Inflammation-Associated Stem Cells in Gastrointestinal Cancers: Their Utility as Prognostic Biomarkers and Therapeutic Targets
Abstract
Simple Summary
Abstract
1. Introduction
2. Stem Cells in the Development of the Gut
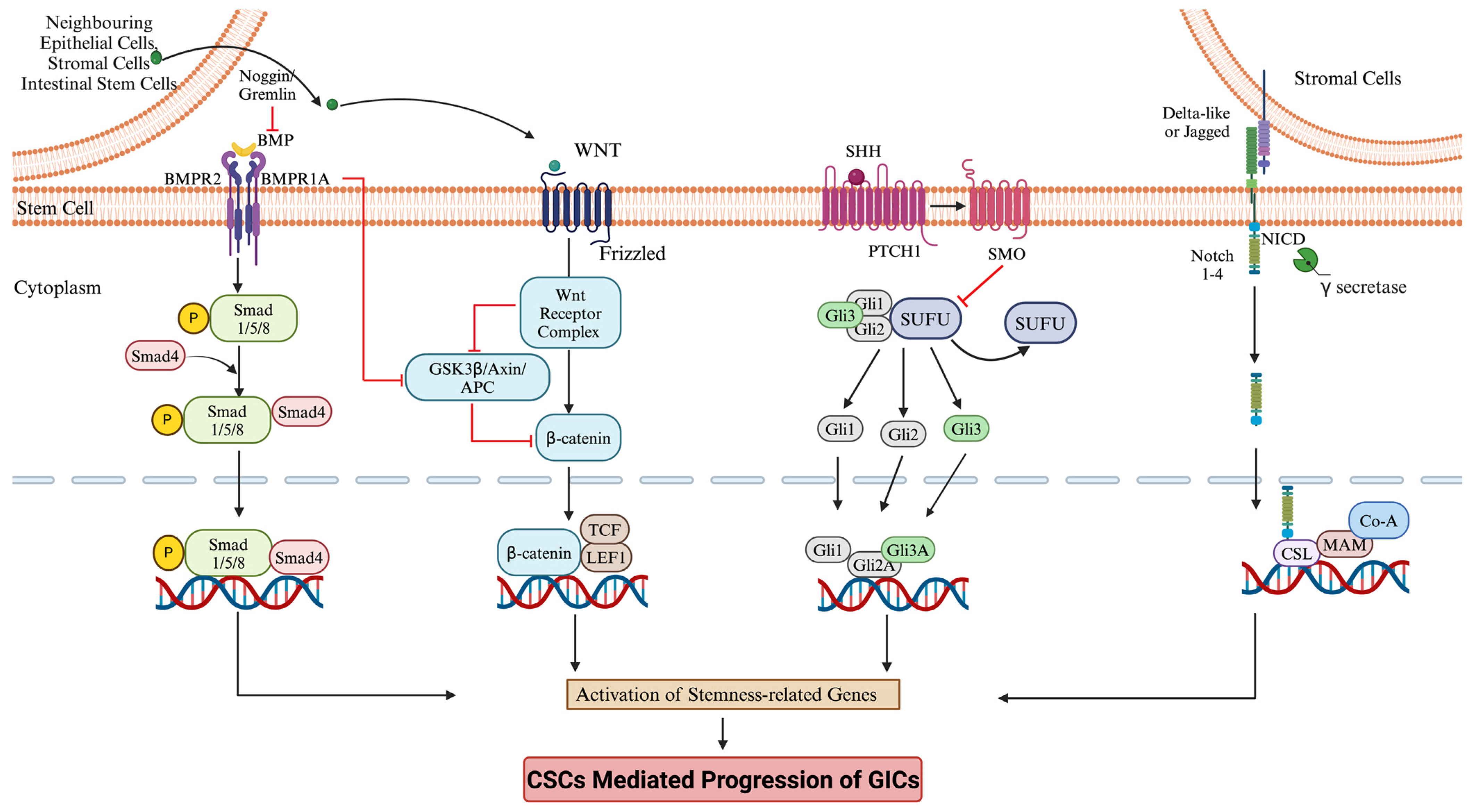
3. Signaling Pathway in Stem Cell Regulation
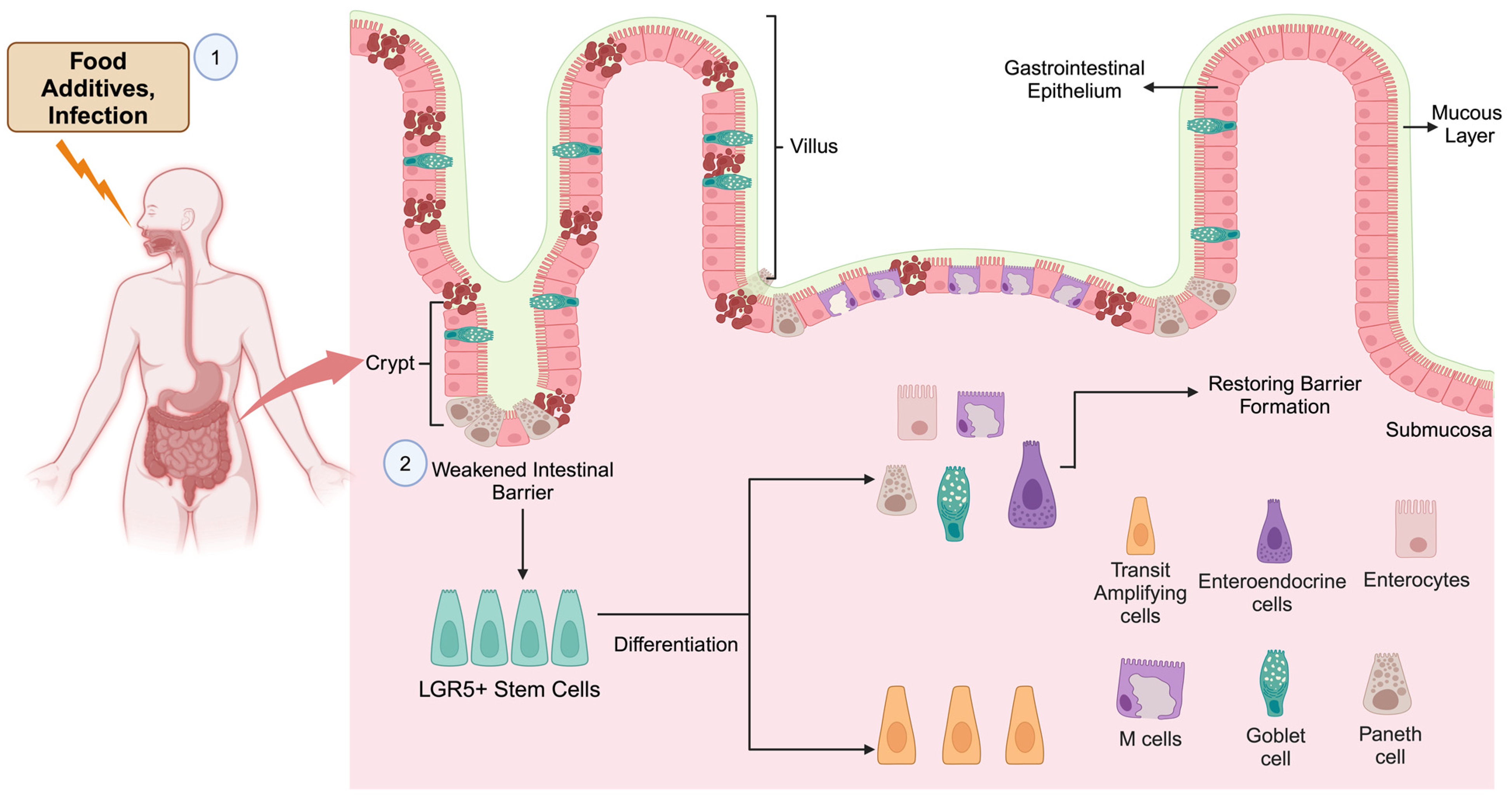
4. GI Damage and Its Repair by Stem Cells
5. Inflammation, Stem Cells, Cancer Initiation, and Progression
6. CSC-Mediated Drug Resistance in Gastrointestinal Cancer
7. Stem Cell-Based Prognostic Marker for GICs
8. Cancer Stem Cell-Based Targeted Therapies and Clinical Trials
Clinical Trials
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capobianco, I.; Di Vincenzo, F.; Puca, P.; Becherucci, G.; Mentella, M.C.; Petito, V.; Scaldaferri, F. Adverse Food Reactions in Inflammatory Bowel Disease: State of the Art and Future Perspectives. Nutrients 2024, 16, 351. [Google Scholar] [CrossRef]
- Abegunde, A.T.; Muhammad, B.H.; Bhatti, O.; Ali, T. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J. Gastroenterol. 2016, 22, 6296–6317. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; Costa, P.D.S.S.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Kuraishi, T.; Lemaitre, B. DrosophilaEGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.R.; Davies, P.S.; Silk, A.D.; Wong, M.H. Epithelial and Mesenchymal Contribution to the Niche: A Safeguard for Intestinal Stem Cell Homeostasis. Gastroenterology 2012, 143, 1426–1430. [Google Scholar] [CrossRef]
- Lopez-Garcia, C.; Klein, A.M.; Simons, B.D.; Winton, D.J. Intestinal Stem Cell Replacement Follows a Pattern of Neutral Drift. Science 2010, 330, 822–825. [Google Scholar] [CrossRef]
- Snippert, H.J.; van der Flier, L.G.; Sato, T.; van Es, J.H.; Born, M.v.D.; Kroon-Veenboer, C.; Barker, N.; Klein, A.M.; van Rheenen, J.; Simons, B.D.; et al. Intestinal Crypt Homeostasis Results from Neutral Competition between Symmetrically Dividing Lgr5 Stem Cells. Cell 2010, 143, 134–144. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M. Anti-aging: Myth or Reality. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128012383113704. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128012383113704 (accessed on 2 August 2024).
