Adrenal Insufficiency following Stereotactic Ablative Radiotherapy (SAbR) of Adrenal Gland Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subjects
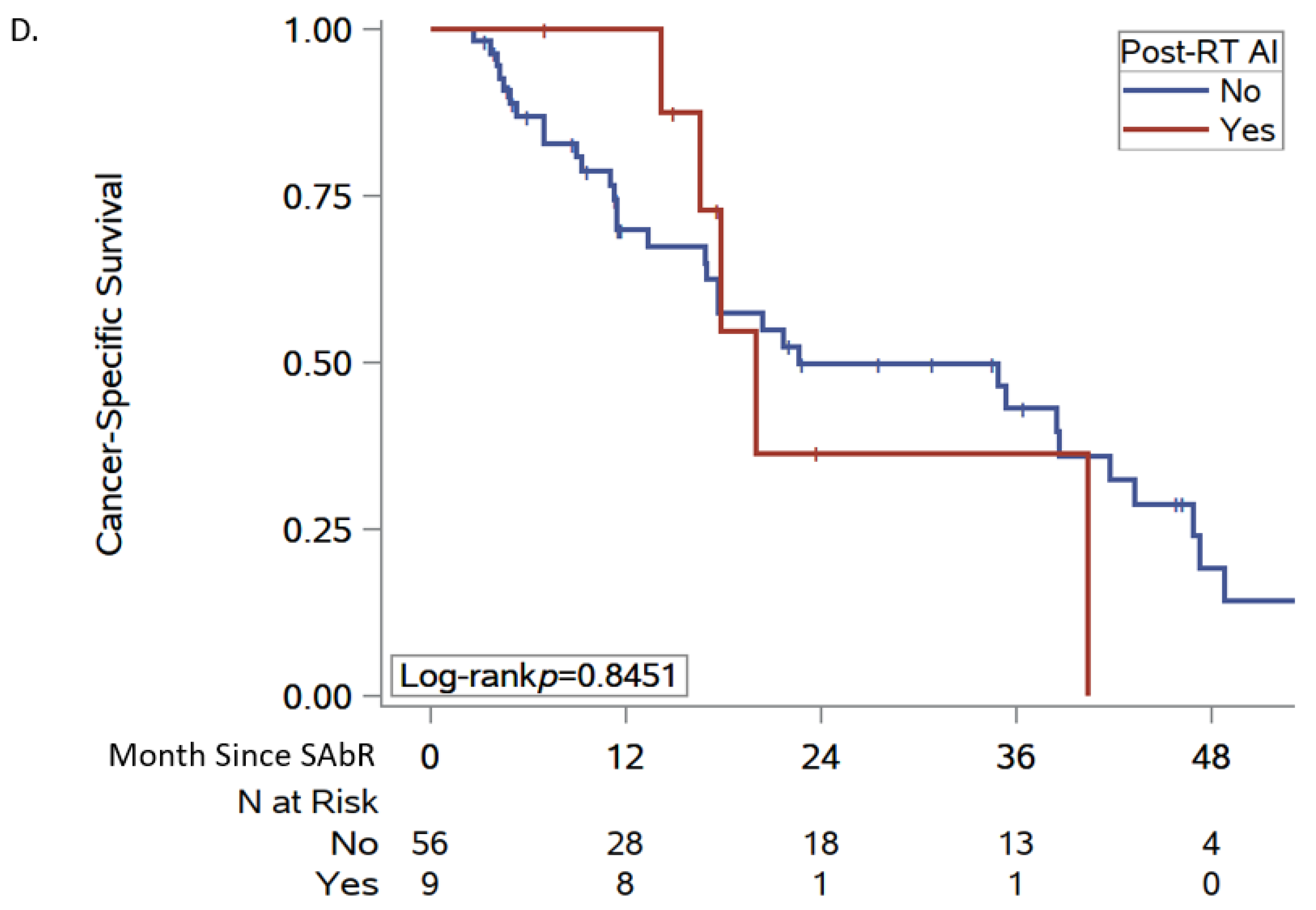
2.2. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. SAbR for Adrenal Metastasis
3.3. Factors Associated with Post-SAbR PAI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, A.K.Y. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Kasperlik-Załuska, A.; Rosłonowska, E.; Słowińska-Srzednicka, J.; Migdalska, B.; Jeske, W.; Makowska, A.; Snochowska, H. Incidentally discovered adrenal mass (incidentaloma): Investigation and management of 208 patients. Clin. Endocrinol. 1997, 46, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; de la Quintana Basarrate, A.; Musholt, T.J.; Paunovic, I.; Puccini, M.; Vidal, Ó.; Ortega, J.; Kraimps, J.-L.; Arocena, E.B.; Rodríguez, J.M. Adrenalectomy for solid tumor metastases: Results of a multicenter European study. Surgery 2013, 154, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Chen, Y.; Katz, A.W.; Muhs, A.G.; Philip, A.; Okunieff, P.; Milano, M.T. Stereotactic body radiotherapy for treatment of adrenal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 71–75. [Google Scholar] [CrossRef]
- Casamassima, F.; Livi, L.; Masciullo, S.; Menichelli, C.; Masi, L.; Meattini, I.; Bonucci, I.; Agresti, B.; Simontacchi, G.; Doro, R. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Cingam, S.R.; Mukkamalla, S.K.R.; Karanchi, H. Adrenal metastasis. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Kung, A.W.; Pun, K.; Lam, K.; Wang, C.; Leung, C. Addisonian crisis as presenting feature in malignancies. Cancer 1990, 65, 177–179. [Google Scholar] [CrossRef]
