Radiomics-Based Computed Tomography Urogram Approach for the Prediction of Survival and Recurrence in Upper Urinary Tract Urothelial Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
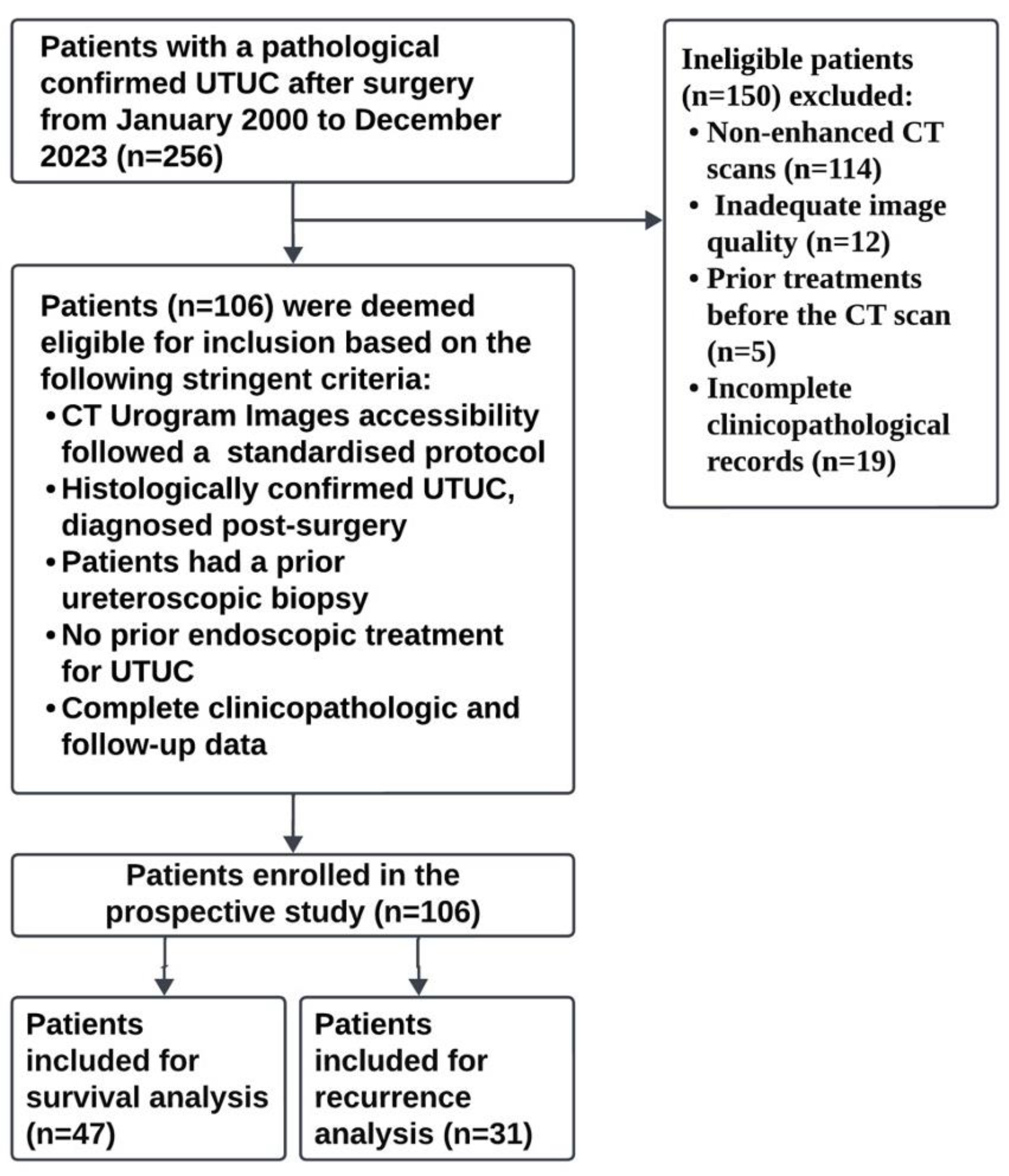
2.1. Study Design and Data
2.2. CT Protocol
2.3. Follow-Up
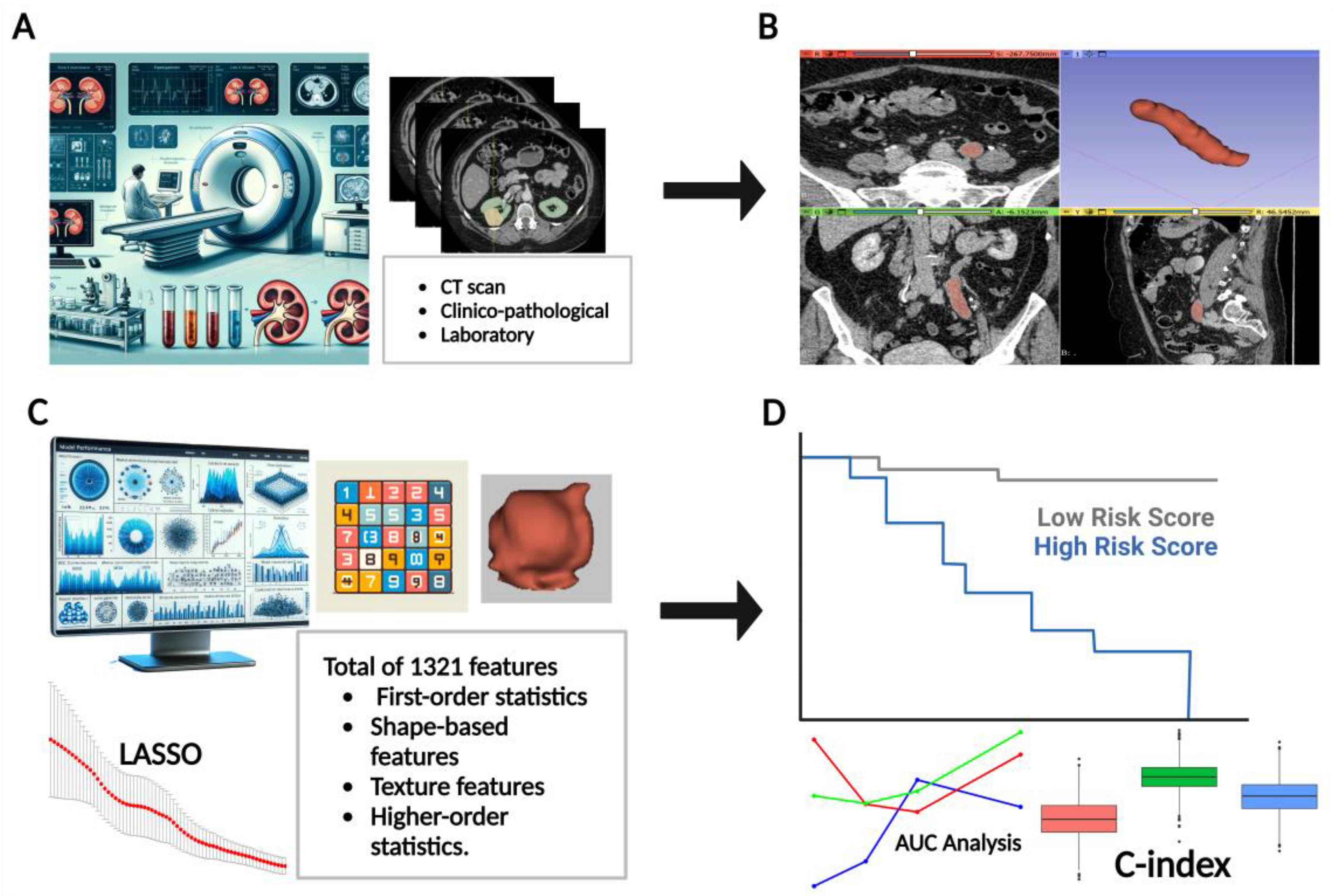
2.4. Three-Dimensional Slicer for Tumor Segmentation
2.5. Radiomics Feature Extraction and Selection
2.6. Data Preparation and Modeling
2.7. Evaluation and Statistical Analysis
3. Results
3.1. Patient Characteristics
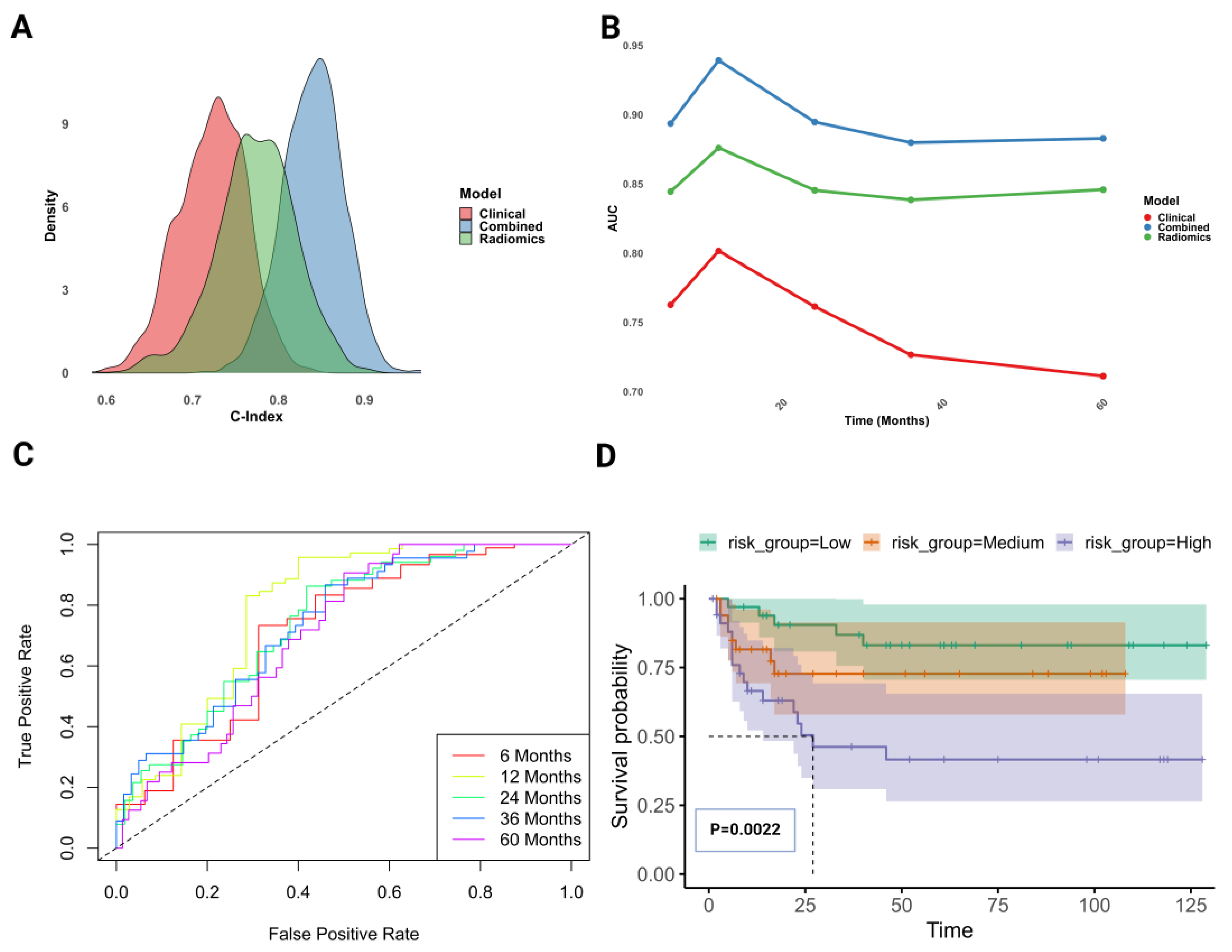
3.2. Development of Prognostic Models
3.3. Advanced Clinico-Radiomics Prognostic Modeling for UTUC Survival
3.4. Assessment of Combined Clinico-Radiomics Model for UTUC Survival Prediction
3.5. Sub-Analysis: Interaction between Radiomics Features and Clinical Predictors
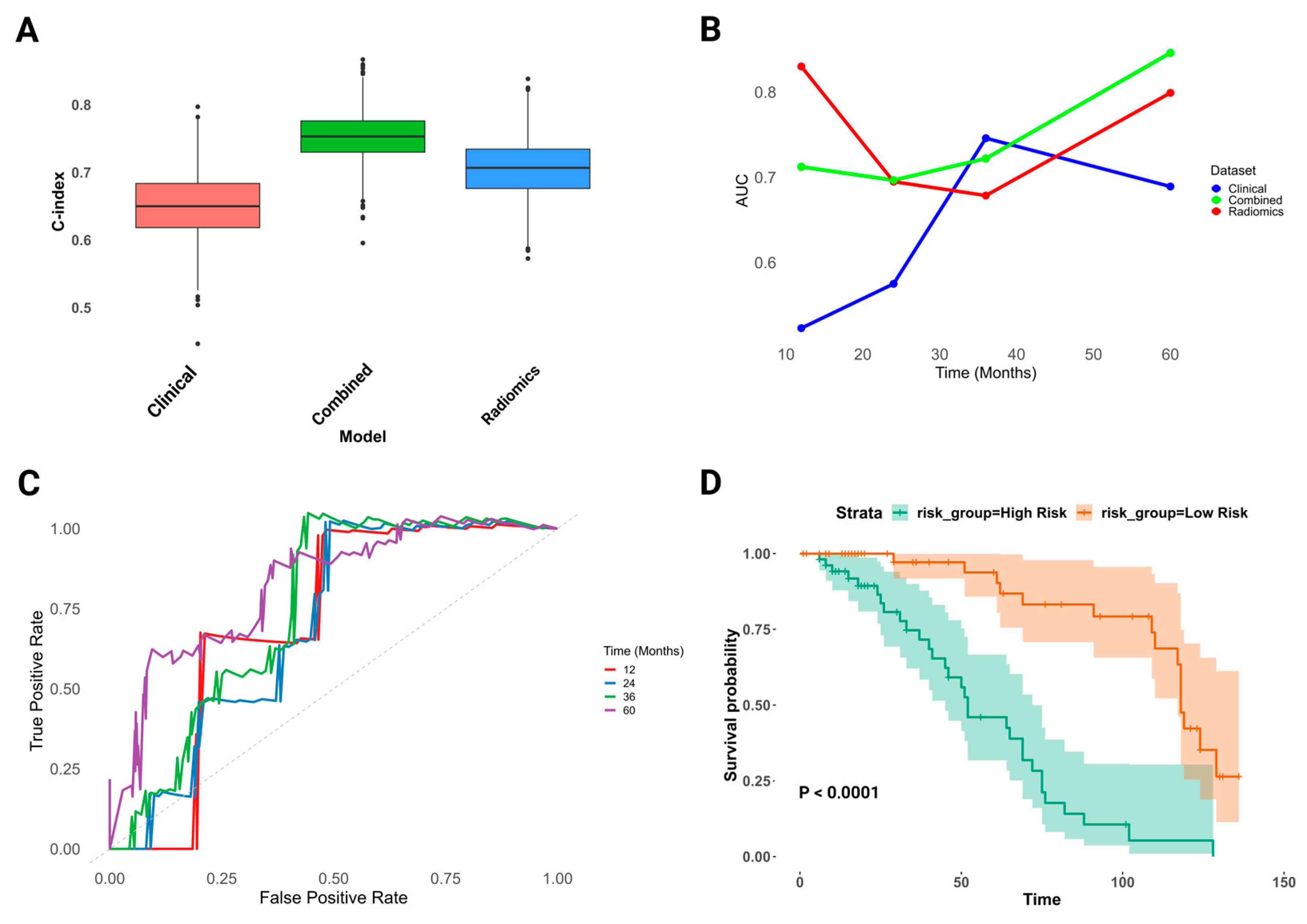
3.6. Advanced Clinico-Radiomics Prognostic Modeling for UTUC Recurrence
3.7. Assessment of Combined Clinico-Radiomics Model for UTUC Recurrence Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Fajkovic, H.; Cha, E.K.; Xylinas, E.; Rink, M.; Pycha, A.; Seitz, C.; Bolenz, C.; Dunning, A.; Novara, G.; Trinh, Q.-D.; et al. Disease-free survival as a surrogate for overall survival in upper tract urothelial carcinoma. World J. Urol. 2013, 31, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Hassler, M.R.; Bray, F.; Catto, J.W.F.; Grollman, A.P.; Hartmann, A.; Margulis, V.; Matin, S.F.; Roupret, M.; Sfakianos, J.P.; Shariat, S.F.; et al. Molecular characterization of upper tract urothelial carcinoma in the era of next-generation sequencing: A systematic review of the current literature. Eur. Urol. 2020, 78, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Kluth, L.; Passoni, N.; Trinh, Q.-D.; Rieken, M.; Lee, R.K.; Fajkovic, H.; Novara, G.; Margulis, V.; Raman, J.D.; et al. Prediction of intravesical recurrence after radical nephroureterectomy: Development of a clinical decision-making tool. Eur. Urol. 2014, 65, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.F.J.; Meehan, C.P.; Colville, J.; Power, R.; Torreggiani, W.C. Transitional cell carcinoma of the upper urinary tract: Spectrum of imaging findings. RadioGraphics 2005, 25, 1609–1627. [Google Scholar] [CrossRef]
- Jinzaki, M.; Matsumoto, K.; Kikuchi, E.; Sato, K.; Horiguchi, Y.; Nishiwaki, Y.; Silverman, S.G. Comparison of CT urography and excretory urography in the detection and localization of urothelial carcinoma of the upper urinary tract. Am. J. Roentgenol. 2011, 196, 1102–1109. [Google Scholar] [CrossRef]