- Sellem, L.; Srour, B.; Javaux, G.; Chazelas, E.; Chassaing, B.; Viennois, E.; Debras, C.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; et al. Food additive emulsifiers and cancer risk: Results from the French prospective NutriNet-Santé cohort. PLoS Med. 2024, 21, e1004338. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Diet, Nutrition, Physical Activity, and Stomach Cancer; Continuous Update Project Report: World Cancer Research Fund; American Institute for Cancer Research: Washington, DC, USA, 2016.
- Diet, Nutrition, Physical Activity, and Colorectal Cancer; Continuous Update Project Expert Report; World Cancer Research Fund; American Institute for Cancer Research: Washington, DC, USA, 2018.
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.E.; Wang, Y.; Li, Y.; Poulin, E.J.; Means, A.L.; Washington, M.K.; Higginbotham, J.N.; Juchheim, A.; Prasad, N.; Levy, S.E.; et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 2012, 149, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Weng, K.; Lin, M.; Jiang, M.; Fang, Y.; Chung, S.S.; Huang, X.; Zhong, Q.; Liu, Z.; Huang, Z.; et al. SOX9 Modulates the Transformation of Gastric Stem Cells Through Biased Symmetric Cell Division. Gastroenterology 2023, 164, 1119–1136.e12. [Google Scholar] [CrossRef]
- Sphyris, N.; Hodder, M.C.; Sansom, O.J. Subversion of Niche-Signalling Pathways in Colorectal Cancer: What Makes and Breaks the Intestinal Stem Cell. Cancers 2021, 13, 1000. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, L.; Dai, L.; Zuo, D.; Li, X.; Zhu, H.; Xu, F. TNF-α promotes the malignant transformation of intestinal stem cells through the NF-κB and Wnt/β-catenin signaling pathways. Oncol. Rep. 2020, 44, 577–588. [Google Scholar] [CrossRef]
- Vries, R.G.; Huch, M.; Clevers, H. Stem cells and cancer of the stomach and intestine. Mol. Oncol. 2010, 4, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kapoor-Narula, U.; Lenka, N. Cancer stem cells and tumor heterogeneity: Deciphering the role in tumor progression and metastasis. Cytokine 2022, 157, 155968. [Google Scholar] [CrossRef]
- Lei, X.; He, Q.; Li, Z.; Zou, Q.; Xu, P.; Yu, H.; Ding, Y.; Zhu, W. Cancer stem cells in colorectal cancer and the association with chemotherapy resistance. Med. Oncol. 2021, 38, 43. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.S.; Ganesan, T.S. Role of Lymphangiogenesis in Cancer. J. Clin. Oncol. 2007, 25, 4298–4307. [Google Scholar] [CrossRef]
- Khosravi-Far, R.; White, E. Programmed Cell Death in Cancer Progression and Therapy; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2008; Volume 615, Available online: http://link.springer.com/10.1007/978-1-4020-6554-5 (accessed on 2 August 2024).
- Han, M.-E.; Oh, S.-O. Gastric stem cells and gastric cancer stem cells. Anat. Cell Biol. 2013, 46, 8–18. [Google Scholar] [CrossRef]
- Calvert, R.; Pothier, P. Migration of fetal intestinal intervillous cells in neonatal mice. Anat. Rec. 1990, 227, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.H.; Winton, D.J.; Ponder, B.A.J. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development 1988, 103, 785–790. [Google Scholar] [CrossRef]
- Langlands, A.J.; Almet, A.A.; Appleton, P.L.; Newton, I.P.; Osborne, J.M.; Näthke, I.S. Paneth Cell-Rich Regions Separated by a Cluster of Lgr5+ Cells Initiate Crypt Fission in the Intestinal Stem Cell Niche. PLOS Biol. 2016, 14, e1002491. [Google Scholar] [CrossRef]
- Cotsarelis, G.; Sun, T.-T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the Epithelial Stem Cell Niche in Skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat. Rec. 1993, 236, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, M.; Cheng, H. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G767–G777. [Google Scholar] [CrossRef]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-Renewal, Multipotency, and the Existence of Two Cell Populations within an Epithelial Stem Cell Niche. Cell 2004, 118, 635–648. [Google Scholar] [CrossRef]
- Morris, R.J.; Potten, C.S. Highly Persistent Label-Retaining Cells in the Hair Follicles of Mice and Their Fate Following Induction of Anagen. J. Investig. Dermatol. 1999, 112, 470–475. [Google Scholar] [CrossRef]
- He, X.C.; Zhang, J.; Tong, W.G.; Tawfik, O.; Ross, J.; Scoville, D.H.; Tian, Q.; Zeng, X.; He, X.; Wiedemann, L.M.; et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 2004, 36, 1117–1121. [Google Scholar] [CrossRef]
- Kosinski, C.; Li, V.S.W.; Chan, A.S.Y.; Zhang, J.; Ho, C.; Tsui, W.Y.; Chan, T.L.; Mifflin, R.C.; Powell, D.W.; Yuen, S.T.; et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl. Acad. Sci. USA 2007, 104, 15418–15423. [Google Scholar] [CrossRef]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ Stem Cells Are Indispensable for Radiation-Induced Intestinal Regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef]
- Clevers, H.; Watt, F.M. Defining Adult Stem Cells by Function, not by Phenotype. Annu. Rev. Biochem. 2018, 87, 1015–1027. [Google Scholar] [CrossRef]
- Fuchs, E.; Chen, T. A matter of life and death: Self-renewal in stem cells. Embo Rep. 2013, 14, 39–48. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Martens, E.C.; Chiang, H.C.; Gordon, J.I. Mucosal Glycan Foraging Enhances Fitness and Transmission of a Saccharolytic Human Gut Bacterial Symbiont. Cell Host Microbe 2008, 4, 447–457. [Google Scholar] [CrossRef]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef]
- Gerbe, F.; van Es, J.H.; Makrini, L.; Brulin, B.; Mellitzer, G.; Robine, S.; Romagnolo, B.; Shroyer, N.F.; Bourgaux, J.-F.; Pignodel, C.; et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 2011, 192, 767–780. [Google Scholar] [CrossRef]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef]
- von Moltke, J.; Ji, M.; Liang, H.-E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Gerbe, F.; Brulin, B.; Makrini, L.; Legraverend, C.; Jay, P. DCAMKL-1 Expression Identifies Tuft Cells Rather Than Stem Cells in the Adult Mouse Intestinal Epithelium. Gastroenterology 2009, 137, 2179–2180. [Google Scholar] [CrossRef]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. The potter’s wheel: The host’s role in sculpting its microbiota. Cell Mol. Life Sci. 2011, 68, 3675–3685. [Google Scholar] [CrossRef]
- Sato, T.; Clevers, H. Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef]
- Van Der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef]
- Barbara, P.d.S.; Brink, G.R.v.D.; Roberts, D.J. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. 2003, 60, 1322–1332. [Google Scholar] [CrossRef]