- Tallis, P.H.; Rushworth, R.L.; Torpy, D.J.; Falhammar, H. Adrenal insufficiency due to bilateral adrenal metastases—A systematic review and meta-analysis. Heliyon 2019, 5, e01783. [Google Scholar] [CrossRef]
- Mao, J.J.; Dages, K.N.; Suresh, M.; Bancos, I. Presentation, disease progression and outcomes of adrenal gland metastases. Clin. Endocrinol. 2020, 93, 546–554. [Google Scholar] [CrossRef]
- Muth, A.; Persson, F.; Jansson, S.; Johanson, V.; Ahlman, H.; Wängberg, B. Prognostic factors for survival after surgery for adrenal metastasis. Eur. J. Surg. Oncol. 2010, 36, 699–704. [Google Scholar] [CrossRef]
- Mitchell, J.; Barbosa, G.; Tsinberg, M.; Milas, M.; Siperstein, A.; Berber, E. Unrecognized adrenal insufficiency in patients undergoing laparoscopic adrenalectomy. Surg. Endosc. 2009, 23, 248–254. [Google Scholar] [CrossRef]
- Wardak, Z.; Meyer, J.; Ghayee, H.; Wilfong, L.; Timmerman, R. Adrenal insufficiency after stereotactic body radiation therapy for bilateral adrenal metastases. Pract. Radiat. Oncol. 2015, 5, e177–e181. [Google Scholar] [CrossRef] [PubMed]
- Salama, J.K.; Hasselle, M.D.; Chmura, S.J.; Malik, R.; Mehta, N.; Yenice, K.M.; Villaflor, V.M.; Stadler, W.M.; Hoffman, P.C.; Cohen, E.E. Stereotactic body radiotherapy for multisite extracranial oligometastases: Final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 2012, 118, 2962–2970. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, X.; Lin, J.; Wang, W.; Zhang, X.; Liu, M.; Li, X.; Huang, G.; Liu, B.; Xie, X. Percutaneous radiofrequency ablation of adrenal metastases from hepatocellular carcinoma: A single-center experience. Cancer Imaging 2019, 19, 44. [Google Scholar] [CrossRef]
- König, L.; Häfner, M.F.; Katayama, S.; Koerber, S.A.; Tonndorf-Martini, E.; Bernhardt, D.; von Nettelbladt, B.; Weykamp, F.; Hoegen, P.; Klüter, S. Stereotactic body radiotherapy (SBRT) for adrenal metastases of oligometastatic or oligoprogressive tumor patients. Radiat. Oncol. 2020, 15, 30. [Google Scholar] [CrossRef]