- Wang, L.J.; Wong, Y.C.; Huang, C.C.; Wu, C.H.; Hung, S.C.; Chen, H.W. Multidetector computerized tomography urography is more accurate than excretory urography for diagnosing transitional cell carcinoma of the upper urinary tract in adults with hematuria. J. Urol. 2010, 183, 48–55. [Google Scholar] [CrossRef]
- Akita, H.; Jinzaki, M.; Kikuchi, E.; Sugiura, H.; Akita, A.; Mikami, S.; Kuribayashi, S. Preoperative T categorization and prediction of histopathologic grading of urothelial carcinoma in renal pelvis using diffusion-weighted MRI. Am. J. Roentgenol. 2011, 197, 1130–1136. [Google Scholar] [CrossRef]
- Yoshida, R.; Yoshizako, T.; Maruyama, M.; Mori, H.; Ishikawa, N.; Tamaki, Y.; Kitagaki, H. The value of adding diffusion-weighted images for tumor detection and preoperative staging in renal pelvic carcinoma for the reader’s experience. Abdom. Radiol. 2017, 42, 2297–2304. [Google Scholar] [CrossRef]
- Subiela, J.D.; Territo, A.; Mercadé, A.; Balañà, J.; Aumatell, J.; Calderon, J.; Gallioli, A.; González-Padilla, D.A.; Gaya, J.M.; Palou, J.; et al. Diagnostic accuracy of ureteroscopic biopsy in predicting stage and grade at final pathology in upper tract urothelial carcinoma: Systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 1989–1997. [Google Scholar] [CrossRef]
- Gillan, A.; El-Mokadem, I.; Rai, B.; Lang, S.; Alcorn, J.; Din, A.S.U.; Dasgupta, R.; Biyani, C.S.; Nabi, G. Carcinoma in situ is significantly underdetected by prenephroureterectomy ureteroscopy in the management of upper tract urothelial cancers. BioMed Res. Int. 2015, 2015, 547586. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.C.; Shariat, S.F.; Herman, M.P.; Ng, C.K.; Scherr, D.S.; Scoll, B.; Uzzo, R.G.; Wille, M.; Eggener, S.E.; Terrell, J.D.; et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J. Urol. 2010, 184, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Pierconti, F.; Martini, M.; Fiorentino, V.; Cenci, T.; Racioppi, M.; Foschi, N.; Di Gianfrancesco, L.; Sacco, E.; Rossi, E.; Larocca, L.M.; et al. Upper urothelial tract high-grade carcinoma: Comparison of urine cytology and DNA methylation analysis in urinary samples. Hum. Pathol. 2021, 118, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Syed, J.S.; Nguyen, K.A.; Suarez-Sariemento, A.; Leung, C.; Casilla-Lennon, M.; Raman, J.D.; Shuch, B. Outcomes of upper tract urothelial cancer managed non-surgically. Can. J. Urol. 2019, 26, 9699–9707. [Google Scholar] [PubMed]
- Syed, J.S.; Nguyen, K.A.; Suarez-Sarmiento, A.; Johnson, K.; Leapman, M.S.; Raman, J.D.; Shuch, B. Survival outcomes for patients with localised upper tract urothelial carcinoma managed with non-definitive treatment. BJU Int. 2018, 121, 124–129. [Google Scholar] [CrossRef]
