in cancer cells. Oncol. Lett. 2019, 17, 1723–1731. [Google Scholar] [CrossRef]
- Ipsen Biopharmaceuticals, I. Highlights of Prescribing Information Somatuline® Depot (Lanreotide) Injection. 2019. [Google Scholar]
- Tesei, A.; Ricotti, L.; De Paola, F.; Casini-Raggi, C.; Barzanti, F.; Frassineti, G.L.; Zoli, W. Lanreotide-induced modulation of 5-fluorouracil or mitomycin C cytotoxicity in human colon cancer cell lines: A preclinical study. J. Chemother. 2000, 12, 421–430. [Google Scholar] [CrossRef]
- Huang, W.; Lin, W.; Chen, B.; Zhang, J.; Gao, P.; Fan, Y.; Lin, Y.; Wei, P. NFAT and NF-kappaB dynamically co-regulate TCR and CAR signaling responses in human T cells. Cell Rep. 2023, 42, 112663. [Google Scholar] [CrossRef]
- Krug, S.; Mordhorst, J.P.; Moser, F.; Theuerkorn, K.; Ruffert, C.; Egidi, M.; Rinke, A.; Gress, T.M.; Michl, P. Interaction between somatostatin analogues and targeted therapies in neuroendocrine tumor cells. PLoS ONE 2019, 14, e0218953. [Google Scholar] [CrossRef]
- Chen, Y.; Ning, J.; Cao, W.; Wang, S.; Du, T.; Jiang, J.; Feng, X.; Zhang, B. Research Progress of TXNIP as a Tumor Suppressor Gene Participating in the Metabolic Reprogramming and Oxidative Stress of Cancer Cells in Various Cancers. Front. Oncol. 2020, 10, 568574. [Google Scholar] [CrossRef]
- Qin, S.; Shi, X.; Wang, C.; Jin, P.; Ma, F. Transcription Factor and miRNA Interplays Can Manifest the Survival of ccRCC Patients. Cancers 2019, 11, 1668. [Google Scholar] [CrossRef]
- Yang, R.; Wang, M.; Zhang, G.; Li, Y.; Wang, L.; Cui, H. POU2F2 regulates glycolytic reprogramming and glioblastoma progression via PDPK1-dependent activation of PI3K/AKT/mTOR pathway. Cell Death Dis. 2021, 12, 433. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Liu, Y.; Su, P.; Liang, Y.; Li, Y.; Wang, X.; Chen, T.; Song, X.; Sang, Y.; et al. Epigenetic Regulation of NAMPT by NAMPT-AS Drives Metastatic Progression in Triple-Negative Breast Cancer. Cancer Res. 2019, 79, 3347–3359. [Google Scholar] [CrossRef] [PubMed]
- Velinovic, M.; Jankovic, R.; Jovanovic, D.; Skodric Trifunovic, V.; Gavrilovic, D.; Stojsic, J.; Cavic, M. Tumor characteristics, expressions of ERCC1, Bax, p53, IGF1R, Bcl2, Bcl2/Bax and prognostic factors for overall survival in patients with lung carcinoid. J. Buon 2019, 24, 256–266. [Google Scholar] [PubMed]
- Su, L.C.; Xu, W.D.; Huang, A.F. IRAK family in inflammatory autoimmune diseases. Autoimmun. Rev. 2020, 19, 102461. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, Y.; Song, Y.; Zhang, J.; Wu, C.; Chen, Y.; Miao, Y.; Lin, C.; Lin, Y.; Yan, D.; et al. CCRL2 promotes antitumor T-cell immunity via amplifying TLR4-mediated immunostimulatory macrophage activation. Proc. Natl. Acad. Sci. USA 2021, 118, e2024171118. [Google Scholar] [CrossRef]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Rinke, A.; Muller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Blaker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Hwang, J.R.; Byeon, Y.; Kim, D.; Park, S.G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef]
- Katz, S.C.; Donkor, C.; Glasgow, K.; Pillarisetty, V.G.; Gonen, M.; Espat, N.J.; Klimstra, D.S.; D’Angelica, M.I.; Allen, P.J.; Jarnagin, W.; et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB 2010, 12, 674–683. [Google Scholar] [CrossRef]