- Hannan, R.; Christensen, M.; Christie, A.; Garant, A.; Pedrosa, I.; Robles, L.; Mannala, S.; Wang, C.; Hammers, H.; Arafat, W. Stereotactic Ablative Radiation for Systemic Therapy–naïve Oligometastatic Kidney Cancer. Eur. Urol. Oncol. 2022, 5, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Hannan, R.; Christensen, M.; Hammers, H.; Christie, A.; Paulman, B.; Lin, D.; Garant, A.; Arafat, W.; Courtney, K.; Bowman, I. Phase II trial of stereotactic ablative radiation for oligoprogressive metastatic kidney cancer. Eur. Urol. Oncol. 2022, 5, 216–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Schoenhals, J.; Christie, A.; Mohamad, O.; Wang, C.; Bowman, I.; Singla, N.; Hammers, H.; Courtney, K.; Bagrodia, A. Stereotactic ablative radiation therapy (SAbR) used to defer systemic therapy in oligometastatic renal cell cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 367–375. [Google Scholar] [CrossRef]
- Schoenhals, J.E.; Mohamad, O.; Christie, A.; Zhang, Y.; Li, D.; Singla, N.; Bowman, I.; Arafat, W.; Hammers, H.; Courtney, K. Stereotactic ablative radiation therapy for oligoprogressive renal cell carcinoma. Adv. Radiat. Oncol. 2021, 6, 100692. [Google Scholar] [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Toesca, D.A.; Koong, A.J.; von Eyben, R.; Koong, A.C.; Chang, D.T. Stereotactic body radiation therapy for adrenal gland metastases: Outcomes and toxicity. Adv. Radiat. Oncol. 2018, 3, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kothari, G.; Foroudi, F.; Gill, S.; Corcoran, N.M.; Siva, S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol. 2015, 54, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Malik, R.; Ranck, M.C.; Farrey, K.; Golden, D.W.; Hasselle, M.D.; Weichselbaum, R.R.; Salama, J.K. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol. Cancer Res. Treat. 2013, 12, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.; Hallemeier, C.; Jethwa, K.; Shariff, A.; Bancos, I. Radiation of bilateral adrenal metastases is associated with a high risk of primary adrenal insufficiency. Clin. Endocrinol. 2023, 99, 35–42. [Google Scholar] [CrossRef]
- Hannan, R.; McLaughlin, M.F.; Pop, L.M.; Pedrosa, I.; Kapur, P.; Garant, A.; Ahn, C.; Christie, A.; Zhu, J.; Wang, T. Phase 2 trial of stereotactic ablative radiotherapy for patients with primary renal cancer. Eur. Urol. 2023, 84, 275–286. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A. Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef]
- Javorsky, B.R.; Raff, H.; Carroll, T.B.; Algeciras-Schimnich, A.; Singh, R.J.; Colón-Franco, J.M.; Findling, J.W. New cutoffs for the biochemical diagnosis of adrenal insufficiency after ACTH stimulation using specific cortisol assays. J. Endocr. Soc. 2021, 5, bvab022. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Murad, M.H.; Newell-Price, J.; Savage, M.O.; Tabarin, A. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 2807–2831. [Google Scholar] [CrossRef]
- Yoshiji, S.; Shibue, K.; Fujii, T.; Usui, T.; Hirota, K.; Taura, D.; Inoue, M.; Sone, M.; Yasoda, A.; Inagaki, N. Chronic primary adrenal insufficiency after unilateral adrenonephrectomy: A case report. Medicine 2017, 96, e9091. [Google Scholar] [CrossRef]
- Moorman, P.G.; Myers, E.R.; Schildkraut, J.M.; Iversen, E.S.; Wang, F.; Warren, N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet. Gynecol. 2011, 118, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, J.; Higham, C.; Langer, T.; Denzer, C.; Brabant, G. Long-term endocrine and metabolic consequences of cancer treatment: A systematic review. Endocr. Rev. 2019, 40, 711–767. [Google Scholar] [CrossRef] [PubMed]