- Lughezzani, G.; Jeldres, C.; Isbarn, H.; Sun, M.; Shariat, S.F.; Alasker, A.; Pharand, D.; Widmer, H.; Arjane, P.; Graefen, M.; et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: A population-based study of 2299 patients. Eur. J. Cancer 2009, 45, 3291–3297. [Google Scholar] [CrossRef]
- Colin, P.; Ouzzane, A.; Pignot, G.; Ravier, E.; Crouzet, S.; Ariane, M.M.; Audouin, M.; Neuzillet, Y.; Albouy, B.; Hurel, S.; et al. Comparison of oncological outcomes after segmental ureterectomy or radical nephroureterectomy in urothelial carcinomas of the upper urinary tract: Results from a large French multicentre study. BJU Int. 2012, 110, 1134–1141. [Google Scholar] [CrossRef]
- Seisen, T.; Colin, P.; Hupertan, V.; Yates, D.R.; Xylinas, E.; Nison, L.; Cussenot, O.; Neuzillet, Y.; Bensalah, K.; Novara, G.; et al. Postoperative nomogram to predict cancer-specific survival after radical nephroureterectomy in patients with localised and/or locally advanced upper tract urothelial carcinoma without metastasis. BJU Int. 2014, 114, 733–740. [Google Scholar] [CrossRef]
- Fukui, T.; Kanno, T.; Kobori, G.; Moroi, S.; Yamada, H. Preoperative hydronephrosis as a predictor of postnephroureterectomy survival in patients with upper tract urothelial carcinoma: A two-center study in Japan. Int. J. Clin. Oncol. 2020, 25, 456–463. [Google Scholar] [CrossRef]
- Rink, M.; Xylinas, E.; Margulis, V.; Cha, E.K.; Ehdaie, B.; Raman, J.D.; Chun, F.K.; Matsumoto, K.; Lotan, Y.; Furberg, H.; et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur. Urol. 2013, 63, 1082–1090. [Google Scholar] [CrossRef]
- Moss, T.J.; Qi, Y.; Xi, L.; Peng, B.; Kim, T.-B.; Ezzedine, N.E.; Mosqueda, M.E.; Guo, C.C.; Czerniak, B.A.; Ittmann, M.; et al. Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur. Urol. 2017, 72, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, R.L.; Bahadori, A.; Mathieu, R.; Haitel, A.; Grubmüller, B.; Margulis, V.; Karam, J.A.; Rouprêt, M.; Seitz, C.; Karakiewicz, P.I.; et al. Prognostic role of decreased E-cadherin expression in patients with upper tract urothelial carcinoma: A multi-institutional study. World J. Urol. 2017, 35, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, L.-M.; Heitplatz, B.; Preuss, S.; Hutchinson, R.C.; Woldu, S.L.; Singla, N.; Boegemann, M.; Wood, C.G.; Karam, J.A.; Weizer, A.Z.; et al. Prognostic value of PD-1 and PD-L1 expression in patients with high grade upper tract urothelial carcinoma. J. Urol. 2017, 198, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Grob, G.; Rogers, D.; Pandolfo, S.D.; Vourganti, S.; Buscarini, M.; Mehrazin, R.; Grob, B.M.; Mir, M.C.; Perdonà, S.; Derweesh, I.H.; et al. Oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma: A literature review. Transl. Androl. Urol. 2023, 12, 1351–1362. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef]
