Prognostic and Predictive Roles of HER2 Status in Non-Breast and Non-Gastroesophageal Carcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. HER2 Role in Different Types of Epithelial Tumors
2.1. Salivary Gland Carcinoma
2.1.1. Epidemiology and Frequency of HER2 Alterations
2.1.2. HER2 Evaluation Criteria
2.1.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.1.4. Predictive Role of HER2 Alterations and Clinical Trials
2.2. Head and Neck Carcinoma
2.2.1. Epidemiology and Frequency of HER2 Alterations
2.2.2. HER2 Evaluation Criteria
2.2.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.2.4. Predictive Role of HER2 Alterations and Clinical Trials
2.3. Lung Cancer
2.3.1. Epidemiology and Frequency of HER2 Alterations
2.3.2. HER2 Evaluation Criteria
2.3.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.3.4. Predictive Role of HER2 Alterations and Clinical Trials
2.4. Biliary Tract Cancer
2.4.1. Epidemiology and Frequency of HER2 Alterations
2.4.2. HER2 Evaluation Criteria
2.4.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.4.4. Predictive Role of HER2 Alterations and Clinical Trials
2.5. Colorectal Cancer
2.5.1. Epidemiology and Frequency of HER2 Alterations
2.5.2. HER2 Evaluation Criteria
2.5.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.5.4. Predictive Role of HER2 Alterations and Clinical Trials
2.6. Bladder Cancer
2.6.1. Epidemiology and Frequency of HER2 Alterations
2.6.2. HER2 Evaluation Criteria
2.6.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.6.4. Predictive Role of HER2 Alterations and Clinical Trials
2.7. Prostate Cancer
2.7.1. Epidemiology and Frequency of HER2 Alterations
2.7.2. HER2 Evaluation Criteria
2.7.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.7.4. Predictive Role of HER2 Alterations and Clinical Trials
2.8. Gynecologic Cancer
2.8.1. Epidemiology and Frequency of HER2 Alterations
2.8.2. HER2 Evaluation Criteria
2.8.3. Prognostic Role of HER2 Alterations and Association with Clinicopathologic Features
2.8.4. Predictive Role of HER2 Alterations and Clinical Trials
2.9. Other Malignancies
2.9.1. Thyroid Cancer
2.9.2. Renal Cell Carcinoma
2.9.3. Pancreatic Ductal Adenocarcinoma
2.9.4. Hepatocellular Carcinoma
2.9.5. Small Bowel Adenocarcinoma
2.9.6. Anal Cancer
2.9.7. Non-Melanoma Skin Cancers
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; McShane, L.M.; Dowsett, M. HER2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Summary. J. Oncol. Pract. 2018, 14, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; Benson, A.B., 3rd; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. Her2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar] [CrossRef] [PubMed]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, N.; Tsurutani, J.; Kawakami, H.; Yonesaka, K.; Kato, R.; Haratani, K.; Hayashi, H.; Takeda, M.; Nonagase, Y.; Maenishi, O.; et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int. J. Cancer 2019, 145, 3414–3424. [Google Scholar] [CrossRef]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Global Cancer Observatory (GLOBOCAN). Available online: https://gco.iarc.who.int/today/en/fact-sheets-cancers (accessed on 23 May 2024).
- Gargano, S.M.; Senarathne, W.; Feldman, R.; Florento, E.; Stafford, P.; Swensen, J.; Vranic, S.; Gatalica, Z. Novel therapeutic targets in salivary duct carcinoma uncovered by comprehensive molecular profiling. Cancer Med. 2019, 8, 7322–7329. [Google Scholar] [CrossRef]
- Egebjerg, K.; Dupont Harwood, C.; Woller, N.C.; Kristensen, C.A.; Mau-Sørensen, M. HER2 Positivity in Histological Subtypes of Salivary Gland Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 693394. [Google Scholar] [CrossRef]
- Dalin, M.G.; Desrichard, A.; Katabi, N.; Makarov, V.; Walsh, L.A.; Lee, K.W.; Wang, Q.; Armenia, J.; West, L.; Dogan, S.; et al. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin. Cancer Res. 2016, 22, 4623–4633. [Google Scholar] [CrossRef]
- Cavalieri, S.; Nuzzolese, I.; Ottini, A.; Bergamini, C.; Resteghini, C.; Colombo, E.; Alfieri, S.; Quattrone, P.; Calareso, G.; Iacovelli, N.A.; et al. HER2 status in recurrent/metastatic androgen receptor overexpressing salivary gland carcinoma patients. Front. Oncol. 2023, 12, 1096068. [Google Scholar] [CrossRef]
- Haddad, R.; Colevas, A.D.; Krane, J.F.; Cooper, D.; Glisson, B.; Amrein, P.C.; Weeks, L.; Costello, R.; Posner, M. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003, 39, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Rinaldi, G.; Bossi, P.; Dagrada, G.P.; Quattrone, P.; Cantú, G.; Licitra, L. Herceptin plus chemotherapy in relapsed and/or metastatic salivary gland cancer. Oral Oncol. 2005, 41, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Limaye, S.A.; Posner, M.R.; Krane, J.F.; Fonfria, M.; Lorch, J.H.; Dillon, D.A.; Shreenivas, A.V.; Tishler, R.B.; Haddad, R.I. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist 2013, 18, 294–300. [Google Scholar] [CrossRef]
- Perissinotti, A.J.; Lee Pierce, M.; Pace, M.B.; El-Naggar, A.; Kies, M.S.; Kupferman, M. The role of trastuzumab in the management of salivary ductal carcinomas. Anticancer Res. 2013, 33, 2587–2591. [Google Scholar]
- De Block, K.; Vander Poorten, V.; Dormaar, T.; Nuyts, S.; Hauben, E.; Floris, G.; Deroose, C.M.; Schöffski, P.; Clement, P.M. Metastatic HER-2-positive salivary gland carcinoma treated with trastuzumab and a taxane: A series of six patients. Acta Clin. Belg. 2016, 71, 383–388. [Google Scholar] [CrossRef]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef]
- Kurzrock, R.; Bowles, D.W.; Kang, H.; Meric-Bernstam, F.; Hainsworth, J.; Spigel, D.R.; Bose, R.; Burris, H.; Sweeney, C.J.; Beattie, M.S.; et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: Results from MyPathway, a phase IIa multiple basket study. Ann. Oncol. 2020, 31, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Wang, X.V.; Makker, V.; Luoh, S.W.; Mitchell, E.P.; Zwiebel, J.A.; Sharon, E.; Gray, R.J.; Li, S.; McShane, L.M.; et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: Results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann. Oncol. 2019, 30, 1821–1830. [Google Scholar] [CrossRef]
- Kawakita, D.; Nagao, T.; Takahashi, H.; Kano, S.; Honma, Y.; Hirai, H.; Saigusa, N.; Akazawa, K.; Tani, K.; Ojiri, H.; et al. Survival benefit of HER2-targeted or androgen deprivation therapy in salivary duct carcinoma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221119538. [Google Scholar] [CrossRef]