| N (%) (n = 66) | |
|---|---|
| Median age at cancer diagnosis (IQR) | 61 (55–66) |
| Sex | |
| Male | 48 (73%) |
| Female | 18 (27%) |
| Primary tumor site | |
| Kidney | 27 (41%) |
| Lung | 25 (38%) |
| Colorectal | 6 (9%) |
| Melanoma | 3 (5%) |
| Bladder | 2 (3%) |
| Angiosarcoma | 1 (1.5%) |
| Hepatocellular | 1 (1.5%) |
| Thyroid | 1 (1.5%) |
| T stage a | |
| 1 | 3 (6.5%) |
| 2 | 12 (26.1%) |
| 3 | 19 (41.3%) |
| 4 | 12 (26.1%) |
| Nodal involvement a | |
| No | 16 (42.1%) |
| Nodes not sampled | 7 (18.4%) |
| Yes | 15 (39.5%) |
| M stage a | |
| 0 | 21 (58.3%) |
| 1 | 15 (41.7%) |
| N = 66 | |
|---|---|
| Median size of largest adrenal metastasis, cm (IQR) a | 2.8 (2.1–4.5) |
| Median total size of adrenal metastasis, cm (IQR) a | 2.8 (2.1–4.9) |
| Median months to adrenal metastasis (IQR) | 14 (5–37) |
| Metastatic adrenal laterality (overall) | |
| Bilateral | 25 (37.9%) |
| Left | 17 (25.8%) |
| Right | 24 (36.4%) |
| Timing of bilateral adrenal involvement | |
| Metachronous | 14 (21.2%) |
| Synchronous | 11 (16.7%) |
| Unilateral | 41 (62.1%) |
| Median months to contralateral adrenal metastasis (IQR) | 6 (0–23) |
| FDG Avid | 29 (43.9%) |
| Median SUV (IQR) | 9.8 (5.7–15.5) |
| Adrenal biopsy | 13 (19.7%) |
| Adrenalectomy | 11 (16.7%) |
| Median months to adrenalectomy (IQR) | 13 (0–19) |
| Status of contralateral adrenal gland at the time of SAbR | |
| Bilateral adrenal metastases | 16 (24.2%) |
| Prior adrenalectomy | 9 (13.6%) |
| Unaffected | 41 (62.1%) |
| Median months to SAbR from initial cancer diagnosis (IQR) | 30 (16–53) |
| Pre-SAbR systemic therapy | 49 (74.2%) |