- Abouassaly, R.; Alibhai, S.M.H.; Shah, N.; Timilshina, N.; Fleshner, N.; Finelli, A. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology 2010, 76, 895–901. [Google Scholar] [CrossRef]
- Collà Ruvolo, C.; Nocera, L.; Stolzenbach, L.F.; Wenzel, M.; Califano, G.; Tian, Z.; Verze, P.; Shariat, S.F.; Saad, F.; Briganti, A.; et al. Tumor size predicts muscle-invasive and non–organ-confined disease in upper tract urothelial carcinoma at radical nephroureterectomy. Eur. Urol. Focus 2022, 8, 498–505. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Sun, J.; Jeliazkova, N.; Chupakhin, V.; Golib-Dzib, J.-F.; Engkvist, O.; Carlsson, L.; Wegner, J.; Ceulemans, H.; Georgiev, I.; Jeliazkov, V.; et al. ExCAPE-DB: An integrated large scale dataset facilitating Big Data analysis in chemogenomics. J. Cheminform. 2017, 9, 17. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.-M.; Orton, M.R. Radiomics in oncology: A practical guide. RadioGraphics 2021, 41, 1717–1732. [Google Scholar] [CrossRef]
- Eloyan, A.; Yue, M.S.; Khachatryan, D. Tumor heterogeneity estimation for radiomics in cancer. Stat. Med. 2020, 39, 4704–4723. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; Vosough, A.; Khalvati, F.; Kiss, A.; Ganeshan, B.; Bjarnason, G.A. CT texture analysis: A potential tool for prediction of survival in patients with metastatic clear cell carcinoma treated with sunitinib. Cancer Imaging 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.G.; Stabo, N.; Abel, E.J.; del Rio, A.M.; Pickhardt, P.J. CT textural analysis of large primary renal cell carcinomas: Pretreatment tumor heterogeneity correlates with histologic findings and clinical outcomes. Am. J. Roentgenol. 2016, 207, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tabibu, S.; Vinod, P.K.; Jawahar, C.V. Pan-renal cell carcinoma classification and survival prediction from histopathology images using deep learning. Sci. Rep. 2019, 9, 10509. [Google Scholar] [CrossRef]
- Edalat-Javid, M.; Shiri, I.; Hajianfar, G.; Abdollahi, H.; Arabi, H.; Oveisi, N.; Javadian, M.; Shamsaei Zafarghandi, M.; Malek, H.; Bitarafan-Rajabi, A.; et al. Cardiac SPECT radiomic features repeatability and reproducibility: A multi-scanner phantom study. J. Nucl. Cardiol. 2021, 28, 2730–2744. [Google Scholar] [CrossRef]
- Neisius, U.; El-Rewaidy, H.; Nakamori, S.; Rodriguez, J.; Manning, W.J.; Nezafat, R. Radiomic analysis of myocardial native T1 imaging discriminates between hypertensive heart disease and hypertrophic cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1946–1954. [Google Scholar] [CrossRef]