- Korinek, V.; Barker, N.; Moerer, P.; van Donselaar, E.; Huls, G.; Peters, P.J.; Clevers, H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998, 19, 379–383. [Google Scholar] [CrossRef]
- Kim, B.; Mao, J.; Taketo, M.M.; Shivdasani, R.A. Phases of Canonical Wnt Signaling During the Development of Mouse Intestinal Epithelium. Gastroenterology 2007, 133, 529–538. [Google Scholar] [CrossRef]
- Madison, B.B.; Braunstein, K.; Kuizon, E.; Portman, K.; Qiao, X.T.; Gumucio, D.L. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 2005, 132, 279–289. [Google Scholar] [CrossRef]
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995, 172, 126–138. [Google Scholar] [CrossRef]
- Haramis, A.-P.G.; Begthel, H.; Born, M.; van Es, J.; Jonkheer, S.; Offerhaus, G.J.A.; Clevers, H. De Novo Crypt Formation and Juvenile Polyposis on BMP Inhibition in Mouse Intestine. Science 2004, 303, 1684–1686. [Google Scholar] [CrossRef]
- Batts, L.E.; Polk, D.B.; Dubois, R.N.; Kulessa, H. Bmp signaling is required for intestinal growth and morphogenesis. Dev. Dyn. 2006, 235, 1563–1570. [Google Scholar] [CrossRef]
- Al Hargan, A.; Daghestani, M.H.; Harrath, A.H. Alterations in APC, BECN1, and TP53 gene expression levels in colon cancer cells caused by monosodium glutamate. Braz. J. Biol. 2021, 83, e246970. [Google Scholar] [CrossRef]
- Csáki, K.F. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med. Hypotheses 2011, 76, 676–681. [Google Scholar] [CrossRef]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: An overview. Toxicol. Rep. 2021, 8, 938–961. [Google Scholar] [CrossRef]
- Powell, J.J.; Ainley, C.C.; Harvey, R.S.; Mason, I.M.; Kendall, M.D.; Sankey, E.A.; Dhillon, A.P.; Thompson, R.P. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut 1996, 38, 390–395. [Google Scholar] [CrossRef]
- Lomer, M.C.E.; Hutchinson, C.; Volkert, S.; Greenfield, S.M.; Catterall, A.; Thompson, R.P.H.; Powell, J.J. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br. J. Nutr. 2004, 92, 947–955. [Google Scholar] [CrossRef]
- Arnold, A.R.; Chassaing, B. Maltodextrin, Modern Stressor of the Intestinal Environment. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 475–476. [Google Scholar] [CrossRef]
- Guo, J.; Shang, X.; Chen, P.; Huang, X. How does carrageenan cause colitis? A review. Carbohydr. Polym. 2023, 302, 120374. [Google Scholar] [CrossRef]
- Shil, A.; Olusanya, O.; Ghufoor, Z.; Forson, B.; Marks, J.; Chichger, H. Artificial Sweeteners Disrupt Tight Junctions and Barrier Function in the Intestinal Epithelium through Activation of the Sweet Taste Receptor, T1R3. Nutrients 2020, 12, 1862. [Google Scholar] [CrossRef]
- Becker, H.M.; Bertschinger, M.M.; Rogler, G. Microparticles and their impact on intestinal immunity. Dig. Dis. 2012, 30 (Suppl. 3), 47–54. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Gill, R.; Chen, M.L.; Zhang, F.; Linhardt, R.J.; Dudeja, P.K.; Tobacman, J.K. Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J. Biol. Chem. 2008, 283, 10550–10558. [Google Scholar] [CrossRef]
- Santos, P.S.; Caria, C.R.P.; Gotardo, E.M.F.; Ribeiro, M.L.; Pedrazzoli, J.; Gambero, A. Artificial sweetener saccharin disrupts intestinal epithelial cells’ barrier function in vitro. Food Funct. 2018, 9, 3815–3822. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Serena, G.; D’Avino, P.; Fasano, A. Celiac Disease and Non-celiac Wheat Sensitivity: State of Art of Non-dietary Therapies. Front. Nutr. 2020, 7, 152. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Nejad, M.R.; Aldulaimi, D.; Ishaq, S.; Ehsani-Ardakani, M.J.; Zali, M.R.; Malekzadeh, R.; Rostami, K. Geographic trends and risk of gastrointestinal cancer among patients with celiac disease in Europe and Asian-Pacific region. Gastroenterol. Hepatol. Bed Bench 2013, 6, 170–177. [Google Scholar]
- Libby, P.; Ebert, B.L. CHIP (Clonal Hematopoiesis of Indeterminate Potential): Potent and Newly Recognized Contributor to Cardiovascular Risk. Circulation 2018, 138, 666–668. [Google Scholar] [CrossRef]
- Krishnan, T.; Vasconcelos, J.P.S.; Titmuss, E.; Topham, J.T.; Schaeffer, D.F.; Karsan, A.; Lim, H.J.; Ho, C.; Gill, S.; Kennecke, H.F.; et al. Clonal hematopoiesis of indeterminate potential (CHIP), treatment outcomes and adverse events in gastrointestinal cancers: A pooled analysis of clinical trial and real-world data. J. Clin. Oncol. 2024, 42, 169. [Google Scholar] [CrossRef]
- Sturm, A.; Dignass, A.U. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 348–353. [Google Scholar] [CrossRef]
- Dignass, A.U.; Podolsky, D.K. Cytokine modulation of intestinal epithelial cell restitution: Central role of transforming growth factor beta. Gastroenterology 1993, 105, 1323–1332. [Google Scholar] [CrossRef]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef]
- Dignass, A.U.; Stow, J.L.; Babyatsky, M.W. Acute epithelial injury in the rat small intestine in vivo is associated with expanded expression of transforming growth factor alpha and beta. Gut 1996, 38, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Travis, S.P.L. Mucosal healing in inflammatory bowel diseases: A systematic review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.-A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ey, B.; Eyking, A.; Gerken, G.; Podolsky, D.K.; Cario, E. TLR2 Mediates Gap Junctional Intercellular Communication through Connexin-43 in Intestinal Epithelial Barrier Injury. J. Biol. Chem. 2009, 284, 22332–22343. [Google Scholar] [CrossRef]
- Podolsky, D.K.; Gerken, G.; Eyking, A.; Cario, E. Colitis-Associated Variant of TLR2 Causes Impaired Mucosal Repair Because of TFF3 Deficiency. Gastroenterology 2009, 137, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Lueschow, S.R.; McElroy, S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Immunol. 2020, 11, 587. [Google Scholar] [CrossRef]
- Sanman, L.E.; Chen, I.W.; Bieber, J.M.; Steri, V.; Trentesaux, C.; Hann, B.; Klein, O.D.; Wu, L.F.; Altschuler, S.J. Transit-Amplifying Cells Coordinate Changes in Intestinal Epithelial Cell-Type Composition. Dev. Cell 2021, 56, 356–365.e9. [Google Scholar] [CrossRef]
- Jenny, M.; Uhl, C.; Roche, C.; Duluc, I.; Guillermin, V.; Guillemot, F.; Jensen, J.; Kedinger, M.; Gradwohl, G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002, 21, 6338–6347. [Google Scholar] [CrossRef]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.A.; Yang, V.W.; Kaestner, K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002, 129, 2619–2628. [Google Scholar] [CrossRef]