- Bousquet, C.; Lasfargues, C.; Chalabi, M.; Billah, S.M.; Susini, C.; Vezzosi, D.; Caron, P.; Pyronnet, S. Clinical review: Current scientific rationale for the use of somatostatin analogs and mTOR inhibitors in neuroendocrine tumor therapy. J. Clin. Endocrinol. Metab. 2012, 97, 727–737. [Google Scholar] [CrossRef]
- Patel, Y.C.; Greenwood, M.T.; Panetta, R.; Demchyshyn, L.; Niznik, H.; Srikant, C.B. The somatostatin receptor family. Life Sci. 1995, 57, 1249–1265. [Google Scholar] [CrossRef]
- Reisine, T.; Bell, G.I. Molecular biology of somatostatin receptors. Endocr. Rev. 1995, 16, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V. Oncological applications of somatostatin analogues. Cancer Res. 1988, 48, 6977–6985. [Google Scholar]
- Susini, C.; Buscail, L. Rationale for the use of somatostatin analogs as antitumor agents. Ann. Oncol. 2006, 17, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Buscail, L.; Delesque, N.; Esteve, J.P.; Saint-Laurent, N.; Prats, H.; Clerc, P.; Robberecht, P.; Bell, G.I.; Liebow, C.; Schally, A.V.; et al. Stimulation of tyrosine phosphatase and inhibition of cell proliferation by somatostatin analogues: Mediation by human somatostatin receptor subtypes SSTR1 and SSTR2. Proc. Natl. Acad. Sci. USA 1994, 91, 2315–2319. [Google Scholar] [CrossRef]
- Buscail, L.; Esteve, J.P.; Saint-Laurent, N.; Bertrand, V.; Reisine, T.; O’Carroll, A.M.; Bell, G.I.; Schally, A.V.; Vaysse, N.; Susini, C. Inhibition of cell proliferation by the somatostatin analogue RC-160 is mediated by somatostatin receptor subtypes SSTR2 and SSTR5 through different mechanisms. Proc. Natl. Acad. Sci. USA 1995, 92, 1580–1584. [Google Scholar] [CrossRef]
- Cordelier, P.; Esteve, J.P.; Bousquet, C.; Delesque, N.; O’Carroll, A.M.; Schally, A.V.; Vaysse, N.; Susini, C.; Buscail, L. Characterization of the antiproliferative signal mediated by the somatostatin receptor subtype sst5. Proc. Natl. Acad. Sci. USA 1997, 94, 9343–9348. [Google Scholar] [CrossRef]
- Lahlou, H.; Saint-Laurent, N.; Esteve, J.P.; Eychene, A.; Pradayrol, L.; Pyronnet, S.; Susini, C. sst2 Somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J. Biol. Chem. 2003, 278, 39356–39371. [Google Scholar] [CrossRef]
- Sharma, K.; Patel, Y.C.; Srikant, C.B. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol. Endocrinol. 1996, 10, 1688–1696. [Google Scholar] [CrossRef]
- Teijeiro, R.; Rios, R.; Costoya, J.A.; Castro, R.; Bello, J.L.; Devesa, J.; Arce, V.M. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol. Biochem. 2002, 12, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Aguila, M.C.; Rodriguez, A.M.; Aguila-Mansilla, H.N.; Lee, W.T. Somatostatin antisense oligodeoxynucleotide-mediated stimulation of lymphocyte proliferation in culture. Endocrinology 1996, 137, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Casnici, C.; Lattuada, D.; Perego, C.; Franco, P.; Marelli, O. Inhibitory effect of somatostatin on human T lymphocytes proliferation. Int. J. Immunopharmacol. 1997, 19, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Lattuada, D.; Casnici, C.; Crotta, K.; Mastrotto, C.; Franco, P.; Schmid, H.A.; Marelli, O. Inhibitory effect of pasireotide and octreotide on lymphocyte activation. J. Neuroimmunol. 