- Sousa, L.G.; Wang, K.; Torman, D.; Binks, B.J.; Rubin, M.L.; Andersen, C.R.; Lewis, W.E.; Rivera, M.J.; Kaya, D.; El-Naggar, A.K.; et al. Treatment patterns and outcomes of palliative systemic therapy in patients with salivary duct carcinoma and adenocarcinoma, not otherwise specified. Cancer 2022, 128, 509–518. [Google Scholar] [CrossRef]
- Uijen, M.J.M.; Lassche, G.; van Engen-van Grunsven, A.C.H.; Driessen, C.M.L.; van Herpen, C.M.L. Case series of docetaxel, trastuzumab, and pertuzumab (DTP) as first line anti-HER2 therapy and ado-trastuzumab emtansine (T-DM1) as second line for recurrent or metastatic HER2-positive salivary duct carcinoma. Oral Oncol. 2022, 125, 105703. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.; Jung, H.A.; Lee, S.H.; Seo, S.; Kim, S.B.; Kim, J.W.; Lee, K.W.; Kang, E.J.; Kim, J.W.; et al. A phase 2 multicenter study of docetaxel-PM and trastuzumab-pkrb combination therapy in recurrent or metastatic salivary gland carcinomas. Cancer 2023, 129, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Pagani, R.; Tober, N.; Lorini, L.; Riefolo, M.; Molinari, G.; Burato, A.; Alfieri, S.; Bossi, P.; Presutti, L. HER2-targeted therapies for salivary gland cancers. Oral Oncol. 2024, 148, 106612. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Peters, S.; Lepage, B.; Cortot, A.B.; Barlesi, F.; Beau-Faller, M.; Besse, B.; Blons, H.; Mansuet-Lupo, A.; Urban, T.; et al. Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 2013, 31, 1997–2003. [Google Scholar] [CrossRef]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef]
- Besse, B.; Soria, J.; Yao, B.; Kris, M.; Chao, B.; Cortot, A.; Mazieres, J.; Socinski, M.A.; Horn, L.; Waqar, S.; et al. Neratinib (N) with our without temsirolimus (TEM) in patients (pts) with non-small cell lung cancer (NSCLC) carrying HER2 somatic mutations: An international randomized phase II study. Ann. Oncol. 2014, 25, v1. [Google Scholar] [CrossRef]
- Kris, M.G.; Camidge, D.R.; Giaccone, G.; Hida, T.; Li, B.T.; O’Connell, J.; Taylor, I.; Zhang, H.; Arcila, M.E.; Goldberg, Z.; et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann. Oncol. 2015, 26, 1421–1427. [Google Scholar] [CrossRef]
- Peters, S.; Curioni-Fontecedro, A.; Nechushtan, H.; Shih, J.Y.; Liao, W.Y.; Gautschi, O.; Spataro, V.; Unk, M.; Yang, J.C.; Lorence, R.M.; et al. Activity of Afatinib in Heavily Pretreated Patients with ERBB2 Mutation-Positive Advanced NSCLC: Findings from a Global Named Patient Use Program. J. Thorac. Oncol. 2018, 13, 1897–1905. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Wang, Q.; Gao, G.; Zhang, Y.; Chen, J.; Shu, Y.; Hu, Y.; Fan, Y.; Fang, J.; et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm. Phase II Study. J. Clin. Oncol. 2020, 38, 2753–2761. [Google Scholar] [CrossRef]
- Song, Z.; Li, Y.; Chen, S.; Ying, S.; Xu, S.; Huang, J.; Wu, D.; Lv, D.; Bei, T.; Liu, S.; et al. Efficacy and safety of pyrotinib in advanced lung adenocarcinoma with HER2 mutations: A multicenter, single-arm, phase II trial. BMC Med. 2022, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, H.; Yang, Y.; Zhang, S.; Xu, F.; Hao, X.; Li, J.; Xing, P.; Hu, X.; Liu, Y.; et al. Pyrotinib combined with apatinib for targeting metastatic non-small cell lung cancer with HER2 alterations: A prospective, open-label, single-arm phase 2 study (PATHER2). BMC Med. 2022, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Cornelissen, R.; Yang, Y.; Zhang, S.; Xu, F.; Hao, X.; Li, J.; Xing, P.; Hu, X.; Liu, Y.; et al. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial. J. Clin. Oncol. 2022, 40, 710–718. [Google Scholar] [CrossRef]
- Elamin, Y.Y.; Robichaux, J.P.; Carter, B.W.; Altan, M.; Gibbons, D.L.; Fossella, F.V.; Lam, V.K.; Patel, A.B.; Negrao, M.V.; Le, X.; et al. Poziotinib for Patients with HER2 Exon 20 Mutant Non–Small-Cell Lung Cancer: Results from a Phase II Trial. J. Clin. Oncol. 2022, 40, 702–709. [Google Scholar] [CrossRef]
- Sun, S.; Prelaj, A.; Baik, C.; Le, X.; Garassino, M.; Wollner, M.; Haura, E.; Piotrowska, Z.; Socinski, M.; Dreiling, L.; et al. 26MO-Efficacy and safety of poziotinib in treatment-naïve HER2 exon 20 insertion (ex20ins) mutated non-small cell lung cancer (NSCLC): ZENITH20-4. Ann. Oncol. 2022, 33, S13–S23. [Google Scholar] [CrossRef]
- Mazières, J.; Barlesi, F.; Filleron, T.; Besse, B.; Monnet, I.; Beau-Faller, M.; Peters, S.; Dansin, E.; Früh, M.; Pless, M.; et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann. Oncol. 2016, 27, 281–286. [Google Scholar] [CrossRef]
- Li, B.T.; Michelini, F.; Misale, S.; Cocco, E.; Baldino, L.; Cai, Y.; Shifman, S.; Tu, H.Y.; Myers, M.L.; Xu, C.; et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020, 10, 674–687. [Google Scholar] [CrossRef]
- Iwama, E.; Zenke, Y.; Sugawara, S.; Daga, H.; Morise, M.; Yanagitani, N.; Sakamoto, T.; Murakami, H.; Kishimoto, J.; Matsumoto, S.; et al. Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur. J. Cancer 2022, 162, 99–106. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.W.; Planchard, D.; Ahn, M.J.; Smit, E.F.; de Langen, A.J.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients with HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results from the Randomized, Phase II DESTINY-Lung02 Trial. J. Clin. Oncol. 2023, 41, 4852–4863. [Google Scholar] [CrossRef]
- Mazieres, J.; Lafitte, C.; Ricordel, C.; Greillier, L.; Negre, E.; Zalcman, G.; Domblides, C.; Madelaine, J.; Bennouna, J.; Mascaux, C.; et al. Combination of Trastuzumab, Pertuzumab, and Docetaxel in Patients with Advanced Non–Small-Cell Lung Cancer Harboring HER2 Mutations: Results From the IFCT-1703 R2D2 Trial. J. Clin. Oncol. 2022, 40, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Guisier, F.; Dubos-Arvis, C.; Viñas, F.; Doubre, H.; Ricordel, C.; Ropert, S.; Janicot, H.; Bernardi, M.; Fournel, P.; Lamy, R.; et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients with Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J. Thorac. Oncol. 2020, 15, 628–636. [Google Scholar] [CrossRef]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, Y.; Liu, R.; Li, W.; Xu, H.; Hao, X.; Li, J.; Xing, P.; Zhang, S.; Ai, X.; et al. First-line immunotherapy or angiogenesis inhibitor plus chemotherapy for HER2 -altered NSCLC: A retrospective real-world POLISH study. Ther. Adv. Med. Oncol. 2022, 14, 175883592210823. [Google Scholar] [CrossRef]