- Taniguchi, K.; Wu, L.W.; Grivennikov, S.I.; de Jong, P.R.; Lian, I.; Yu, F.X.; Wang, K.; Ho, S.B.; Boland, B.S.; Chang, J.T.; et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015, 519, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Gola, A.; Fuchs, E. Environmental control of lineage plasticity and stem cell memory. Curr. Opin. Cell Biol. 2021, 69, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; Van De Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut 2020, 70, 1383–1395. [Google Scholar] [CrossRef]
- Kayisoglu, O.; Weiss, F.; Niklas, C.; Pierotti, I.; Pompaiah, M.; Wallaschek, N.; Germer, C.-T.; Wiegering, A.; Bartfeld, S. Location-specific cell identity rather than exposure to GI microbiota defines many innate immune signalling cascades in the gut epithelium. Gut 2021, 70, 687–697. [Google Scholar] [CrossRef]
- Naik, S.; Larsen, S.B.; Cowley, C.J.; Fuchs, E. Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell 2018, 175, 908–920. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, X.; Wu, J.; He, L.; Lu, T.; Wang, Y.; Liu, B.; Ye, B.; Sun, L.; Fan, D.; et al. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat. Immunol. 2019, 20, 183–194. [Google Scholar] [CrossRef]
- Quante, M.; Wang, T.C. Inflammation and Stem Cells in Gastrointestinal Carcinogenesis. Physiology 2008, 23, 350–359. [Google Scholar] [CrossRef]
- Ahsan, A.; Liu, Z.; Su, R.; Liu, C.; Liao, X.; Su, M. Potential Chemotherapeutic Effect of Selenium for Improved Canceration of Esophageal Cancer. Int. J. Mol. Sci. 2022, 23, 5509. [Google Scholar] [CrossRef]
- Wanebo, H.J.; LeGolvan, M.; Paty, P.B.; Saha, S.; Zuber, M.; D’angelica, M.I.; Kemeny, N.E. Meeting the biologic challenge of colorectal metastases. Clin. Exp. Metastasis 2012, 29, 821–839. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Ridgway, R.A.; Van Es, J.H.; Van De Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Wu, Y.C.; Chi, C.C.; Su, W.C.; Chang, P.J.; Lee, K.F.; Tung, T.-H.; Wang, J.; Liu, J.-J.; Tung, S.-Y.; et al. Activation of IL6/IGFIR confers poor prognosis of HBV-related hepatocellular carcinoma through induction of OCT4/NANOG expression. Clin. Cancer Res. 2015, 21, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Koch, S. Wnt signaling in intestinal inflammation. Differentiation 2019, 108, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Katoh, M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (Review). Int. J. Mol. Med. 2006, 18, 1019–1023. Available online: http://www.spandidos-publications.com/10.3892/ijmm.18.6.1019 (accessed on 25 May 2024). [CrossRef] [PubMed]
- Xie, Z.; Zhang, M.; Zhou, G.; Lin, L.; Han, J.; Wang, Y.; Li, L.; He, Y.; Zeng, Z.; Chen, M.; et al. Emerging roles of the Hedgehog signalling pathway in inflammatory bowel disease. Cell Death Discov. 2021, 7, 314. [Google Scholar] [CrossRef]
- Blanchet, M.R.; McNagny, K.M. Stem cells, inflammation and allergy. Allergy Asthma Clin. Immunol. 2009, 5, 13. [Google Scholar] [CrossRef]
- Kandasamy, J.; Huda, S.; Ambalavanan, N.; Jilling, T. Inflammatory signals that regulate intestinal epithelial renewal, differentiation, migration and cell death: Implications for necrotizing enterocolitis. Pathophysiology 2014, 21, 67–80. [Google Scholar] [CrossRef]
- Kuo, K.-K.; Lee, K.-T.; Chen, K.-K.; Yang, Y.-H.; Lin, Y.-C.; Tsai, M.-H.; Wuputra, K.; Lee, Y.-L.; Ku, C.-C.; Miyoshi, H.; et al. Positive Feedback Loop of OCT4 and c-JUN Expedites Cancer Stemness in Liver Cancer. Stem Cells 2016, 34, 2613–2624. [Google Scholar] [CrossRef]
- Kumar, D.B.U.; Chen, C.-L.; Liu, J.-C.; Feldman, D.E.; Sher, L.S.; DiNorcia, J.; French, S.W.; Naini, B.V.; Junrungsee, S.; Agopian, V.G.; et al. TLR4 Signaling via NANOG Cooperates with STAT3 to Activate Twist1 and Promote Formation of Tumor-Initiating Stem-Like Cells in Livers of Mice. Gastroenterology 2016, 150, 707–719. [Google Scholar] [CrossRef]
- Takasugi, M.; Yoshida, Y.; Hara, E.; Ohtani, N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2022, 290, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, L. Senescence Promotes the Recovery of Stemness among Cancer Cells via Reprograming. Biomolecules 2024, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.-P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Tato-Costa, J.; Casimiro, S.; Pacheco, T.; Pires, R.; Fernandes, A.; Alho, I.; Pereira, P.; Costa, P.; Castelo, H.B.; Ferreira, J.; et al. Therapy-Induced Cellular Senescence Induces Epithelial-to-Mesenchymal Transition and Increases Invasiveness in Rectal Cancer. Clin. Color. Cancer 2016, 15, 170–178.e3. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Nacarelli, T.; Fukumoto, T.; Zundell, J.A.; Fatkhutdinov, N.; Jean, S.; Cadungog, M.G.; Borowsky, M.E.; Zhang, R. NAMPT Inhibition Suppresses Cancer Stem-like Cells Associated with Therapy-Induced Senescence in Ovarian Cancer. Cancer Res. 2020, 80, 890–900. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, Y.W.; Lee, J.; Soh, E.Y.; Kim, J.-H.; Park, T.J. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat. Commun. 2017, 8, 15208. [Google Scholar] [CrossRef]
- Vernot, J.P. Senescence-Associated Pro-inflammatory Cytokines and Tumor Cell Plasticity. Front. Mol. Biosci. 2020, 7, 63. [Google Scholar] [CrossRef]
- Xu, Q.; Long, Q.; Zhu, D.; Fu, D.; Zhang, B.; Han, L.; Qian, M.; Guo, J.; Xu, J.; Cao, L.; et al. Targeting amphiregulin (AREG) derived from senescent stromal cells diminishes cancer resistance and averts programmed cell death 1 ligand (PD-L1)-mediated immunosuppression. Aging Cell 2019, 18, e13027. [Google Scholar] [CrossRef]
- Vermeulen, L.; de Sousa e Melo, F.; Richel, D.J.; Medema, J.P. The developing cancer stem-cell model: Clinical challenges and opportunities. Lancet Oncol. 2012, 13, e83–e89. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; Sprick, M.R.; Kemper, K.; Stassi, G.; Medema, J.P. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008, 15, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Giraud, J.; Chambonnier, L.; Dubus, P.; Wittkop, L.; Belleannée, G.; Collet, D.; Soubeyran, I.; Evrard, S.; Rousseau, B.; et al. Characterization of Biomarkers of Tumorigenic and Chemoresistant Cancer Stem Cells in Human Gastric Carcinoma. Clin. Cancer Res. 2017, 23, 1586–1597. [Google Scholar] [CrossRef]