2007, 182, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Lupoli, G.; Guarrasi, R.; Colao, A.; Dicitore, A.; Gaudenzi, G.; Misso, G.; Castellano, M.; Addeo, R.; Facchini, G.; et al. Interleukin-2 and lanreotide in the treatment of medullary thyroid cancer: In vitro and in vivo studies. J. Clin. Endocrinol. Metab. 2013, 98, E1567–E1574. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.; Halperin, D.M.; Uronis, H.S.; Hsu, D.S.; Hurwitz, H.; Bolch, E.; Warren, D.; Haley, S.; John, L.; Moyer, A.; et al. Phase Ib/II study of pembrolizumab with lanreotide depot for advanced, progressive gastroenteropancreatic neuroendocrine tumors (PLANET). J. Clin. Oncol. 2021, 39, 369. [Google Scholar] [CrossRef]
- Burel, J.G.; Pomaznoy, M.; Lindestam Arlehamn, C.S.; Weiskopf, D.; da Silva Antunes, R.; Jung, Y.; Babor, M.; Schulten, V.; Seumois, G.; Greenbaum, J.A.; et al. Circulating T cell-monocyte complexes are markers of immune perturbations. Elife 2019, 8, e46045. [Google Scholar] [CrossRef] [PubMed]


| Responders (N = 9) | Non-Responders (N = 8) | |
|---|---|---|
| Age Median (range), y | 69 (34–79) | 66 (50–81) |
| Origin | ||
| pNET | 5 | 4 |
| Intestinal NET | 4 | 3 |
| Unknown | 0 | 1 |
| Metastatic sites, N | ||
| Liver | 6 | 6 |
| Nodes | 1 | 2 |
| Lung | 0 | 1 |
| Skin | 0 | 1 |
| Peritoneum | 1 | 0 |
| Ki 67, N | ||
| Not specified | 1 | 2 |
| <3% | 5 | 0 |
| 3–20% | 3 | 4 |
| >20% | 0 | 2 |
| Differentiation, N | ||
| Not specified | 1 | 0 |
| Well-differentiated | 8 | 7 |
| Moderately differentiated | 0 | 1 |
| Patient Effect | Treatment Effect | Interaction Effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Tc | Th | Treg | Tc | Th | Treg | Tc | Th | Treg |
| IRAK2 | POU2F2 | IGF1R | CCRL2 | LAG3 | MAP2K1 | CCRL2 | IL5RA | HLA_DRB3 |
| TXNIP | TNFRSF14 | ECSIT | BCL2L1 | MS4A1 | PDCD1 | TNFRSF13C | IRAK2 | IRF5 |
| IL6 | NUP107 | CCND3 | FCGR2A | TFEB | KIR_Inhibiting _Subgroup_1 | HLA_DRB4 | IFI16 | FCER1G |
| TRAF6 | ATG5 | ITGA4 | MRC1 | CASP3 | EP300 | IRAK2 | NUP107 | MFGE8 |
| VEGFC | TFEB | C1QBP | TLR6 | EGR1 | TP53 | FEZ1 | C1QBP | ATF2 |
| SPA17 | IL21R | RELA | KLRC2 | GTF3C1 | NFKBIA | TXNIP | LAG3 | HLA_DPB1 |
| KLRC2 | CREB1 | MERTK | CLEC4C | BCL10 | FCER1G | CMKLR1 | BID | LAMP3 |
| SIGIRR | AKT3 | CD7 | HLA_DRB4 | PSMD7 | CD80 | CDKN1A | BCL10 | KIR_Inhibiting _Subgroup_1 |
| TNFSF12 | IL17RA | CREB1 | CD79B | TRAF6 | TYK2 | CLEC4A | PSMD7 | PDCD1 |
| TNFRSF13C | TXNIP | TXNIP | MASP1 | XCL2 | HLA_DPB1 | MICA | MS4A1 | IGF1R |
| CREB1 | GZMK | LAMP3 | NFKBIA | DDX58 | CCR2 | TMEFF2 | TXNIP | TNFRSF10C |
| MAP2K4 | BCL2 | PIN1 | TLR8 | NFKBIA | NUP107 | CD70 | CCL4 | RELA |
| CD3E | CASP3 | CXCR5 | TNFRSF13C | CXCR6 | SH2D1A | FAS | TRAF6 | EP300 |
| MIF | MAP2K1 | ITGAL | DMBT1 | CD3EAP | TFRC | TNFRSF12A | CXCR3 | TXNIP |
| FOS | HLA_DPB1 | BCL2 | LILRB1 | CR1 | CCR9 | TRAF6 | JAM3 | IL13 |
| CARD11 | ATM | SMAD3 | YTHDF2 | IRAK2 | IL8 | FOXJ1 | IL18RAP | ECSIT |
| ITGB2 | IRAK4 | CARD11 | IL6ST | IL5RA | TLR10 | TNFSF13 | NOTCH1 | IL1R1 |
| IL11 | CD40LG | TCF7 | FUT7 | CXCL5 | IRF5 | IFIT1 | PTGDR2 | SPACA3 |
| SMAD3 | ITGAL | RUNX1 | MAP2K4 | EOMES | MFGE8 | IFIH1 | TNFRSF14 | XCR1 |
| FAS | PSMB9 | IRAK4 | KIR3DL2 | S100A8 | IL6ST | LILRB1 | GTF3C1 | BCL10 |
| Pathway | NES | p Adjusted | |
|---|---|---|---|
| Patient Effect | |||
| Th | NOD-like receptor signaling pathway | −1.527 | 0.0469 |
| Yersinia infection | −1.594 | 0.0369 | |