- Song, Z.; Lv, D.; Chen, S.Q.; Huang, J.; Li, Y.; Ying, S.; Wu, X.; Hua, F.; Wang, W.; Xu, C.; et al. Pyrotinib in Patients with HER2-Amplified Advanced Non-Small Cell Lung Cancer: A Prospective, Multicenter Single-Arm Trial. Clin. Cancer Res. 2022, 28, 461–467. [Google Scholar] [CrossRef]
- Warren, E.A.K.; Anil, J.; Castro, P.D.; Kemnade, J.; Suzuki, M.; Hegde, M.; Hicks, J.; Yu, W.; Sandulache, V.; Sikora, A.G. Human epidermal growth factor receptor 2 expression in head and neck squamous cell carcinoma: Variation within and across primary tumor sites, and implications for antigen-specific immunotherapy. Head Neck 2021, 43, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Saddawi-Konefka, R.; Schokrpur, S.; Lui, A.J.; Gutkind, J.S. HER2 and HER3 as Therapeutic Targets in Head and Neck Cancer. Cancer J. 2022, 28, 339–345. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Li, H.; Fan, Y. Targeting HER2 alterations in non-small cell lung cancer: Therapeutic breakthrough and challenges. Cancer Treat. Rev. 2023, 114, 102520. [Google Scholar] [CrossRef]
- Barlesi, F.; Blons, H.; Beau-Faller, M.; Rouquette, I.; Ouafik, L.; Mosser, J.; Merlio, J.; Bringuier, P.; Jonveaux, P.; Maréchal, C.; et al. Biomarkers (BM) France: Results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts). J. Clin. Oncol. 2013, 31, 8000. [Google Scholar] [CrossRef]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef]
- Hiraoka, N.; Nitta, H.; Ohba, A.; Yoshida, H.; Morizane, C.; Okusaka, T.; Nara, S.; Esaki, M.; Kishi, Y.; Shimada, K.; et al. Details of human epidermal growth factor receptor 2 status in 454 cases of biliary tract cancer. Hum. Pathol. 2020, 105, 9–19. [Google Scholar] [CrossRef]
- Albrecht, T.; Rausch, M.; Rössler, S.; Albrecht, M.; Braun, J.D.; Geissler, V.; Mehrabi, A.; Vogel, M.N.; Pathil-Warth, A.; Mechtersheimer, G.; et al. HER2 gene (ERBB2) amplification is a rare event in non-liver-fluke associated cholangiocarcinogenesis. BMC Cancer 2019, 19, 1191. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef]
- Pedica, F.; Grassini, G. Pathology and molecular pathology of cholangiocarcinoma. Hepatoma Res. 2021, 7, 71. [Google Scholar] [CrossRef]
- Vivaldi, C.; Fornaro, L.; Ugolini, C.; Niccoli, C.; Musettini, G.; Pecora, I.; Cacciato Insilla, A.; Salani, F.; Pasquini, G.; Catanese, S.; et al. HER2 overexpression as a poor prognostic determinant in resected biliary tract cancer. Oncologist 2020, 25, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, R.; Kim, H.R.; Jo, H.; Kim, H.; Ha, S.Y.; Park, J.O.; Park, Y.S.; Kim, S.T. HER2 Aberrations as a Novel Marker in Advanced Biliary Tract Cancer. Front. Oncol. 2022, 12, 834104. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Harding, J.J.; Piha-Paul, S.A.; Shah, R.H.; Murphy, J.J.; Cleary, J.M.; Shapiro, G.I.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.; et al. Antitumour activity of neratinib in patients with HER2-mutant advanced biliary tract cancers. Nat. Commun. 2023, 14, 630. [Google Scholar] [CrossRef]
- Lee, C.K.; Chon, H.J.; Cheon, J.; Lee, M.A.; Im, H.S.; Jang, J.S.; Kim, M.H.; Park, S.; Kang, B.; Hong, M.; et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: A multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol. Hepatol. 2023, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mizuno, N.; Sunakawa, Y.; Canon, J.L.; Galsky, M.D.; Hamilton, E.; Hayashi, H.; Jerusalem, G.; Kim, S.T.; Lee, K.W.; et al. Tucatinib and Trastuzumab for Previously Treated Human Epidermal Growth Factor Receptor 2-Positive Metastatic Biliary Tract Cancer (SGNTUC-019): A Phase II Basket Study. J. Clin. Oncol. 2023, 41, 5569–5578. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.Y.; Hanna, D.L.; Kang, Y.K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef]
- Harding, J.J.; Fan, J.; Oh, D.Y.; Choi, H.J.; Kim, J.W.; Chang, H.M.; Bao, L.; Sun, H.C.; Macarulla, T.; Xie, F.; et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): A multicentre, single-arm, phase 2b study. Lancet Oncol. 2023, 24, 772–782. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef]
- Clark, J.W.; Niedzwiecki, D.; Hollis, D.; Mayer, R. Phase II trial of 5-fluorouracil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzumab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy [abstract]. Proc. Am. Soc. Clin. Oncol. 2003, 22, 3584. [Google Scholar]
- Ramanathan, R.K.; Hwang, J.J.; Zamboni, W.C.; Sinicrope, F.A.; Safran, H.; Wong, M.K.; Earle, M.; Brufsky, A.; Evans, T.; Troetschel, M.; et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Investig. 2004, 22, 858–865. [Google Scholar] [CrossRef]
- Hagemann, I.S.; Bridge, J.A.; Tafe, L.J.; Hameed, M.R.; Moncur, J.T.; Bellizzi, A.M.; Dolan, M.; Vasalos, P.; Kane, M.E.; Souers, R.J.; et al. Current laboratory testing practices for assessment of ERBB2/HER2 in endometrial serous carcinoma and colorectal carcinoma. Arch. Pathol. Lab. Med. 2023, 147, 1148–1157. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, 000911. [Google Scholar] [CrossRef]
- Gupta, R.; Meric-Bernstam, F.; Rothe, M.; Garrett-Mayer, E.; Mangat, P.K.; D’Andre, S.; Ahn, E.R.; O’Lone, R.; Halabi, S.; Grantham, G.N.; et al. Pertuzumab Plus Trastuzumab in Patients with Colorectal Cancer With ERBB2 Amplification or ERBB2/3 Mutations: Results from the TAPUR Study. JCO Precis. Oncol. 2022, 6, 2200306. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Hasegawa, H.; Kato, K.; Iwasa, S.; Esaki, T.; Komatsu, Y.; Masuishi, T.; Nishina, T.; et al. TRIUMPH: Primary efficacy of a phase II trial of trastuzumab (T) and pertuzumab (P) in patients (pts) with metastatic colorectal cancer (mCRC) with HER2 (ERBB2) amplification (amp) in tumour tissue or circulating tumour DNA (ctDNA): A GOZILA sub-study. Ann. Oncol. 2019, 30, v199–v200. [Google Scholar] [CrossRef]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef]
- Chang, J.; Xu, M.; Wang, C.; Huang, D.; Zhang, Z.; Chen, Z.; Zhu, X.; Li, W. Dual HER2 Targeted Therapy with Pyrotinib and Trastuzumab in Refractory HER2 Positive Metastatic Colorectal Cancer: A Result from HER2-FUSCC-G Study. Clin. Color. Cancer 2022, 21, 347–353. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Vijayvergia, N.; Xiu, J.; Scicchitano, A.; Lim, B.; Yee, N.S.; Harvey, H.A.; Gatalica, Z.; Reddy, S. Molecular profiling of 6,892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol. Ther. 2015, 16, 1726–1737. [Google Scholar] [CrossRef]