- Li, W.; Zhang, N.; Jin, C.; Long, M.D.; Rajabi, H.; Yasumizu, Y.; Fushimi, A.; Yamashita, N.; Hagiwara, M.; Zheng, R.; et al. MUC1-C drives stemness in progression of colitis to colorectal cancer. J. Clin. Investig. 2020, 5, e137112. [Google Scholar] [CrossRef]
- Sheng, Y.H.; Wong, K.Y.; Seim, I.; Wang, R.; He, Y.; Wu, A.; Patrick, M.; Lourie, R.; Schreiber, V.; Giri, R.; et al. MUC13 promotes the development of colitis-associated colorectal tumors via β-catenin activity. Oncogene 2019, 38, 7294–7310. [Google Scholar] [CrossRef]
- Garg, A.D.; Kaczmarek, A.; Krysko, O.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. ER stress-induced inflammation: Does it aid or impede disease progression? Trends Mol. Med. 2012, 18, 589–598. [Google Scholar] [CrossRef]
- Hua, F.; Shang, S.; Yang, Y.-W.; Zhang, H.-Z.; Xu, T.-L.; Yu, J.-J.; Zhou, D.-D.; Cui, B.; Li, K.; Lv, X.-X.; et al. TRIB3 Interacts with β-Catenin and TCF4 to Increase Stem Cell Features of Colorectal Cancer Stem Cells and Tumorigenesis. Gastroenterology 2019, 156, 708–721.e15. [Google Scholar] [CrossRef]
- Jo, J.H.; Park, S.B.; Chung, J.; Oh, T.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Jung, D.E.; et al. Transgelin-2, a novel cancer stem cell-related biomarker, is a diagnostic and therapeutic target for biliary tract cancer. BMC Cancer 2024, 24, 357. [Google Scholar] [CrossRef] [PubMed]
- Elsafadi, M.; Manikandan, M.; Almalki, S.; Mahmood, A.; Shinwari, T.; Vishnubalaji, R.; Mobarak, M.; Alfayez, M.; Aldahmash, A.; Kassem, M.; et al. Transgelin is a poor prognostic factor associated with advanced colorectal cancer (CRC) stage promoting tumor growth and migration in a TGFβ-dependent manner. Cell Death Dis. 2020, 11, 341. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Sun, P. DOT1L promotes expression of CD44 through the Wnt/β-catenin signaling pathway in early gastric carcinoma. J. Cancer 2024, 15, 2276–2291. [Google Scholar] [CrossRef]
- Jinushi, M.; Chiba, S.; Yoshiyama, H.; Masutomi, K.; Kinoshita, I.; Dosaka-Akita, H.; Yagita, H.; Takaoka, A.; Tahara, H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12425–12430. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.C.; Kim, J.H. Cancer stem cell metabolism: Target for cancer therapy. BMB Rep. 2018, 51, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Fan, Z. Cancer stem cells and tumorigenesis. Biophys. Rep. 2018, 4, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Schöning, J.P.; Monteiro, M.; Gu, W. Drug resistance and cancer stem cells: The shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α. Clin. Exp. Pharmacol. Physiol. 2017, 44, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Mächtel, R.; Narducci, A.; Griffith, D.A.; Cordes, T.; Orelle, C. An integrated transport mechanism of the maltose ABC importer. Res. Microbiol. 2019, 170, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Begicevic, R.-R.; Falasca, M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef] [PubMed]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The Role of ABC Transporters in Drug Resistance, Metabolism and Toxicity. Curr. Drug Deliv. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Fu, Y.; Du, P.; Zhao, J.; Hu, C.; Qin, Y.; Huang, G. Gastric Cancer Stem Cells: Mechanisms and Therapeutic Approaches. Yonsei Med. J. 2018, 59, 1150–1158. [Google Scholar] [CrossRef]
- Alisi, A.; Cho, W.C.; Locatelli, F.; Fruci, D. Multidrug Resistance and Cancer Stem Cells in Neuroblastoma and Hepatoblastoma. Int. J. Mol. Sci. 2013, 14, 24706–24725. [Google Scholar] [CrossRef]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, X.; Lu, W.; Ding, Y.; Tang, Y. Blockade of integrin β3 signals to reverse the stem-like phenotype and drug resistance in melanoma. Cancer Chemother. Pharmacol. 2019, 83, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-M.; Xu, P.; Chen, Q.; Feng, S.-L.; Xie, Y. A multiple-targets alkaloid nuciferine overcomes paclitaxel-induced drug resistance in vitro and in vivo. Phytomedicine 2020, 79, 153342. [Google Scholar] [CrossRef]
- Zhu, G.-X.; Gao, D.; Shao, Z.-Z.; Chen, L.; Ding, W.-J.; Yu, Q.-F. Wnt/β-catenin signaling: Causes and treatment targets of drug resistance in colorectal cancer (Review). Mol. Med. Rep. 2020, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Okada, M.; Sanomachi, T.; Togashi, K.; Seino, S.; Sato, A.; Yamamoto, M.; Kitanaka, C. Therapeutic targeting of pancreatic cancer stem cells by dexamethasone modulation of the MKP-1–JNK axis. J. Biol. Chem. 2020, 295, 18328–18342. [Google Scholar] [CrossRef]
- Liu, X. ABC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 13–100. [Google Scholar]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar]
- Zhai, J.; Shen, J.; Xie, G.; Wu, J.; He, M.; Gao, L.; Zhang, Y.; Yao, X.; Shen, L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019, 454, 37–43. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Z.; Liu, Y.; Liu, Z. Forkhead box O3 promotes colon cancer proliferation and drug resistance by activating MDR1 expression. Mol. Genet. Genom. Med. 2019, 7, e554. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.B.; Deng, Q.M.; Deng, H.Q.; Dong, C.C.; Li, L.; He, C.G.; Wang, T.; Xu, S.; Mai, W. Siva-1 regulates multidrug resistance of gastric cancer by targeting MDR1 and MRP1 via the NF-κB pathway. Mol. Med. Rep. 2020, 22, 1558–1566. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.Y.; Li, X.; Chen, X.Y.; Wu, W.X. Role of ubiquitin-specific peptidase 22 in multidrug resistance of colorectal cancer and its correlation with multidrug resistance gene P-gp. Zhonghua Yi Xue Za Zhi 2021, 101, 3944–3949. [Google Scholar]
- Shi, X.; Valizadeh, A.; Mir, S.M.; Asemi, Z.; Karimian, A.; Majidina, M.; Safa, A.; Yosefi, B. miRNA-29a reverses P-glycoprotein-mediated drug resistance and inhibits proliferation via up-regulation of PTEN in colon cancer cells. Eur. J. Pharmacol. 2020, 880, 173138. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Zhang, Y.; Yan, X.; Tang, D.; Li, X.; Ni, Q.; Wang, D.; Zhu, J. Syndecan-2, negatively regulated by miR-20b-5p, contributes to 5-fluorouracil resistance of colorectal cancer cells via the JNK/ERK signaling pathway. Acta Biochim. Biophys. Sin. 2021, 53, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lin, C.; Zhang, Y.; Zhang, X.; Zhang, C.; Zhang, P.; Xie, X.; Ren, Z. miR-506 enhances the sensitivity of human colorectal cancer cells to oxaliplatin by suppressing MDR1/P-gp expression. Cell Prolif. 2017, 50, e12341. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Mo, Z.; Shi, X.; Qu, C. Clinical significance and correlation of miR-200c and P-gp expression in gastric cancer and the effects on multidrug resistance. J. Gastrointest. Oncol. 2022, 13, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Liu, H.; Pan, Y.; Sun, L.; Yu, L.; Sun, L.; Yang, Z.; Ran, Y. Distinct biological characterization of the CD44 and CD90 phenotypes of cancer stem cells in gastric cancer cell lines. Mol. Cell. Biochem. 2019, 459, 35–47. [Google Scholar] [CrossRef]