- Shiiba, T.; Arimura, Y.; Nagano, M.; Takahashi, T.; Takaki, A. Improvement of classification performance of Parkinson’s disease using shape features for machine learning on dopamine transporter single photon emission computed tomography. PLoS ONE 2020, 15, e0228289. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, Y.; Liao, Z.; Jiang, H.; Mao, D.; Wang, M.; Yu, E.; Ding, Z. Corpus callosum radiomics-based classification model in Alzheimer’s disease: A case-control study. Front. Neurol. 2018, 9, 618. [Google Scholar] [CrossRef]
- Molina, D.; Pérez-Beteta, J.; Martínez-González, A.; Martino, J.; Velásquez, C.; Arana, E.; Pérez-García, V.M. Influence of gray level and space discretization on brain tumor heterogeneity measures obtained from magnetic resonance images. Comput. Biol. Med. 2016, 78, 49–57. [Google Scholar] [CrossRef]
- Alqahtani, A.; Bhattacharjee, S.; Almopti, A.; Li, C.; Nabi, G. Radiomics-based machine learning approach for the prediction of grade and stage in upper urinary tract urothelial carcinoma: A step towards virtual biopsy. Int. J. Surg. 2024, 110, 3258–3268. [Google Scholar] [CrossRef]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Gürses Andersson, I.; Liedberg, F.; Mariappan, P.; Hugh Mostafid, A.; et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Miles, K.A. Quantifying tumour heterogeneity with CT. Cancer Imaging 2013, 13, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Salemi, M.; Marchese, G.; Salluzzo, M.G.; Lanza, G.; Marino, S.; Schillaci, F.; Truda, A.; Pepe, L.; Pennisi, M. Transcriptome analysis in patients with muscle-invasive bladder cancer. In Vivo 2024, 38, 1660. [Google Scholar] [CrossRef] [PubMed]
- Florez, E.; Fatemi, A.; Claudio, P.P.; Howard, C.M. Emergence of radiomics: Novel methodology identifying imaging biomarkers of disease in diagnosis, response, and progression. SM J. Clin. Med. Imaging 2018, 4, 1019. [Google Scholar]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Shayesteh, S.P.; Shiri, I.; Karami, A.H.; Hashemian, R.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Predicting lung cancer patients’ survival time via logistic regression-based models in a quantitative radiomic framework. J. Biomed. Phys. Eng. 2020, 10, 479–492. [Google Scholar] [CrossRef]
- Wang, X.-H.; Long, L.-H.; Cui, Y.; Jia, A.Y.; Zhu, X.-G.; Wang, H.-Z.; Wang, Z.; Zhan, C.-M.; Wang, Z.-H.; Wang, W.-H. MRI-based radiomics model for preoperative prediction of 5-year survival in patients with hepatocellular carcinoma. Br. J. Cancer 2020, 122, 978–985. [Google Scholar] [CrossRef]