- Loree, J.M.; Bailey, A.M.; Johnson, A.M.; Yu, Y.; Wu, W.; Bristow, C.A.; Davis, J.S.; Shaw, K.R.; Broaddus, R.; Banks, K.C.; et al. Molecular landscape of ERBB2/ERBB3 mutated colorectal cancer. J. Natl. Cancer Inst. 2018, 110, 1409–1417. [Google Scholar] [CrossRef]
- Ivanova, M.; Venetis, K.; Guerini-Rocco, E.; Bottiglieri, L.; Mastropasqua, M.G.; Garrone, O.; Fusco, N.; Ghidini, M. HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes. Life 2022, 12, 1403. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Liu, F.; Ren, C.; Jin, Y.; Xi, S.; He, C.; Wang, F.; Wang, Z.; Xu, R.H.; Wang, F. Assessment of two different HER2 scoring systems and clinical relevance for colorectal cancer. Virchows Arch. 2020, 476, 391–398. [Google Scholar] [CrossRef]
- Lang-Schwarz, C.; Vieth, M.; Dregelies, T.; Sterlacci, W. Frequency of Her2-low in colorectal cancer and its relations with the tumor microenvironment. Pathol. Res. Pract. 2023, 244, 154417. [Google Scholar] [CrossRef] [PubMed]
- Angerilli, V.; Parente, P.; Campora, M.; Ugolini, C.; Battista, S.; Cassoni, P.; Gambella, A.; Cavallin, F.; De Lisi, G.; Vanoli, A.; et al. HER2-low in gastro-oesophageal adenocarcinoma: A real-world pathological perspective. J. Clin. Pathol. 2023, 76, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.N.; Kwak, Y.; Kim, D.W.; Kang, S.B.; Choe, G.; Kim, W.H.; Lee, H.S. HER2 status in colorectal cancer: Its clinical significance and the relationship between HER2 gene amplification and expression. PLoS ONE 2014, 9, 98528. [Google Scholar] [CrossRef] [PubMed]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Corà, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef]
- Scherrer, E.; Kang, A.; Bloudek, L.M.; Koshkin, V.S. HER2 expression in urothelial carcinoma, a systematic literature review. Front. Oncol. 2022, 12, 1011885. [Google Scholar] [CrossRef]
- Galsky, M.D.; Von Hoff, D.D.; Neubauer, M.; Anderson, T.; Fleming, M.; Nagarwala, Y.; Mahoney, J.M.; Midwinter, D.; Vocila, L.; Zaks, T.Z. Target-specific, histology-independent, randomized discontinuation study of lapatinib in patients with HER2-amplified solid tumors. Investig. New Drugs 2012, 30, 695–701. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Bisagni, A.; Zizzo, M.; Ascani, S.; Pedicillo, M.C.; Cormio, A.; Falagario, U.G.; Carrieri, G.; et al. HER2 Expression in Bladder Cancer: A Focused View on Its Diagnostic, Prognostic, and Predictive Role. Int. J. Mol. Sci. 2023, 24, 3720. [Google Scholar] [CrossRef]
- Garczyk, S.; Ortiz-Brüchle, N.; Schneider, U.; Lurje, I.; Guricova, K.; Gaisa, N.T.; Lorsy, E.; Lindemann-Docter, K.; Heidenreich, A.; Knüchel, R. Next-Generation Sequencing Reveals Potential Predictive Biomarkers and Targets of Therapy for Urothelial Carcinoma in Situ of the Urinary Bladder. Am. J. Pathol. 2020, 190, 323–332. [Google Scholar] [CrossRef]
- Kiss, B.; Wyatt, A.W.; Douglas, J.; Skuginna, V.; Mo, F.; Anderson, S.; Rotzer, D.; Fleischmann, A.; Genitsch, V.; Hayashi, T.; et al. Her2 alterations in muscle-invasive bladder cancer: Patient selection beyond protein expression for targeted therapy. Sci. Rep. 2017, 7, 42713. [Google Scholar] [CrossRef]
- Behzatoğlu, K.; Yörükoğlu, K.; Demir, H.; Bal, N. Human Epidermal Growth Factor Receptor 2 Overexpression in Micropapillary and Other Variants of Urothelial Carcinoma. Eur. Urol. Focus 2018, 4, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.; Gao, Y.; Liu, K.; Xu, B.; Qin, W. The Clinical Significance and Prognostic Value of HER2 Expression in Bladder Cancer: A Meta-Analysis and a Bioinformatic Analysis. Front. Oncol. 2021, 11, 653491. [Google Scholar] [CrossRef] [PubMed]
- Helal, D.S.; Darwish, S.A.; Awad, R.A.; Ali, D.A.; El-Guindy, D.M. Immunohistochemical based molecular subtypes of muscle-invasive bladder cancer: Association with HER2 and EGFR alterations, neoadjuvant chemotherapy response and survival. Diagn. Pathol. 2023, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, W.; Zhang, Z.; Song, R.; Zeng, S.; Sun, Y.; Xu, C. Prognostic role of HER2 expression in bladder cancer: A systematic review and meta-analysis. Int. Urol. Nephrol. 2015, 47, 87–94. [Google Scholar] [CrossRef]
- Hussain, M.H.; MacVicar, G.R.; Petrylak, D.P.; Dunn, R.L.; Vaishampayan, U.; Lara, P.N., Jr.; Chatta, G.S.; Nanus, D.M.; Glode, L.M.; Trump, D.L.; et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: Results of a multicenter phase II National Cancer Institute trial. J. Clin. Oncol. 2008, 26, 3295. [Google Scholar] [CrossRef] [PubMed]
- Wülfing, C.; Machiels, J.P.; Richel, D.J.; Grimm, M.O.; Treiber, U.; De Groot, M.R.; Beuzeboc, P.; Parikh, R.; Pétavy, F.; El-Hariry, I.A. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 2009, 115, 2881–2890. [Google Scholar] [CrossRef]
- Oudard, S.; Culine, S.; Vano, Y.; Goldwasser, F.; Théodore, C.; Nguyen, T.; Voog, E.; Banu, E.; Vieillefond, A.; Priou, F.; et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur. J. Cancer 2015, 51, 45–54. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Campanile, A.; Antic, T.; Yap, K.L.; Fitzpatrick, C.A.; Wade, J.L., 3rd; Karrison, T.; Stadler, W.M.; Nakamura, Y.; O’Donnell, P.H. Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients with ERBB Alterations. J. Clin. Oncol. 2017, 35, 478. [Google Scholar] [CrossRef]
- Powles, T.; Huddart, R.A.; Elliott, T.; Sarker, S.J.; Ackerman, C.; Jones, R.; Hussain, S.; Crabb, S.; Jagdev, S.; Chester, J.; et al. Phase III, Double-Blind, Randomized Trial That Compared Maintenance Lapatinib Versus Placebo After First-Line Chemotherapy in Patients with Human Epidermal Growth Factor Receptor 1/2-Positive Metastatic Bladder Cancer. J. Clin. Oncol. 2017, 35, 48–55. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Hu, C.; Pham, H.T.; Dahl, D.M.; Lee-Wu, C.; Swanson, G.P.; Vuky, J.; Lee, R.J.; Souhami, L.; Chang, B.; et al. A Phase 1/2 Trial of a Combination of Paclitaxel and Trastuzumab with Daily Irradiation or Paclitaxel Alone with Daily Irradiation after Transurethral Surgery for Noncystectomy Candidates with Muscle-Invasive Bladder Cancer (Trial NRG Oncology RTOG 0524). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 995–1001. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results from MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2019, 37, 360. [Google Scholar] [CrossRef] [PubMed]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Gong, J.; Zhang, X.; Peng, Z.; Sheng, X.; Mao, C.; Fan, Q.; Bai, Y.; Ba, Y.; et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer 2021, 24, 913–925. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.G.E.; Rüschoff, J.; Lolkema, M.; Tabernero, J.; Gianni, L.; Voest, E.; de Groot, D.J.A.; Castellano, D.; Erb, G.; Naab, J.; et al. Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med. 2023, 12, 12071–12083. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients with Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J. Clin. Oncol. 2024, 42, 1391–1402. [Google Scholar] [CrossRef]