- Lu, L.; Wu, M.; Sun, L.; Li, W.; Fu, W.; Zhang, X.; Liu, T. Clinicopathological and prognostic significance of cancer stem cell markers CD44 and CD133 in patients with gastric cancer: A comprehensive meta-analysis with 4729 patients involved. Medicine 2016, 95, e5163. [Google Scholar] [CrossRef]
- Yiming, L.; Yunshan, G.; Bo, M.; Yu, Z.; Tao, W.; Gengfang, L.; Dexian, F.; Shiqian, C.; Jianli, J.; Juan, T.; et al. CD133 overexpression correlates with clinicopathological features of gastric cancer patients and its impact on survival: A systematic review and meta-analysis. Oncotarget 2015, 6, 42019–42027. [Google Scholar] [CrossRef]
- Gómez-Gallegos, A.A.; Ramírez-Vidal, L.; Becerril-Rico, J.; Pérez-Islas, E.; Hernandez-Peralta, Z.J.; Toledo-Guzmán, M.E.; García-Carrancá, A.; Langley, E.; Hernández-Guerrero, A.; López-Casillas, F.; et al. CD24+CD44+CD54+EpCAM+ gastric cancer stem cells predict tumor progression and metastasis: Clinical and experimental evidence. Stem Cell Res. Ther. 2023, 14, 16. [Google Scholar] [CrossRef]
- Akbari, M.; Shomali, N.; Faraji, A.; Shanehbandi, D.; Asadi, M.; Mokhtarzadeh, A.; Shabani, A.; Baradaran, B. CD133: An emerging prognostic factor and therapeutic target in colorectal cancer. Cell Biol. Int. 2019, 44, 368–380. [Google Scholar] [CrossRef]
- Lin, T.; Peng, W.; Mai, P.; Zhang, E.; Peng, L. Human Gastric Cancer Stem Cell (GCSC) Markers Are Prognostic Factors Correlated with Immune Infiltration of Gastric Cancer. Front. Mol. Biosci. 2021, 8, 626966. [Google Scholar] [CrossRef]
- Senel, F.; Kökenek Unal, T.D.; Karaman, H.; Inanç, M.; Aytekin, A. Prognostic Value of Cancer Stem Cell Markers CD44 and ALDH1/2 in Gastric Cancer Cases. Asian Pac. J. Cancer Prev. 2017, 18, 2527–2531. [Google Scholar] [PubMed]
- Nishikawa, S.; Konno, M.; Hamabe, A.; Hasegawa, S.; Kano, Y.; Ohta, K.; Fukusumi, T.; Sakai, D.; Kudo, T.; Haraguchi, N.; et al. Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy. Int. J. Oncol. 2013, 42, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Mou, Y.-P.; Chen, K.; Cai, J.-Q.; Zhou, Y.-C.; Pan, Y.; Xu, X.-W.; Zhou, W.; Gao, J.-Q.; Chen, D.-W.; et al. Aldehyde dehydrogenase 3A1 is robustly upregulated in gastric cancer stem-like cells and associated with tumorigenesis. Int. J. Oncol. 2016, 49, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Manikandan, M.; Fahad, M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Alajez, N.M. Molecular profiling of ALDH1+ colorectal cancer stem cells reveals preferential activation of MAPK, FAK, and oxidative stress pro-survival signalling pathways. Oncotarget 2018, 9, 13551–13564. [Google Scholar] [CrossRef]
- Kalantari, E.; Taheri, T.; Fata, S.; Abolhasani, M.; Mehrazma, M.; Madjd, Z.; Asgari, M. Significant co-expression of putative cancer stem cell markers, EpCAM and CD166, correlates with tumor stage and invasive behavior in colorectal cancer. World J. Surg. Oncol. 2022, 20, 15. [Google Scholar] [CrossRef]
- Jo, J.H.; Park, S.B.; Park, S.; Lee, H.S.; Kim, C.; Jung, D.E.; Song, S.Y. Novel Gastric Cancer Stem Cell-Related Marker LINGO2 Is Associated with Cancer Cell Phenotype and Patient Outcome. Int. J. Mol. Sci. 2019, 20, 555. [Google Scholar] [CrossRef]
- Li, H.; Piao, L.; Xu, D.; Xuan, Y. LETM1 is a potential biomarker that predicts poor prognosis in gastric adenocarcinoma. Exp. Mol. Pathol. 2020, 112, 104333. [Google Scholar] [CrossRef]
- Piao, L.; Feng, Y.; Yang, Z.; Qi, W.; Li, H.; Han, H.; Xuan, Y. LETM1 is a potential cancer stem-like cell marker and predicts poor prognosis in colorectal adenocarcinoma. Pathol. Res. Pract. 2019, 215, 152437. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.; Shi, Y.; Li, L.; Guo, X. Increased musashi 2 expression indicates a poor prognosis and promotes malignant phenotypes in gastric cancer. Oncol. Lett. 2019, 17, 2599–2606. [Google Scholar] [CrossRef]
- Lin, T.; Ding, Y.-Q.; Li, J.-M. Overexpression of Nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med. Oncol. 2012, 29, 878–885. [Google Scholar] [CrossRef]
- Basati, G.; Mohammadpour, H.; Razavi, A.E. Association of High Expression Levels of SOX2, NANOG, and OCT4 in Gastric Cancer Tumor Tissues with Progression and Poor Prognosis. J. Gastrointest. Cancer 2019, 51, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Yang, T.; Pang, B.-Y.; Zhu, Y.; Liu, Y.-N. The Progress and Prospects of Putative Biomarkers for Liver Cancer Stem Cells in Hepatocellular Carcinoma. Stem Cells Int. 2016, 2016, 7614971. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, B.; Wang, J.; Li, J.; Gong, Y.; Li, S.; Wang, C.; Cui, B.; Xue, X.; Yang, M.; et al. Reduction of NANOG Mediates the Inhibitory Effect of Aspirin on Tumor Growth and Stemness in Colorectal Cancer. Cell. Physiol. Biochem. 2017, 44, 1051–1063. [Google Scholar] [CrossRef]
- Xu, F.; Dai, C.; Zhang, R.; Zhao, Y.; Peng, S.; Jia, C. Nanog: A Potential Biomarker for Liver Metastasis of Colorectal Cancer. Dig. Dis. Sci. 2012, 57, 2340–2346. [Google Scholar] [CrossRef]
- Zhang, X.; Hua, R.; Wang, X.; Huang, M.; Gan, L.; Wu, Z.; Zhang, J.; Wang, H.; Cheng, Y.; Li, J.; et al. Identification of stem-like cells and clinical significance of candidate stem cell markers in gastric cancer. Oncotarget 2016, 7, 9815–9831. [Google Scholar] [CrossRef]
- Chen, X.-L.; Chen, X.-Z.; Wang, Y.-G.; He, D.; Lu, Z.-H.; Liu, K.; Zhang, W.-H.; Wang, W.; Li, C.-C.; Xue, L.; et al. Clinical significance of putative markers of cancer stem cells in gastric cancer: A retrospective cohort study. Oncotarget 2016, 7, 62049–62069. [Google Scholar] [CrossRef]
- Zhao, R.C.; Zhou, J.; Chen, K.F.; Gong, J.; Liu, J.; He, J.Y.; Guan, P.; Li, B.; Qin, Y. The prognostic value of combination of CD90 and OCT4 for hepatocellular carcinoma after curative resection. Neoplasma 2016, 63, 288–298. [Google Scholar] [CrossRef]
- Fujino, S.; Miyoshi, N. Oct4 Gene Expression in Primary Colorectal Cancer Promotes Liver Metastasis. Stem Cells Int. 2019, 2019, 7896524. [Google Scholar] [CrossRef] [PubMed]