- Shiri, I.; Maleki, H.; Hajianfar, G.; Abdollahi, H.; Ashrafinia, S.; Hatt, M.; Zaidi, H.; Oveisi, M.; Rahmim, A. Next-generation radiogenomics sequencing for prediction of EGFR and KRAS mutation status in NSCLC patients using multimodal imaging and machine learning algorithms. Mol. Imaging Biol. 2020, 22, 1132–1148. [Google Scholar] [CrossRef]
- Rallis, K.S.; Kleeman, S.O.; Grant, M.; Ordidge, K.L.; Sahdev, A.; Powles, T. Radiomics for renal cell carcinoma: Predicting outcomes from immunotherapy and targeted therapies—A narrative review. Eur. Urol. Focus 2021, 7, 717–721. [Google Scholar] [CrossRef]

| Clinical Variables | Entire Cohort (n = 106) |
|---|---|
| Age—mean age; age range | 74 years (49–93 years) |
| Gender # (%) | |
| Female | 44 (41%) |
| Male | 62 (58%) |
| Smoker # (%) | |
| Never | 25 (23%) |
| Smoker | 32 (30%) |
| Ex-Smoker | 49 (46%) |
| BMI # (%) | |
| Normal range | 35 (33%) |
| Overweight (pre-obese) | 36 (34%) |
| Obese | 35 (33%) |
| Cytology # (%) | |
| Positive | 22 (20%) |
| Negative | 31 (29%) |
| Suspicious | 10 (9%) |
| Nondiagnostic | 43 (40%) |
| Deceased, yes, # (%) | 58 (54%) |
| Metastasis, yes, # (%) | 12 (11%) |
| CTU findings positive, yes, # (%) | 106 (100%) |
| Laterality # (%) | |
| Left | 43 (40%) |
| Right | 63 (59%) |
| Location # (%) | |
| Renal Pelvis | 60 (56%) |
| Ureter | 66 (62%) |
| Histological grade # (%) | |
| Low grade | 31 (29%) |
| High grade | 75 (70%) |
| T stage # (%) | |
| T1 | 59 (55%) |
| T2 | 19 (17%) |
| T3 or T4 | 28 (26%) |
| Carcinoma in situ, yes, # (%) | 25 (23%) |
| Hydronephrosis, yes, # (%) | 25 (23%) |
| Multifocal, yes, # (%) | 38 (35%) |
| Tumor size (cm) | |
| Mean (standard deviation) | 1.97 (0.83) |
| Median (interquartile range) | 1.87 (1.42–2.36) |
| Range | 0.63–5.32 |
| Survival, yes, # (%) | 47 (44%) |
| Recurrence, yes, # (%) | 31 (29%) |
| Models | Variables | Coefficient | Exp. Coef. | Std. Error | Z | p-Value | 95% CI |
|---|---|---|---|---|---|---|---|
| Clinical | Tumor size | 0.74 | 2.11 | 0.34 | 2.19 | 0.02 | 2.95–62.19 |
| Size | −0.40 | 0.66 | 0.27 | −1.44 | 0.14 | 0.38–1.15 | |
| Grade | −0.004 | 0.99 | 0.19 | −0.02 | 0.98 | 0.68–1.45 | |
| Smoker | 0.59 | 1.82 | 0.46 | 1.30 | 0.19 | 0.73–4.49 | |
| Cytology | −0.39 | 0.67 | 0.19 | −2.03 | 0.04 | 0.46–0.98 | |
| Metastasis | −0.63 | 0.53 | 0.39 | −1.61 | 0.10 | 0.24–1.14 | |
| Hydronephrosis | 0.61 | 1.85 | 0.69 | 0.89 | 0.37 | 0.47–7.18 | |
| BMI | −0.84 | 0.42 | 0.40 | −2.10 | 0.03 | 0.19–0.94 | |
| Stage | 0.05 | 1.05 | 0.03 | 1.71 | 0.08 | 0.99–1.12 | |
| Multifocal | −0.96 | 0.38 | 0.44 | −2.17 | 0.02 | 0.16–0.90 | |
| Location | −0.08 | 0.91 | 0.38 | −0.22 | 0.82 | 0.43–1.93 | |
| Gender | −0.02 | 0.97 | 0.20 | −0.10 | 0.91 | 0.65–1.45 | |
| Age | −0.17 | 0.83 | 0.17 | −0.99 | 0.32 | 0.58–1.18 | |