- Font, A.; Mellado, B.; Climent, M.A.; Virizuela, J.A.; Oudard, S.; Puente, J.; Castellano, D.; González-Del-Alba, A.; Pinto, A.; Morales-Barrera, R.; et al. Phase II trial of afatinib in patients with advanced urothelial carcinoma with genetic alterations in ERBB1-3 (LUX-Bladder 1). Br. J. Cancer 2024, 130, 434–441. [Google Scholar] [CrossRef]
- Qu, M.; Zhou, L.; Yan, X.; Li, S.; Wu, X.; Xu, H.; Li, J.; Guo, J.; Zhang, X.; Li, H.; et al. Advances in HER2-Targeted Treatment for Advanced/Metastatic Urothelial Carcinoma. Bladder 2023, 10, 21200012. [Google Scholar] [CrossRef]
- Craft, N.; Shostak, Y.; Carey, M.; Sawyers, C.L. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 1999, 5, 280–285. [Google Scholar] [CrossRef]
- Lara, P.N., Jr.; Chee, K.G.; Longmate, J.; Ruel, C.; Meyers, F.J.; Gray, C.R.; Edwards, R.G.; Gumerlock, P.H.; Twardowski, P.; Doroshow, J.H.; et al. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: Final results from the California Cancer Consortium Screening and Phase II Trial. Cancer 2004, 100, 2125–2131. [Google Scholar] [CrossRef]
- Ziada, A.; Barqawi, A.; Glode, L.M.; Varella-Garcia, M.; Crighton, F.; Majeski, S.; Rosenblum, M.; Kane, M.; Chen, L.; Crawford, E.D. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate 2004, 60, 332–337. [Google Scholar] [CrossRef]
- Leslie, K.K.; Sill, M.W.; Lankes, H.A.; Fischer, E.G.; Godwin, A.K.; Gray, H.; Schilder, R.J.; Walker, J.L.; Tewari, K.; Hanjani, P.; et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol. Oncol. 2012, 127, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, Z.S.; Zeng, Z. Clinical efficacy and safety of combination of abraxane and trastuzumab in treatment of recurrent ovarian cancer. Pak. J. Pharm. Sci. 2018, 31, 2831–2834. [Google Scholar] [PubMed]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial.gov. Available online: https://clinicaltrials.gov/study/NCT04482309 (accessed on 24 May 2024).
- Alva, A.S.; Mangat, P.K.; Garrett-Mayer, E.; Halabi, S.; Hansra, D.; Calfa, C.J.; Khalil, M.F.; Ahn, E.R.; Cannon, T.L.; Crilley, P.; et al. Pembrolizumab in Patients with Metastatic Breast Cancer with High Tumor Mutational Burden: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J. Clin. Oncol. 2021, 39, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Mofid, B.; Jalali Nodushan, M.; Rakhsha, A.; Zeinali, L.; Mirzaei, H. Relation between HER-2 gene expression and Gleason score in patients with prostate cancer. Urol. J. 2007, 4, 101–104. [Google Scholar] [CrossRef]
- Kalantari, M.R.; Mahdavi Zafarghandi, R.; Tavakkoli, M.; Kalantari, S.; Aghaee, A.; Mirsani, A.; Soltani, S. Relation between HER-2 gene expression and prognostic prostate cancer parameters in Trans Rectal Ultrasoundguided Biopsies. J. Pat. Saf. Qual. Improv. 2019, 7, 69–74. [Google Scholar]
- Signoretti, S.; Montironi, R.; Manola, J.; Altimari, A.; Tam, C.; Bubley, G.; Balk, S.; Thomas, G.; Kaplan, I.; Hlatky, L.; et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J. Natl. Cancer Inst. 2000, 92, 1918–1925. [Google Scholar] [CrossRef]
- Minner, S.; Jessen, B.; Stiedenroth, L.; Burandt, E.; Köllermann, J.; Mirlacher, M.; Erbersdobler, A.; Eichelberg, C.; Fisch, M.; Brümmendorf, T.H.; et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin. Cancer Res. 2010, 16, 1553–1560. [Google Scholar] [CrossRef]
- Estephan, F.; Lap, C.J.; Banagan, J.; Antonio, M.; Liu, S.; Diao, G.; Rozalen, A.Z.; Rajendran, R.; Krasnow, S.; Subrahmanyam, R.; et al. The prevalence and clinical significance of HER2 expression in prostate adenocarcinoma. Ann. Diagn. Pathol. 2023, 67, 152219. [Google Scholar] [CrossRef]
- Savinainen, K.J.; Saramäki, O.R.; Linja, M.J.; Bratt, O.; Tammela, T.L.; Isola, J.J.; Visakorpi, T. Expression and gene copy number analysis of ERBB2 oncogene in prostate cancer. Am. J. Pathol. 2002, 160, 339–345. [Google Scholar] [CrossRef]
- Morris, M.J.; Reuter, V.E.; Kelly, W.K.; Slovin, S.F.; Kenneson, K.; Verbel, D.; Osman, I.; Scher, H.I. HER-2 profiling and targeting in prostate carcinoma. Cancer 2002, 94, 980–986. [Google Scholar] [CrossRef]
- Jathal, M.K.; Steele, T.M.; Siddiqui, S.; Mooso, B.A.; D’Abronzo, L.S.; Drake, C.M.; Whang, Y.E.; Ghosh, P.M. Dacomitinib, but not lapatinib, suppressed progression in castration-resistant prostate cancer models by preventing HER2 increase. Br. J. Cancer 2019, 121, 237–248. [Google Scholar] [CrossRef]
- Erickson, B.K.; Zeybek, B.; Santin, A.D.; Fader, A.N. Targeting human epidermal growth factor receptor 2 (HER2) in gynecologic malignancies. Curr. Opin. Obs. Gynecol. 2020, 32, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ma, W.; Brown, D.; Da Cruz Paula, A.; Zhou, Q.; Iaosonos, A.; Tessier-Cloutier, B.; Ross, D.S.; Troso-Sandoval, T.; Reis-Filho, J.S.; et al. HER2 Genetic Intratumor Heterogeneity Is Associated with Resistance to Trastuzumab and Trastuzumab Emtansine Therapy in Recurrent High-Grade Endometrial Cancer. Mod. Pathol. 2023, 36, 100299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Finkelman, B.S.; Ettel, M.G.; Velez, M.J.; Turner, B.M.; Hicks, D.G. HER2 evaluation for clinical decision making in human solid tumours: Pearls and pittfalls. Histopathology 2024, 85, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Horeweg, N.; Leon-Castillo, A.; Rutten, T.A.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Powell, M.E.; Singh, N.; Crosbie, E.J.; et al. HER2 Status in High-Risk Endometrial Cancers (PORTEC-3): Relationship with Histotype, Molecular Classification, and Clinical Outcomes. Cancers 2021, 13, 44. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Uterine Neoplasms. Version 1. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 24 May 2024).