- Hütz, K.; Mejías-Luque, R.; Farsakova, K.; Ogris, M.; Krebs, S.; Anton, M.; Vieth, M.; Schüller, U.; Schneider, M.R.; Blum, H.; et al. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis 2013, 35, 942–950. [Google Scholar] [CrossRef]
- Zhao, H.; Bi, M.; Lou, M.; Yang, X.; Sun, L. Downregulation of SOX2-OT Prevents Hepatocellular Carcinoma Progression Through miR-143-3p/MSI2. Front. Oncol. 2021, 11, 685912. [Google Scholar] [CrossRef]
- Li, M.-M.; Tang, Y.-Q.; Gong, Y.-F.; Cheng, W.; Li, H.-L.; Kong, F.-E.; Zhu, W.-J.; Liu, S.-S.; Huang, L.; Guan, X.-Y.; et al. Development of an oncogenic dedifferentiation SOX signature with prognostic significance in hepatocellular carcinoma. BMC Cancer 2019, 19, 851. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.J.; McCoy, M.J.; Lee-Pullen, T.F.; Anyaegbu, C.C.; Hemmings, C.; Bulsara, M.K.; Platell, C.F. The Prognostic and Predictive Value of SOX2+ Cell Densities in Patients Treated for Colorectal Cancer. Cancers 2020, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Khales, S.A.; Abbaszadegan, M.R.; Abdollahi, A.; Raeisossadati, R.; Tousi, M.F.; Forghanifard, M.M. SALL4 as a new biomarker for early colorectal cancers. J. Cancer Res. Clin. Oncol. 2015, 141, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, Q.; Wang, P.; Liu, M.; Xiong, S.; Luo, J.; Huang, H.; Du, Q.; Geller, D.A.; Cheng, B. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget 2016, 7, 5754–5768. [Google Scholar] [CrossRef]
- Cheung, P.F.; Cheung, T.T.; Yip, C.W.; Ng, L.W.; Fung, S.W.; Lo, C.M.; Fan, S.T.; Cheung, S.T. Hepatic cancer stem cell marker granulin-epithelin precursor and β-catenin expression associate with recurrence in hepatocellular carcinoma. Oncotarget 2016, 7, 21644–21657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, W.; Lu, Y.; Dong, X.; Chen, Y.; Lin, B.; Xie, X.; Guo, J.; Li, M. Alpha fetoprotein antagonizes apoptosis induced by paclitaxel in hepatoma cells in vitro. Sci. Rep. 2016, 6, 26472. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Tsuchiya, A.; Watanabe, Y.; Kawata, Y.; Kojima, Y.; Ikarashi, S.; Yanai, H.; Nakamura, K.; Kumaki, D.; Hirano, M.; et al. Clinical outcome of hepatocellular carcinoma can be predicted by the expression of hepatic progenitor cell markers and serum tumour markers. Oncotarget 2018, 9, 21844–21860. [Google Scholar] [CrossRef]
- Jin, J.; Niu, X.; Zou, L.; Li, L.; Li, S.; Han, J.; Zhang, P.; Song, J.; Xiao, F. AFP mRNA level in enriched circulating tumor cells from hepatocellular carcinoma patient blood samples is a pivotal predictive marker for metastasis. Cancer Lett. 2016, 378, 33–37. [Google Scholar] [CrossRef]
- Hassan, W.A.; Muqresh, M.A.; Omar, M. The Potential Role of CD44 and CD133 in Colorectal Stem Cell Cancer. Cureus 2022, 14, e30509. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.; Li, H.-N.; Zhang, L.; Hu, W.; Sun, Y.-M.; Chen, F.-L.; Jin, X.-M. Expressions of putative cancer stem cell markers ABCB1, ABCG2, and CD133 are correlated with the degree of differentiation of gastric cancer. Gastric Cancer 2012, 15, 440–450. [Google Scholar] [CrossRef]
- Ni, T.; Wang, H.; Zhan, D.; Tao, L.; Lv, M.; Wang, W.; Chu, Z.; Zhou, Z.; Sunagawa, M.; Liu, Y. CD133+/CD166+ human gastric adenocarcinoma cells present the properties of neoplastic stem cells and emerge more malignant features. Life Sci. 2021, 269, 119021. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Oshi, M.; Endo, I.; Takabe, K. Clinical relevance of stem cell surface markers CD133, CD24, and CD44 in colorectal cancer. Am. J. Cancer Res. 2021, 11, 5141–5154. [Google Scholar] [PubMed]
- Toh, J.; Hoppe, M.M.; Thakur, T.; Yang, H.; Tan, K.T.; Pang, B.; Ho, S.; Roy, R.; Ho, K.Y.; Yeoh, K.G.; et al. Profiling of gastric cancer cell-surface markers to achieve tumour–normal discrimination. BMJ Open Gastroenterol. 2020, 7, e000452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; He, B.; Bie, Q.; Cao, J.; Lu, H.; Zhang, Z.; Liang, J.; Wei, L.; Xiong, H.; Zhang, B. AQP5 complements LGR5 to determine the fates of gastric cancer stem cells through regulating ULK1 ubiquitination. J. Exp. Clin. Cancer Res. 2022, 41, 322. [Google Scholar] [CrossRef]
- Razmi, M.; Ghods, R.; Vafaei, S.; Sahlolbei, M.; Zanjani, L.S.; Madjd, Z. Clinical and prognostic significances of cancer stem cell markers in gastric cancer patients: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 139. [Google Scholar] [CrossRef]
- Barriuso, J.; Nagaraju, R.T.; Belgamwar, S.; Chakrabarty, B.; Burghel, G.J.; Schlecht, H.; Foster, L.; Kilgour, E.; Wallace, A.J.; Braun, M.; et al. Early Adaptation of Colorectal Cancer Cells to the Peritoneal Cavity Is Associated with Activation of “Stemness” Programs and Local Inflammation. Clin. Cancer Res. 2021, 27, 1119–1130. [Google Scholar] [CrossRef]
- Mesquita, P.; Freire, A.F.; Lopes, N.; Gomes, R.; Azevedo, D.; Barros, R.; Pereira, B.; Cavadas, B.; Pópulo, H.; Boaventura, P.; et al. Expression and Clinical Relevance of SOX9 in Gastric Cancer. Dis. Markers 2019, 2019, 8267021. [Google Scholar] [CrossRef]
- Ricanek, P.; Lunde, L.K.; Frye, S.A.; Støen, M.; Nygård, S.; Morth, J.P.; Rydning, A.; Vatn, M.H.; Amiry-Moghaddam, M.; Tonjum, T. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin. Exp. Gastroenterol. 2015, 8, 49–67. [Google Scholar] [CrossRef]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Asp. Med. 2012, 33, 642–650. [Google Scholar] [CrossRef]
- Huang, W.; Wen, F.; Gu, P.; Liu, J.; Xia, Y.; Li, Y.; Zhou, J.; Song, S.; Ruan, S.; Gu, S.; et al. The inhibitory effect and mechanism of Yi-qi-hua-yu-jie-du decoction on the drug resistance of gastric cancer stem cells based on ABC transporters. Chin. Med. 2022, 17, 93. [Google Scholar] [CrossRef]
- Silva, V.R.; Santos, L.d.S.; Dias, R.B.; Quadros, C.A.; Bezerra, D.P. Emerging agents that target signaling pathways to eradicate colorectal cancer stem cells. Cancer Commun. 2021, 41, 1275–1313. [Google Scholar] [CrossRef]
- Chen, K.; Huang, Y.-H.; Chen, J.-L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef]
- Ma, S.; Lee, T.K.; Zheng, B.-J.; Chan, K.W.; Guan, X.-Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008, 27, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Beachy, P.A. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001, 411, 349–354. [Google Scholar] [CrossRef]