| Herpes simplex virus 1 infection | −1.624 | 0.0208 | |
| Human immunodeficiency virus 1 infection | −1.628 | 0.0234 | |
| Regulation of actin cytoskeleton | −1.780 | 0.0208 | |
| Tc | - | ||
| Treg | - | ||
| Treatment Effect | |||
| Th | Cell surface interactions at the vascular wall | 1.705 | 0.0354 |
| Disease | −1.386 | 0.0354 | |
| RNA Polymerase II Transcription | −1.580 | 0.0354 | |
| Generic Transcription Pathway | −1.610 | 0.0326 | |
| Gene expression (Transcription) | −1.652 | 0.0203 | |
| Cellular responses to stress | −1.780 | 0.0203 | |
| Cellular responses to stimuli | −1.780 | 0.0203 | |
| Deubiquitination | −1.844 | 0.0345 | |
| Post-translational protein modification | −1.852 | 0.0184 | |
| Purinergic signaling in leishmaniasis infection | −1.867 | 0.0394 | |
| Cell recruitment (pro-inflammatory response) | −1.867 | 0.0394 | |
| Metabolism of RNA | −1.892 | 0.0203 | |
| Metabolism of proteins | −1.902 | 0.0054 | |
| Ub-specific processing proteases | −1.908 | 0.0203 | |
| Tc | Metabolism of proteins | −1.765 | 0.0485 |
| cGMP-PKG signaling pathway | 1.833 | 0.0234 | |
| Ras signaling pathway | 1.817 | 0.0234 | |
| Focal adhesion | 1.746 | 0.0432 | |
| Rap1 signaling pathway | 1.716 | 0.0493 | |
| Cell adhesion molecules | 1.678 | 0.0432 | |
| Yersinia infection | 1.663 | 0.0432 | |
| T cell receptor signaling pathway | 1.662 | 0.0432 | |
| PI3K-Akt signaling pathway | 1.632 | 0.0432 | |
| Treg | - | ||
| Interaction Effect | |||
| Th | - | ||
| Tc | Signaling by TGF-beta Receptor Complex | 2.003 | 0.0438 |
| Signaling by TGFB family members | 2.003 | 0.0438 | |
| Treg | Host Interactions of HIV factors | 1.693 | 0.0171 |
| HIV Infection | 1.683 | 0.0171 | |
| TCF dependent signaling in response to WNT | 1.681 | 0.0171 | |
| Cell surface interactions at the vascular wall | 1.651 | 0.0171 | |
| TCR signaling | 1.638 | 0.0276 | |
| Signaling by ROBO receptors | 1.622 | 0.0350 | |
| Signaling by WNT | 1.608 | 0.0350 | |
| Transcriptional regulation by RUNX3 | 1.581 | 0.0350 | |
| Diseases of signal transduction by growth factor receptors and second messengers | 1.548 | 0.0350 | |
| Generic Transcription Pathway | 1.503 | 0.0276 | |
| RNA Polymerase II Transcription | 1.469 | 0.0350 | |
| Gene expression (Transcription) | 1.459 | 0.0469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaklabi, S.; Maguire, O.; Pattnaik, H.; Zhang, Y.; Chow, J.; Wang, J.; Minderman, H.; Iyer, R. Immune Cell Molecular Pharmacodynamics of Lanreotide in Relation to Treatment Response in Patients with Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2024, 16, 3104. https://doi.org/10.3390/cancers16173104
Alaklabi S, Maguire O, Pattnaik H, Zhang Y, Chow J, Wang J, Minderman H, Iyer R. Immune Cell Molecular Pharmacodynamics of Lanreotide in Relation to Treatment Response in Patients with Gastroenteropancreatic Neuroendocrine Tumors. Cancers. 2024; 16(17):3104. https://doi.org/10.3390/cancers16173104
Chicago/Turabian StyleAlaklabi, Sabah, Orla Maguire, Harsha Pattnaik, Yali Zhang, Jacky Chow, Jianmin Wang, Hans Minderman, and Renuka Iyer. 2024. "Immune Cell Molecular Pharmacodynamics of Lanreotide in Relation to Treatment Response in Patients with Gastroenteropancreatic Neuroendocrine Tumors" Cancers 16, no. 17: 3104. https://doi.org/10.3390/cancers16173104