| Pre-SAbR IO therapy | 19 (28.8%) |
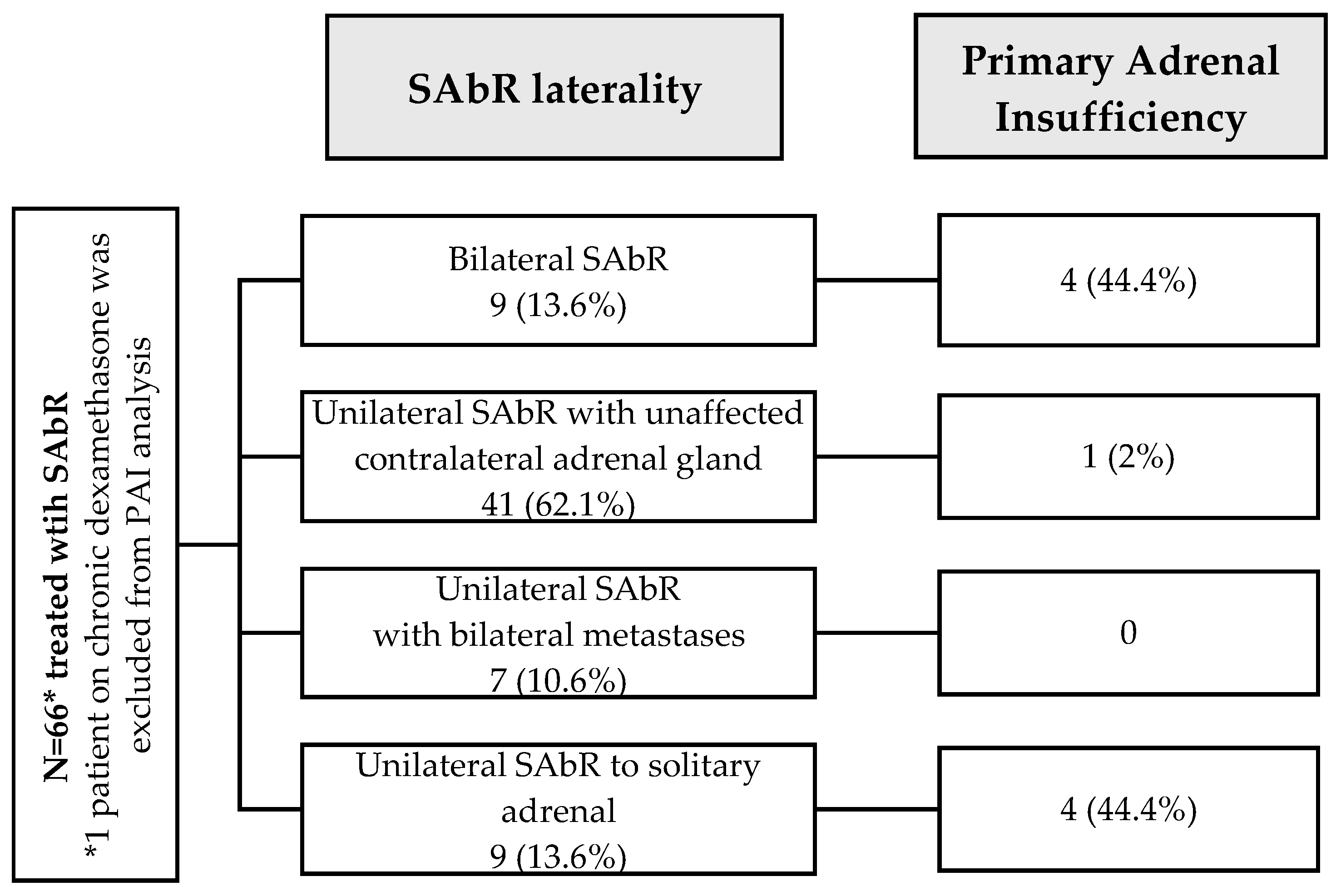
| Adrenal SAbR laterality | |
| Bilateral | 9 (13.6%) |
| Unilateral with unaffected contralateral adrenal gland | 41 (62.1%) |
| Unilateral with bilateral metastases | 7 (10.6%) |
| Solitary unilateral | 9 (13.6%) |
| SAbR fractions, median dose/fraction | |
| 1 | 1 (1.5%), 2000 Gy/fx |
| 3 | 18 (27.3%), 1350 Gy/fx |
| 4 | 1 (1.5%), 600 Gy/fx |
| 5 | 44 (66.7%), 800 Gy/fx |
| 10 | 2 (3.0%), 300 Gy/fx |
| SAbR intent b | |
| Consolidative | 15 (22.7%) |
| Curative | 20 (30.3%) |
| Definitive | 15 (22.7%) |
| Palliative | 16 (24.2%) |
| SAbR approach | |
| IMRT | 2 (3.0%) |
| SBRT | 64 (97.0%) |
| Median BED-LQ, Gy (IQR) | 128.2 (64.2–200.3) |
| Median BED-USC, Gy (IQR) | 84.2 (63.0–160.1) |
| 9 | N with PAI/Total | Odds Ratio (95% CI) | p |
|---|---|---|---|
| Age at diagnosis (per 5 years) | - | 1.11 (0.75, 1.64) | 0.60 |
| Sex | |||
| Female | 2/18 | reference | 0.69 |
| Male | 7/47 | 1.40 (0.26, 7.47) | |
| Primary tumor site | |||
| Lung | 2/25 | reference | 0.28 |
| Renal cell carcinoma | 6/27 | 3.29 (0.60, 18.1) | |
| Other | 1/13 | 0.96 (0.08, 11.7) | |
| M stage | |||
| 0 | 4/21 | reference | 0.94 |
| 1 | 3/15 | 1.06 (0.20, 5.64) | |
| Pre-SAbR systemic therapy | |||
| Yes | 5/48 | 0.38 (0.09, 1.62) | 0.19 |
| No | 4/17 | reference | |
| Pre-SAbR immunotherapy | |||
| Yes | 2/19 | 0.66 (0.12, 3.49) | 0.62 |
| No | 7/46 | reference | |
| Size of largest adrenal metastasis (per cm) | - | 0.84 (0.54, 1.31) | 0.45 |
| Total size of adrenal metastasis (per cm) | - | 1.01 (0.77, 1.34) | 0.92 |
| Timing of bilateral involvement | |||
| Metachronous | 4/14 | 7.80 (1.25, 48.8) | 0.051 |
| Synchronous | 3/10 | 8.36 (1.18, 59.4) | |
| Unilateral | 2/41 | reference | |