- Amisha, F.; Malik, P.; Saluja, P.; Gautam, N.; Harishbhai Patel, T.; Mariam Roy, A.; Singh, S.R.K.; Janarthanam Malapati, S. A Comprehensive Review on the Role of Human Epidermal Growth Factor Receptor 2 (HER2) as a Biomarker in Extra-Mammary and Extra-Gastric Cancers. Onco 2023, 3, 96–124. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Giuffrè, G.; Giovanella, L.; Siracusa, M.; Simone, A.; Branca, G.; Scarfì, R.; Trimarchi, F.; Ieni, A.; et al. HER2 Analysis in Sporadic Thyroid Cancer of Follicular Cell Origin. Int. J. Mol. Sci. 2016, 17, 2040. [Google Scholar] [CrossRef]
- Siraj, A.K.; Beg, S.; Jehan, Z.; Prabhakaran, S.; Al-Sobhi, S.S.; Al-Dawish, M.; Al-Nuaim, A.; Al-Dayel, F.; Sauter, G.; Al-Kuraya, K.S. The role of HER2 overexpression in Middle Eastern papillary thyroid cancer. Trans. Cancer Res. 2017, 6, 366–373. [Google Scholar] [CrossRef]
- Ensinger, C.; Prommegger, R.; Kendler, D.; Gabriel, M.; Spizzo, G.; Mikuz, G.; Kremser, R. Her2/neu expression in poorly-differentiated and anaplastic thyroid carcinomas. Anticancer Res. 2003, 23, 2349–2353. [Google Scholar]
- Sherman, E.J.; Ho, A.L.; Fagin, J.; Haque, S.; Robinson, C.; Ghossein, R.; Chen, H.; Pfister, D. Combination of dabrafenib (DAB) for the treatment of BRAF-mutant thyroid cancer. J. Clin. Oncol. 2018, 36, 6087. [Google Scholar] [CrossRef]
- Naoum, G.E.; Morkos, M.; Kim, B.; Arafat, W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol. Cancer 2018, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Spasova, S.; Petrova, N.; Ghenev, P. HER2 expression in renal cell carcinoma. Trakia J. Sci. 2015, 13, 141–143. [Google Scholar] [CrossRef]
- Zhang, X.H.; Takenaka, I.; Sato, C.; Sakamoto, H. p53 and HER-2 alterations in renal cell carcinoma. Urology 1997, 50, 636–642. [Google Scholar] [CrossRef]
- Latif, Z.; Watters, A.D.; Bartlett, J.M.; Underwood, M.A.; Aitchison, M. Gene amplification and overexpression of HER2 in renal cell carcinoma. BJU Int. 2002, 89, 5–9. [Google Scholar] [CrossRef]
- Seliger, B.; Rongcun, Y.; Atkins, D.; Hammers, S.; Huber, C.; Störkel, S.; Kiessling, R. HER-2/neu is expressed in human renal cell carcinoma at heterogeneous levels independently of tumor grading and staging and can be recognized by HLA-A2.1-restricted cytotoxic T lymphocytes. Int. J. Cancer 2000, 87, 349–359. [Google Scholar] [CrossRef]
- Selli, C.; Amorosi, A.; Vona, G.; Sestini, R.; Travaglini, F.; Bartoletti, R.; Orlando, C. Retrospective evaluation of c-erbB-2 oncogene amplification using competitive PCR in collecting duct carcinoma of the kidney. J. Urol. 1997, 158, 245–247. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Han, J.; Zhen, L.; Zhang, T.; He, X.; Xu, E.; Li, M. HER2 expression in renal cell carcinoma is rare and negatively correlated with that in normal renal tissue. Oncol. Lett. 2012, 4, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Ravaud, A.; Hawkins, R.; Gardner, J.P.; von der Maase, H.; Zantl, N.; Harper, P.; Rolland, F.; Audhuy, B.; Machiels, J.P.; Pétavy, F.; et al. Lapatinib versus hormone therapy in patients with advanced renal cell carcinoma: A randomized phase III clinical trial. J. Clin. Oncol. 2008, 26, 2285–2291. [Google Scholar] [CrossRef]
- Aumayr, K.; Soleiman, A.; Sahora, K.; Schindl, M.; Werba, G.; Schoppmann, S.F.; Birner, P. HER2 gene amplification and protein expression in pancreatic ductal adenocarcinomas. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 146–152. [Google Scholar] [CrossRef]
- Chou, A.; Waddell, N.; Cowley, M.J.; Gill, A.J.; Chang, D.K.; Patch, A.M.; Nones, K.; Wu, J.; Pinese, M.; Johns, A.L.; et al. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Bilici, A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J. Gastroenterol. 2014, 20, 10802–10812. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Gu, J.; Zheng, L. Prognostic role of HER2 amplification based on fluorescence in situ hybridization (FISH) in pancreatic ductal adenocarcinoma (PDAC): A meta-analysis. World J. Surg. Oncol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Han, S.H.; Ryu, K.H.; Kwon, A.Y. The Prognostic Impact of HER2 Genetic and Protein Expression in Pancreatic Carcinoma—HER2 Protein and Gene in Pancreatic Cancer. Diagnostics 2021, 11, 653. [Google Scholar] [CrossRef]
- Harder, J.; Ihorst, G.; Heinemann, V.; Hofheinz, R.; Moehler, M.; Buechler, P.; Kloeppel, G.; Röcken, C.; Bitzer, M.; Boeck, S.; et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br. J. Cancer 2012, 106, 1033–1038. [Google Scholar] [CrossRef]
- Magalhães, D.; Dos Santos, J.; Frutuoso, A.; Mesquita, A. Human Epidermal Growth Factor Receptor 2 (HER2) Expression by Immunohistochemistry and Its Clinical Significance in Hepatocellular Carcinoma: A Single-Center Analysis. Cureus 2023, 15, 34724. [Google Scholar] [CrossRef]
- Xian, Z.H.; Zhang, S.H.; Cong, W.M.; Wu, W.Q.; Wu, M.C. Overexpression/amplification of HER-2/neu is uncommon in hepatocellular carcinoma. J. Clin. Pathol. 2005, 58, 500–503. [Google Scholar] [CrossRef]
- Clinical Trial.gov. Available online: https://clinicaltrials.gov/search?cond=Hepatocellular%20Carcinoma&intr=HER2 (accessed on 24 May 2024).