| Radiomics | original_glcm_Correlation | −0.15 | 0.85 | 0.55 | −0.28 | 0.77 | 1.33–12.87 |
| original_glcm_MCC | 0.23 | 1.26 | 0.56 | 0.41 | 0.6 | 1.51–44.46 | |
| gradient_glcm_Correlation | −0.59 | 0.55 | 0.50 | −1.17 | 0.23 | 1.22–4.41 | |
| gradient_glcm_MCC | 0.49 | 1.64 | 0.43 | 1.15 | 0.24 | 2.02–45.40 | |
| squareroot_firstorder_Kurtosis | 0.21 | 1.23 | 0.21 | 0.97 | 0.32 | 2.24–6.63 | |
| wavelet.LHL_glcm_Correlation | 0.73 | 2.09 | 0.40 | 1.84 | 0.06 | 2.59–98.60 | |
| wavelet.HHL_glcm_Correlation | −0.20 | 0.81 | 0.47 | −0.43 | 0.66 | 1.37–7.98 | |
| wavelet.HHH_glcm_Imc1 | 0.25 | 1.28 | 0.24 | 1.02 | 0.30 | 2.20–8.11 | |
| wavelet.HHH_glcm_Correlation | 0.10 | 1.11 | 0.33 | 0.32 | 0.74 | 1.78–8.41 | |
| wavelet.LHL_firstorder_Skewness | −0.10 | 0.89 | 0.13 | −0.83 | 0.40 | 1.99–3.18 | |
| Combined | Smoker | −0.39 | 0.67 | 0.18 | −2.12 | 0.03 | 0.47–0.96 |
| Hydronephrosis | −0.07 | 0.93 | 0.38 | −0.18 | 0.85 | 0.44–1.97 | |
| wavelet.LLH_glcm_InverseVariance | 0.56 | 1.75 | 0.24 | 2.28 | 0.02 | 1.08–2.84 | |
| wavelet.LHL_glcm_Correlation | 0.58 | 1.78 | 0.15 | 3.80 | 0.0001 | 1.32–2.41 | |
| wavelet.HHL_glcm_Correlation | 0.29 | 1.34 | 0.24 | 1.17 | 0.24 | 0.82–2.18 | |
| wavelet.HHH_glcm_Correlation | 0.41 | 1.51 | 0.31 | 1.33 | 0.18 | 0.82–2.80 | |
| wavelet.LHH_glcm_Imc2 | 0.09 | 1.10 | 0.34 | 0.28 | 0.77 | 0.55–2.18 |
| Models | Variables | Coefficient | Exp. Coef. | Std. Error | Z | p-Value | 95% CI |
|---|---|---|---|---|---|---|---|
| Main analysis | Smoker | −0.391 | 0.67 | 0.18 | −2.12 | 0.03 | 0.47–0.96 |
| Hydronephrosis | −0.07 | 0.93 | 0.38 | −0.184 | 0.85 | 0.44–1.97 | |
| wavelet.LLH_glcm_InverseVariance | 0.56 | 1.75 | 0.24 | 2.28 | 0.023 | 1.08–2.84 | |
| wavelet.LHL_glcm_Correlation | 0.58 | 1.79 | 0.15 | 3.8 | 0.0001 | 1.32–2.41 | |
| Sub-analysis | Hydronephrosis | −1.17 | 0.31 | 0.82 | −1.42 | 0.15 | 0.06–1.55 |
| original_shape_Elongation | −0.78 | 0.45 | 0.41 | −1.86 | 0.06 | 0.2–1.03 | |
| original_shape_Sphericity | 0.26 | 1.29 | 0.34 | 0.75 | 0.44 | 0.66–2.55 | |
| original_gldm_SmallDependenceLowGrayLevelEmphasis | 1.11 | 3.05 | 0.4 | 2.74 | 0.006 | 1.37–6.77 | |
| gradient_glcm_Correlation | 0.7 | 2.02 | 0.43 | 1.62 | 0.1 | 0.86–4.72 | |
| squareroot_firstorder_Kurtosis | 0.24 | 1.27 | 0.49 | 0.49 | 0.61 | 0.48–3.33 | |
| wavelet.LHL_glcm_Idmn | −0.02 | 0.97 | 0.4 | −0.06 | 0.94 | 0.43–2.16 | |
| wavelet.HLL_gldm_SmallDependenceLowGrayLevelEmphasis | −0.29 | 0.74 | 0.32 | −0.9 | 0.36 | 0.39–1.4 | |
| wavelet.HLH_glcm_Correlation | 1.01 | 2.75 | 0.45 | 2.24 | 0.02 | 1.136–6.7 | |
| wavelet.HLH_glcm_Imc1 | 0.56 | 1.75 | 0.32 | 1.71 | 0.08 | 0.92–3.33 | |