| Adrenalectomy | |||
| Yes | 4/11 | 5.60 (1.21, 26.0) | 0.028 |
| No | 5/54 | reference | |
| Status of contralateral adrenal gland | |||
| Bilateral adrenal metastases | 4/15 ¶ | 14.6 (1.47, 144) | 0.016 |
| Prior adrenalectomy | 4/9 | 32.0 (2.96, 346) | |
| Unaffected | 1/41 | reference | |
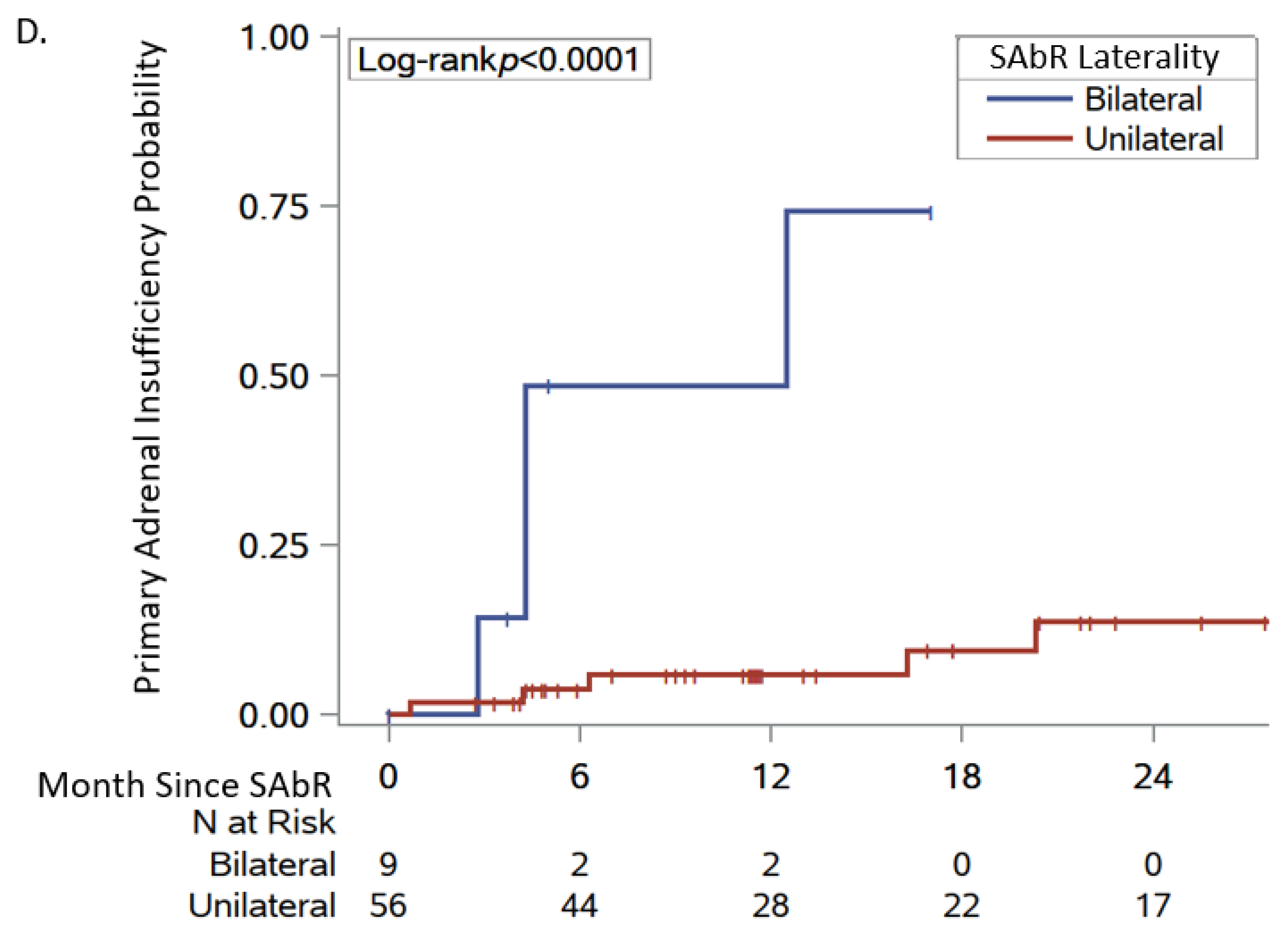
| Adrenal SAbR laterality | |||
| Bilateral | 4/9 | 8.16 (1.64, 40.6) | 0.010 |
| Unilateral | 5/56 | reference | |
| BED-LQ, Gy (per 10 Gy) | - | 1.06 (0.97, 1.15) | 0.19 |
| BED-USC, Gy (per 10 Gy) | - | 1.08 (0.96, 1.21) | 0.19 |
| Pre-SAbR cortisol (per μg/dL) | - | 1.27 (0.93, 1.73) | 0.13 |
| Age at Cancer Diagnosis/Sex | Primary Cancer | Adrenal Metastasis Side | Total Adrenal metastasis Burden (cm) | Adrenalectomy | SAbR | BED-LQ (Gy) | BED-USC (Gy) | Time from SAbR to PAI, Months | PAI CTCAE Grade | MR Deficiency |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1: 60/M | Renal cell | Bilateral | 5.9 | No | Bilateral | 200.3 | 173 | 12.5 | 4 | No |
| Pt 2: 66/F | Renal cell | Right | 1.5 | No | Right | 103.2 | 57.32 | 16.3 | 2 | Yes |
| Pt 3: 75/M | Colorectal | Bilateral | 5.5 | No | Bilateral | 231.8 | 192.23 | 4.3 | 2 | Yes |
| Pt 4: 65/M | Lung | Bilateral | 8.6 | No | Bilateral | 64.2 | 64.18 | 4.3 | 3 | No |
| Pt 5: 45/M | Renal cell | Bilateral | 2.2 | Yes | Left | 202.3 | 86.67 | 0.7 | 2 | No |
| Pt 6: 59/M | Renal cell | Bilateral | 3.1 | Yes | Left | 150.3 | 150.34 | 20.2 | 4 | Yes |
| Pt 7: 69/F | Lung | Bilateral | 2.4 | No | Bilateral | 64.9 | 59.64 | 2.8 | 2 | No |
| Pt 8: 60/M | Renal cell | Bilateral | 2.7 | Yes | Right | 246.1 | 78.42 | 6.3 | 1 | No |
| Pt 9: 66/M | Renal cell | Right | 2.5 | Yes | Right | 50.9 | 50.92 | 4.2 | 2 | Yes |
| 6-Month Survival/ Failure (95% CI) | 1-Year Survival/ Failure (95% CI) | Median Survival/ Failure (95% CI) | |