- Giuffrida, P.; Vanoli, A.; Arpa, G.; Bonometti, A.; Luinetti, O.; Solcia, E.; Corazza, G.R.; Paulli, M.; Di Sabatino, A. Small Bowel Carcinomas Associated with Immune-Mediated Intestinal Disorders: The Current Knowledge. Cancers 2018, 11, 31. [Google Scholar] [CrossRef]
- Adam, L.; San Lucas, F.A.; Fowler, R.; Yu, Y.; Wu, W.; Liu, Y.; Wang, H.; Menter, D.; Tetzlaff, M.T.; Ensor, J., Jr.; et al. DNA Sequencing of Small Bowel Adenocarcinomas Identifies Targetable Recurrent Mutations in the ERBB2 Signaling Pathway. Clin. Cancer Res. 2019, 25, 641–651. [Google Scholar] [CrossRef]
- Laforest, A.; Aparicio, T.; Zaanan, A.; Silva, F.P.; Didelot, A.; Desbeaux, A.; Le Corre, D.; Benhaim, L.; Pallier, K.; Aust, D.; et al. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur. J. Cancer 2014, 50, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Svrcek, M.; Henriques, J.; Afchain, P.; Lièvre, A.; Tougeron, D.; Gagniere, J.; Terrebonne, E.; Piessen, G.; Legoux, J.L.; et al. Panel gene profiling of small bowel adenocarcinoma: Results from the NADEGE prospective cohort. Int. J. Cancer 2021, 48, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.J.; Hong, S.M.; Jung, S.J.; Korean Small Intestinal Cancer Study Group. HER2 protein expression and HER2 gene amplification are infrequent in small intestinal carcinomas. Virchows Arch. 2013, 462, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, A.; Di Sabatino, A.; Furlan, D.; Klersy, C.; Grillo, F.; Fiocca, R.; Mescoli, C.; Rugge, M.; Nesi, G.; Fociani, P.; et al. Small Bowel Carcinomas in Coeliac or Crohn’s Disease: Clinico-pathological, Molecular, and Prognostic Features. A Study from the Small Bowel Cancer Italian Consortium. J. Crohns Colitis 2017, 11, 942–953. [Google Scholar] [CrossRef]
- Braga, V.M.; de Oliveira, M.B.; Netto, C.C.; Ibrahim, R.E.; Peixoto, R.D. Human Epidermal Growth Factor Receptor 2-Positive Duodenal Adenocarcinoma: A Case Report and Review of the Literature. Case Rep. Oncol. 2015, 8, 285–289. [Google Scholar] [CrossRef]
- Hamad, A.; Singhi, A.D.; Bahary, N.; McGrath, K.; Amarin, R.; Zeh, H.J.; Zureikat, A.H. Neoadjuvant Treatment with Trastuzumab and FOLFOX Induces a Complete Pathologic Response in a Metastatic ERBB2 (HER2)-Amplified Duodenal Cancer. J. Natl. Compr. Cancer Netw. 2017, 15, 983–988. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Wei, Y.; An, L.; Su, S.; Xi, C.; Wang, K.; Hong, D.; Shi, Y. A HER2-mutant patient with late-stage duodenal adenocarcinoma benefited from anti-HER2 therapy and PD-1 inhibition: A case report. J. Gastrointest. Oncol. 2021, 12, 1939–1943. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Malley, R.; Wang, H.; Lenz, H.J.; Arguello, D.; El-Deiry, W.S.; Xiu, J.; Gatalica, Z.; Hwang, J.J.; Philip, P.A.; et al. Molecular characterization of squamous cell carcinoma of the anal canal. J. Gastrointest. Oncol. 2021, 12, 2423–2437. [Google Scholar] [CrossRef] [PubMed]
- Krähn, G.; Leiter, U.; Kaskel, P.; Udart, M.; Utikal, J.; Bezold, G.; Peter, R.U. Coexpression patterns of EGFR, HER2, HER3 and HER4 in non-melanoma skin cancer. Eur. J. Cancer 2001, 37, 251–259. [Google Scholar] [CrossRef]
- Masuguchi, S.; Jinnin, M.; Fukushima, S.; Makino, T.; Sakai, K.; Inoue, Y.; Igata, T.; Ihn, H. The expression of HER-2 in extramammary Paget’s disease. Biosci. Trends 2011, 5, 151–155. [Google Scholar] [CrossRef]
- Pérez, J.C.; Salgado, A.C.; Pérez-Mies, B.; Rullán, J.A.D.; Ajuria-Illarramendi, O.; Alia, E.M.G.; Serrano Domingo, J.J. Extramammary Paget Disease: A Therapeutic Challenge, for a Rare Entity. Curr. Oncol. Rep. 2023, 25, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
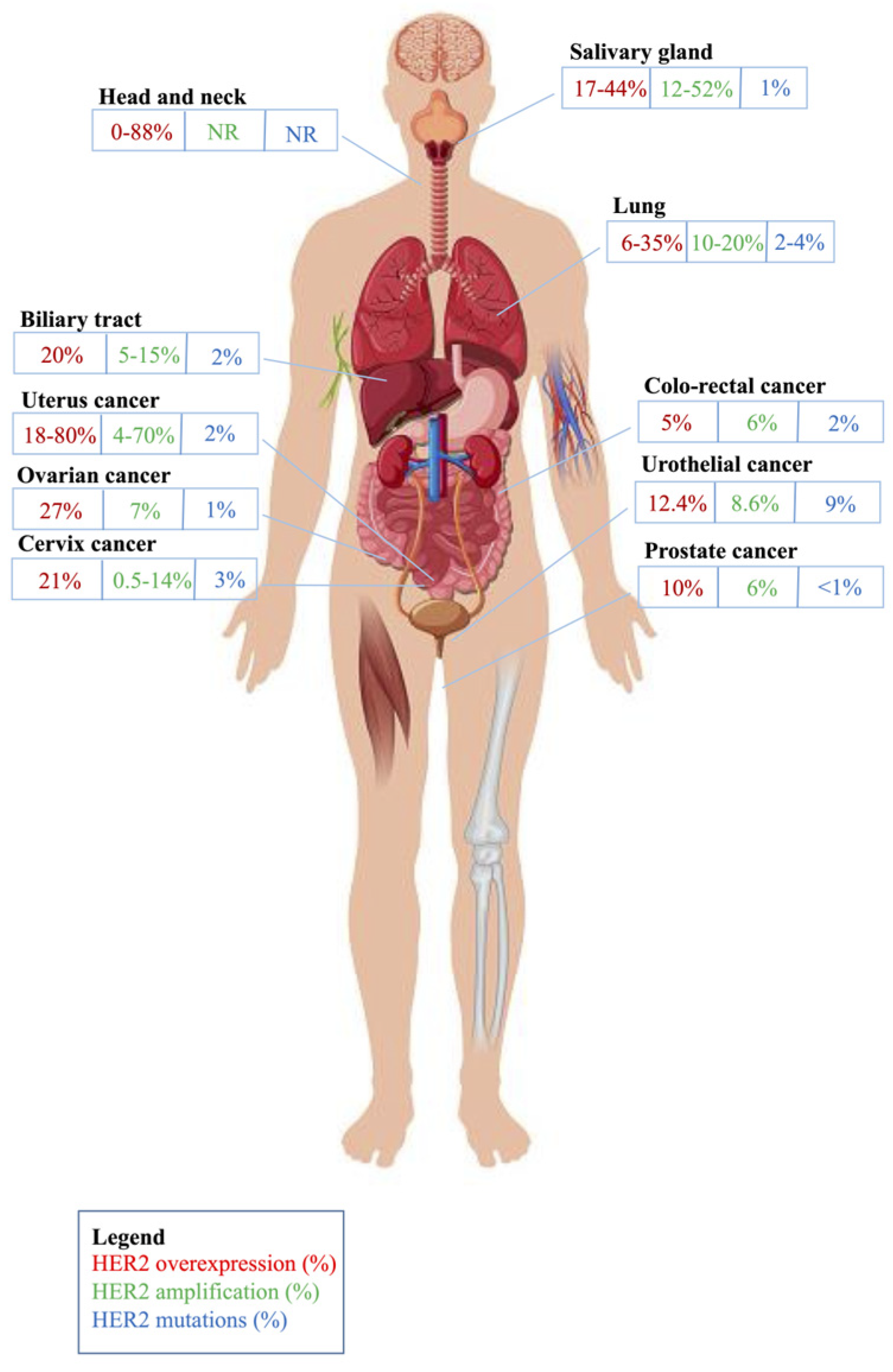
| Type of Tumor | Recommended HER2 IHC Scoring Systems | Interpretation of HER2 IHC | Interpretation of ISH |
|---|---|---|---|
| Salivary gland carcinoma | Breast cancer criteria with Hercept test: score 0: no staining observed or Incomplete membrane staining that is faint or barely perceptible and within ≤10% of the invasive tumor cells score 1+: Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells score 2+: Weak to moderate complete membrane staining observed in >10% of tumor cells score 3+: Circumferential membrane staining that is complete, intense, and in >10% of tumor cells | Score 0 and 1+: negative Score 2+: equivocal, need ISH confirmation Score 3+: positive | Breast cancer criteria: ISH negative:
|
| HNSCC | See salivary gland carcinoma | See salivary gland carcinoma | See salivary gland carcinoma |