- Lewis, A.; Segditsas, S.; Deheragoda, M.; Pollard, P.; Jeffery, R.; Nye, E.; Lockstone, H.; Davis, H.; Clark, S.; Stamp, G.; et al. Severe polyposis in Apc(1322T) mice is associated with submaximal Wnt signalling and increased expression of the stem cell marker Lgr5. Gut 2010, 59, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.S.; Karthikeyan, M.; Gnanasekaran, A.; Kaginelli, S.B.; Kuppanna, G.; Kallappa, C.G.; Basalingappa, K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef]
- Klempner, S.J.; Sonbol, B.B.; Wainberg, Z.A.; Uronis, H.E.; Chiu, V.K.; Scott, A.J.; Iqbal, S.; Tejani, M.A.; Stilian, M.C.; Thoma, M.; et al. A phase 2 study (DisTinGuish) of DKN-01 in combination with tislelizumab + chemotherapy as first-line (1L) therapy in patients with advanced gastric or GEJ adenocarcinoma (GEA). J. Clin. Oncol. 2023, 41, 4027. [Google Scholar] [CrossRef]
- Tabernero, J.; Van Cutsem, E.; Garralda, E.; Tai, D.; De Braud, F.; Geva, R.; van Bussel, M.T.J.; Dotti, K.F.; Elez, E.; de Miguel, M.J.; et al. A Phase Ib/II Study of WNT974 + Encorafenib + Cetuximab in Patients with BRAF V600E-Mutant KRAS Wild-Type Metastatic Colorectal Cancer. Oncologist 2023, 28, 230–238. [Google Scholar] [CrossRef]
- Cochrane, C.R.; Szczepny, A.; Watkins, D.N.; Cain, J.E. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers 2015, 7, 1554–1585. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Laterra, J.; Yauch, R.L.; Callahan, C.A.; Fu, L.; Holcomb, T.; Stinson, J.; Gould, S.E.; Coleman, B.; et al. Treatment of Medulloblastoma with Hedgehog Pathway Inhibitor GDC-0449. New Engl. J. Med. 2009, 361, 1173–1178. [Google Scholar] [CrossRef]
- Yauch, R.L.; Dijkgraaf, G.J.P.; Alicke, B.; Januario, T.; Ahn, C.P.; Holcomb, T.; Pujara, K.; Stinson, J.; Callahan, C.A.; Tang, T.; et al. Smoothened Mutation Confers Resistance to a Hedgehog Pathway Inhibitor in Medulloblastoma. Science 2009, 326, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.; de Sauvage, F.J. Hedgehog fights back: Mechanisms of acquired resistance against Smoothened antagonists. Cancer Res. 2011, 71, 5057–5061. [Google Scholar] [CrossRef] [PubMed]
- Lopez Miranda, E.; Stathis, A.; Hess, D.; Racca, F.; Quon, D.; Rodon, J.; Gadea, O.S.S.; Garcia, J.M.P.; Nuciforo, P.; Vivancos, A.; et al. Phase 1 study of CB-103, a novel first-in-class inhibitor of the CSL-NICD gene transcription factor complex in human cancers. J. Clin. Oncol. 2021, 39 (Suppl. 15), 3020. [Google Scholar] [CrossRef]
- Meng, R.D.; Shelton, C.C.; Li, Y.M.; Qin, L.X.; Notterman, D.; Paty, P.B.; Schwartz, G.K. γ-Secretase Inhibitors Abrogate Oxaliplatin-Induced Activation of the Notch-1 Signaling Pathway in Colon Cancer Cells Resulting in Enhanced Chemosensitivity. Cancer Res. 2009, 69, 573–582. [Google Scholar] [CrossRef]
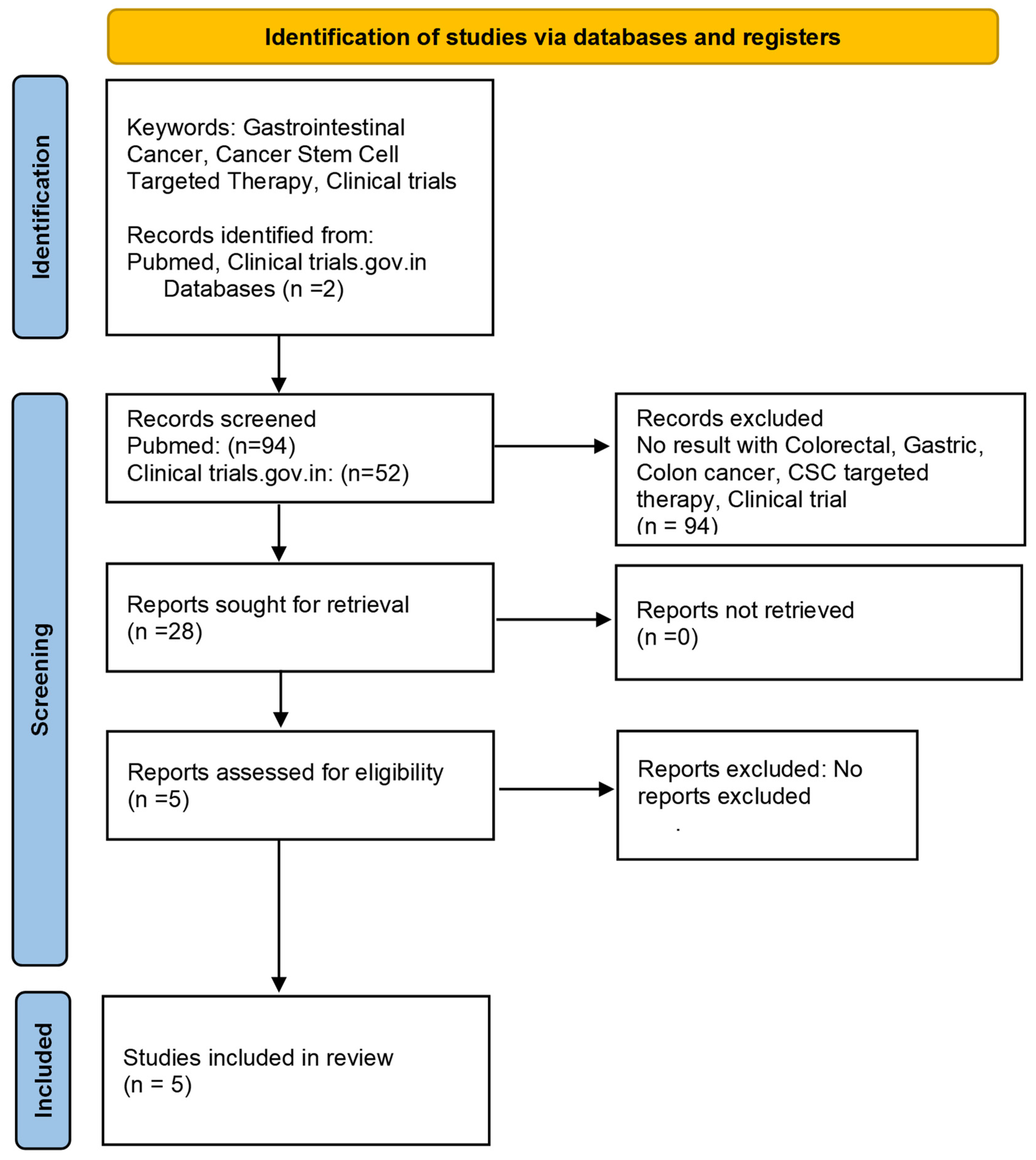
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Cancer Type | Chemotherapy | Pathway Involved in Resistance | Molecules Involved in Resistance | Reference |
|---|---|---|---|---|
| Colorectal Cancer | Doxorubicin | NF-kB | IL8, ABCB1 | [151] |
| Colorectal Cancer | Doxorubicin | - | FOXO3, MDR1 | [151] |
| Colorectal Cancer | Vincristine, 5-fluorouracil, doxorubicin | NF-kB | Siva 1, MDR1, MRP1 | [152,153] |
| Colorectal Cancer | Oxaliplatin | - | USP22, P-glycoprotein, MRP1 | [154] |
| Colon Cancer | Doxorubicin | PI3K/Akt | miR-29a, P-gp, PTEN | [155] |
| Colorectal Cancer | 5-Fluorouracil | - | miR-20b-5p, SDC2 | [156] |
| Colorectal Cancer | Oxaliplatin | Wnt/β-catenin | miR-506, MDR/P-gp | [157] |
| Gastric Cancer | Vincristine | - | miR-200c, ABCB1 (P-gp) | [158] |
| Surface CD Marker | ||||
| Gastric CSCs | Reference | CRC CSCs | Reference | |
| CD24 | + | [162] | - | |
| CD44 | + | [159] | + | [193] |
| CD90 | + | [159] | - | |
| CD133 | + | [194] | + | [193] |
| CD166 | + | [195] | - | |
| Other Surface Biomarker | ||||
| EpCAM | + | [162] | + | [169] |
| LGR5 | + | + | ||
| Intracellular Markers | ||||
| Nanog | + | - | ||
| Oct-3/4 | - | - | ||
| SOX2 | + | [183] | - | |
| Sall4 | - | + | [187] | |
| AFP | - | - |
| Surface CD Biomarkers | ||||
| Gastric CSCs | Reference | CRC CSCs | Reference | |
| CD24 | + | [162] | - | |
| CD44 | + | [165,179] | + | [196] |
| CD54 | + | [180] | - | |
| CD90 | - | - | ||
| CD133 | + | [180] | + | [163] |
| CD166 | + | [164] | - | |
| Other Surface Biomarkers | ||||
| CXCR4 | + | [164] | - | |
| EpCAM | + | [197] | - | |
| LINGO2 | + | [170] | - | |
| MUC13 | - | + | [129] | |
| DOTIL | + | [134] | ||
| AQP5 | + | [198] | ||
| Intracellular Markers | ||||
| ALDH | + | [165,166,167] | + | [168] |
| TRIB3 | - | + | ||
| Letm1 | + | [171] | + | [171] |
| Mushashi2 | + | [173] | - | |
| Nanog | + | [174,175] | + | [178] |
| Oct-3/4 | + | [180] | + | [182] |
| SOX2, SOX9 | + | [16,174,175] | + | [186] |
| Sall4 | - | + | [187] | |
| MUC13 | - | - | + | [129] |
| TAGLN | - | + | [114,115] |
| Cancer Type | Inhibitor Reported | Targeted Pathway | Reported Outcomes | Clinical Trial Identifier |
|---|---|---|---|---|
| Gastric Cancer/Gastroesophageal Cancer | DKN-01, Tislelizumab | Wnt | Currently recruiting, outcomes not reported | NCT04363801 |
| Metastatic Colorectal Cancer | WNT974, LGX818, Cetuximab | Wnt | Completed | NCT02278133 |
| Metastatic Colon Cancer | Vismodegib | Hedgehog | PFS: 9.3 months (Vismodegib), 10.1 months (Placebo) | NCT00636610 |
| Metastatic Colorectal Cancer | RO4929097 (Gamma-secretase inhibitor) | Notch | No benefit | NCT01116687 |
| Colorectal Cancer | CB-103 | Notch | PFS: 21.7 months | NCT03422679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, B.; Tiwari, A.; Meena, S.; Ahirwar, D.K. Inflammation-Associated Stem Cells in Gastrointestinal Cancers: Their Utility as Prognostic Biomarkers and Therapeutic Targets. Cancers 2024, 16, 3134. https://doi.org/10.3390/cancers16183134
Kumari B, Tiwari A, Meena S, Ahirwar DK. Inflammation-Associated Stem Cells in Gastrointestinal Cancers: Their Utility as Prognostic Biomarkers and Therapeutic Targets. Cancers. 2024; 16(18):3134. https://doi.org/10.3390/cancers16183134
Chicago/Turabian StyleKumari, Beauty, Aniket Tiwari, Sakshi Meena, and Dinesh Kumar Ahirwar. 2024. "Inflammation-Associated Stem Cells in Gastrointestinal Cancers: Their Utility as Prognostic Biomarkers and Therapeutic Targets" Cancers 16, no. 18: 3134. https://doi.org/10.3390/cancers16183134
APA StyleKumari, B., Tiwari, A., Meena, S., & Ahirwar, D. K. (2024). Inflammation-Associated Stem Cells in Gastrointestinal Cancers: Their Utility as Prognostic Biomarkers and Therapeutic Targets. Cancers, 16(18), 3134. https://doi.org/10.3390/cancers16183134