|---|---|---|---|
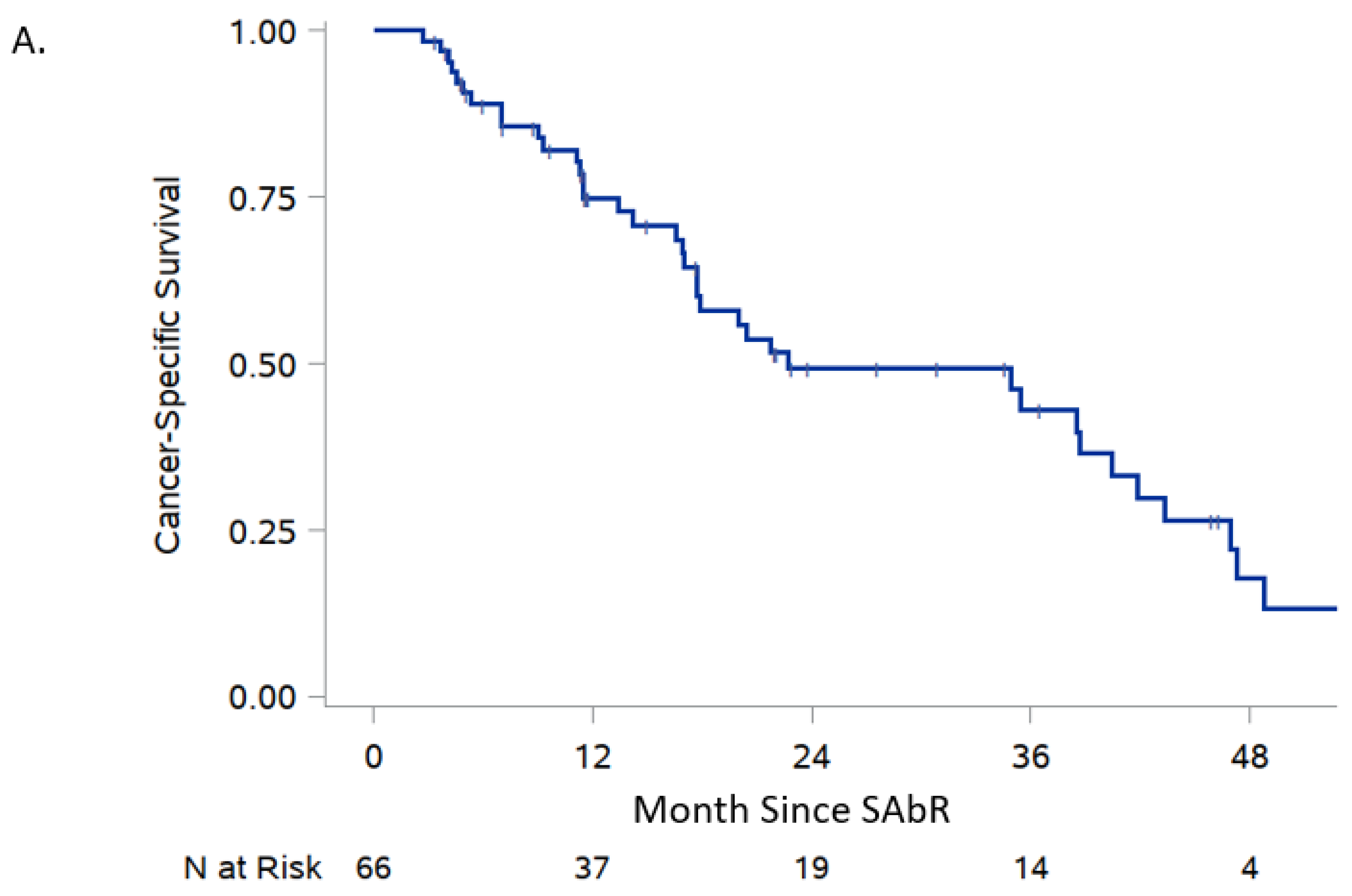
| Cancer-specific survival | 89.0% (78.3, 94.6) | 74.8% (61.6, 84.0) | 22.7 (17.7, 40.4) |
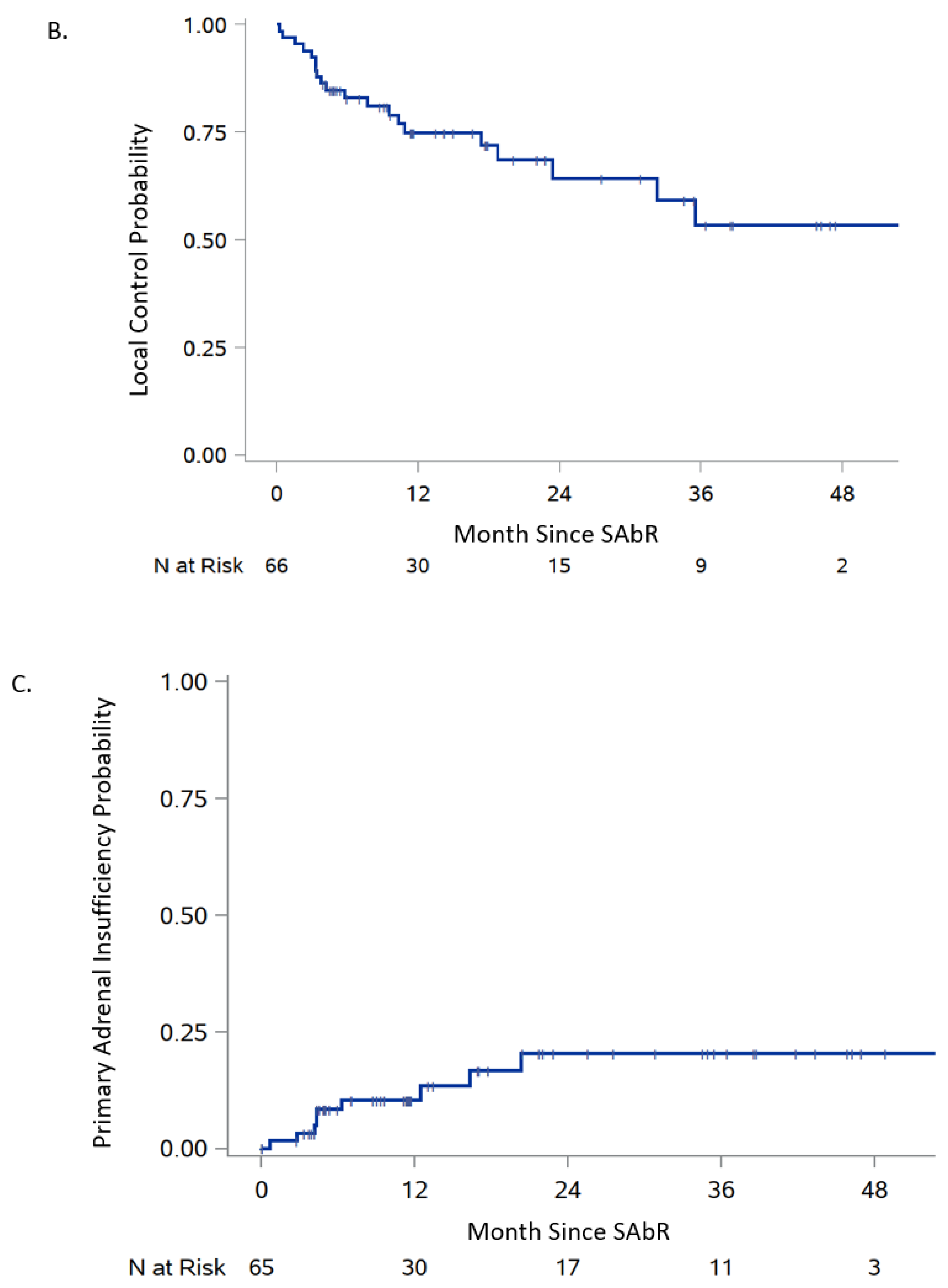
| Progression-free survival | 82.9% (71.2, 90.2) | 74.8% (61.5, 84.1) | 80.0 (23.4-NR) |
| Adrenal insufficiency failure | 8.4% (3.6, 19.1) | 10.4% (4.8, 21.7) | Not reached |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidi, O.; Miljanic, M.; Tumyan, G.; Christie, A.; Mirfakhraee, S.; Ali, S.; Dohopolski, M.; Gottumukkala, S.; Brugarolas, J.; Timmerman, R.; et al. Adrenal Insufficiency following Stereotactic Ablative Radiotherapy (SAbR) of Adrenal Gland Metastases. Cancers 2024, 16, 3140. https://doi.org/10.3390/cancers16183140
Hamidi O, Miljanic M, Tumyan G, Christie A, Mirfakhraee S, Ali S, Dohopolski M, Gottumukkala S, Brugarolas J, Timmerman R, et al. Adrenal Insufficiency following Stereotactic Ablative Radiotherapy (SAbR) of Adrenal Gland Metastases. Cancers. 2024; 16(18):3140. https://doi.org/10.3390/cancers16183140
Chicago/Turabian StyleHamidi, Oksana, Mihailo Miljanic, Gayane Tumyan, Alana Christie, Sasan Mirfakhraee, Sadia Ali, Michael Dohopolski, Sujana Gottumukkala, James Brugarolas, Robert Timmerman, and et al. 2024. "Adrenal Insufficiency following Stereotactic Ablative Radiotherapy (SAbR) of Adrenal Gland Metastases" Cancers 16, no. 18: 3140. https://doi.org/10.3390/cancers16183140
APA StyleHamidi, O., Miljanic, M., Tumyan, G., Christie, A., Mirfakhraee, S., Ali, S., Dohopolski, M., Gottumukkala, S., Brugarolas, J., Timmerman, R., & Hannan, R. (2024). Adrenal Insufficiency following Stereotactic Ablative Radiotherapy (SAbR) of Adrenal Gland Metastases. Cancers, 16(18), 3140. https://doi.org/10.3390/cancers16183140