| wavelet.HLH_glcm_InverseVariance | 0.696 | 2.007 | 0.48 | 1.43 | 0.15 | 0.77–5.19 |
| Models | Variables | Coefficient | Exp. Coef. | Std. Error | Z | p-Value | 95% CI |
|---|---|---|---|---|---|---|---|
| Clinical | Grade | 0.86 | 2.36 | 0.49 | 1.72 | 0.08 | 0.89–6.27 |
| Smoker | 0.71 | 2.59 | 0.23 | 2.18 | 0.02 | 1.37–7.94 | |
| CIS | 0.87 | 2.39 | 0.39 | 2.19 | 0.02 | 1.09–5.20 | |
| Radiomics | original_shape_Elongation | 0.13 | 1.14 | 0.26 | 0.49 | 0.61 | 0.67–1.91 |
| original_shape_MajorAxisLength | 0.31 | 1.36 | 0.24 | 1.29 | 0.19 | 0.85–2.19 | |
| original_shape_Sphericity | −0.02 | 0.97 | 0.24 | −0.11 | 0.90 | 0.60–1.56 | |
| original_glcm_ClusterTendency | 0.35 | 1.42 | 0.20 | 1.71 | 0.08 | 0.95–2.12 | |
| original_gldm_LowGrayLevelEmphasis | −0.42 | 0.65 | 0.27 | −1.55 | 0.11 | 0.38–1.11 | |
| wavelet.LLH_firstorder_Mean | −0.57 | 0.56 | 0.33 | −1.74 | 0.08 | 0.29–1.07 | |
| wavelet.LLH_glrlm_RunEntropy | −0.69 | 0.49 | 0.30 | −2.26 | 0.02 | 0.27–0.91 | |
| Combined | Grade | 0.80 | 2.22 | 0.50 | 1.59 | 0.11 | 0.83–5.97 |
| Smoker | 0.45 | 0.63 | 0.26 | 1.74 | 0.08 | 0.37–1.05 | |
| CIS | 0.75 | 2.12 | 0.41 | 1.83 | 0.06 | 0.94–4.78 | |
| original_shape_Elongation | −0.15 | 0.85 | 0.25 | −0.61 | 0.53 | 0.51–1.41 | |
| original_shape_MajorAxisLength | 0.24 | 1.27 | 0.24 | 1.00 | 0.31 | 0.79–2.04 | |
| original_shape_Sphericity | −0.03 | 0.96 | 0.23 | −0.16 | 0.86 | 0.60–1.53 | |
| original_glcm_ClusterTendency | 0.40 | 1.49 | 0.16 | 2.52 | 0.01 | 1.09–2.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, A.; Bhattacharjee, S.; Almopti, A.; Li, C.; Nabi, G. Radiomics-Based Computed Tomography Urogram Approach for the Prediction of Survival and Recurrence in Upper Urinary Tract Urothelial Carcinoma. Cancers 2024, 16, 3119. https://doi.org/10.3390/cancers16183119
Alqahtani A, Bhattacharjee S, Almopti A, Li C, Nabi G. Radiomics-Based Computed Tomography Urogram Approach for the Prediction of Survival and Recurrence in Upper Urinary Tract Urothelial Carcinoma. Cancers. 2024; 16(18):3119. https://doi.org/10.3390/cancers16183119
Chicago/Turabian StyleAlqahtani, Abdulsalam, Sourav Bhattacharjee, Abdulrahman Almopti, Chunhui Li, and Ghulam Nabi. 2024. "Radiomics-Based Computed Tomography Urogram Approach for the Prediction of Survival and Recurrence in Upper Urinary Tract Urothelial Carcinoma" Cancers 16, no. 18: 3119. https://doi.org/10.3390/cancers16183119
APA StyleAlqahtani, A., Bhattacharjee, S., Almopti, A., Li, C., & Nabi, G. (2024). Radiomics-Based Computed Tomography Urogram Approach for the Prediction of Survival and Recurrence in Upper Urinary Tract Urothelial Carcinoma. Cancers, 16(18), 3119. https://doi.org/10.3390/cancers16183119