| Lung cancer | See salivary gland carcinoma | See salivary gland carcinoma | See salivary gland carcinoma |
| Biliary tract cancer | Gastroesophageal cancer criteria with Hercept test: Score 0: No reactivity or membranous reactivity in <10% of tumor cells Score 1+: Faint or barely perceptible membranous reactivity in ≥10% of tumor cells; cells are reactive only in part of their membrane. Score 2+: Weak to moderate complete, basolateral or lateral membranous reactivity ≥ 10% of tumor cells Score 3+: strong complete, basolateral or lateral membranous staining in ≥10% of tumor cells | Score 0 and 1+: negative Score 2+: equivocal, ISH confirmation is needed Score 3+: positive | Gastroesophageal cancer criteria: ISH positive:
|
| Colorectal cancer | CAP/ASCP/ASCO GEA: Score 0: no reactivity or membranous reactivity in <10% Score 1+: faint/barely perceptible reactivity in ≥10% Score 2+: weak to moderate complete, basolateral, or lateral membranous reactivity in ≥10% but <50% Score 2+: weal to moderate complete, basolateral, or lateral membranous reactivity in ≥50% Score 3+: strong complete, basolateral, or lateral membrane staining in 10–50% Score 3+: strong complete, basolateral, or lateral membrane staining > 50% HERACLES Diagnostic Criteria with Ventana 4B5: Score 0: no staining Score 1+: faint staining, any cellularity, segmental or granular pattern Negative (2+): moderate staining in <50% cells, any pattern Equivocal (2+): moderate staining in ≥50% cells, any pattern with circumferential, basolateral or lateral pattern (IHC mandatory: re-test IHC; if confirmed, proceed with ISH) Negative (3+): intense in ≤10% cells, circumferential, basolateral or lateral pattern Positive (3+): intense in >10% and <50% cells, circumferential, basolateral or lateral pattern (IHC mandatory: re-test IHC; if confirmed, proceed with ISH) Positive (3+): intense in ≥50% cells, circumferential, basolateral, or lateral pattern | CAP/ASCP/ASCO GEA: Score 0 and 1+: negative Score 2+: equivocal, need ISH confirmation HERACLES Diagnostic Criteria with Ventana 4B5: Score 0 and 1+: negative Negative (2+): moderate staining in <50% cells, any pattern Equivocal (2+): moderate staining in ≥50% cells, any pattern with circumferential, basolateral or lateral pattern (IHC mandatory: re-test IHC; if confirmed, proceed with ISH) Negative (3+): intense in ≤10% cells, circumferential, basolateral or lateral pattern Positive (3+): intense in >10% and < 50% cells, circumferential, basolateral or lateral pattern (IHC mandatory: re-test IHC; if confirmed, proceed with ISH) Positive (3+): intense in ≥50% cells, circumferential, basolateral or lateral pattern | CAP/ASCP/ASCO GEA and HERACLES Diagnostic Criteria:
|
| Urothelial cancer | See salivary gland carcinoma and biliary tract cancer | See salivary gland carcinoma and biliary tract cancer | See salivary gland carcinoma and biliary tract cancer |
| Prostate cancer | See salivary gland carcinoma | See salivary gland carcinoma | See salivary gland carcinoma |
| Gynecological cancer | Score 2+: intense complete or basolateral/lateral membrane staining in 30% or fewer tumor cells or weak to moderate staining in greater than or equal to 10% of tumor cells Score 3+: Intense complete or basolateral/lateral membrane staining in over 30% of tumor cells | Score 2+: equivocal, must order reflex test (same specimen using ISH) or order a new test (new specimen if available, using IHC or ISH). Score 3+: positive | ISH negative:
|
| Thyroid cancer | See salivary gland carcinoma | See salivary gland carcinoma | See salivary gland carcinoma |
| RCC | See salivary gland carcinoma | See salivary gland carcinoma | See salivary gland carcinoma |
| PDAC | See biliary tract cancer | See biliary tract cancer | See biliary tract cancer |
| HCC | See biliary tract cancer | See biliary tract cancer | See biliary tract cancer |
| Small bowel adenocarcinoma | See biliary tract cancer | See biliary tract cancer | See biliary tract cancer |
| Anal cancer | See biliary tract cancer | See biliary tract cancer | See biliary tract cancer |
| Non-melanoma skin cancers | NR | NR | NR |
| Type of Tumor | Author, Year [Ref] | Study Design | N° pts | Treatment Line | Type of HER2 Alteration Evaluated | Definition of HER2 Positivity | Drug | Primary Endpoint | Results | Survival Data |
|---|---|---|---|---|---|---|---|---|---|---|
| Salivary gland carcinoma | Haddad R et al., 2003 [12] | Phase I | 14 | First-line | Overexpression | IHC 2+ or 3+ (breast criteria) | Trastuzumab | PFS | 4.2 mo | See primary endpoint |
| Locati LD et al., 2005 [13] | Retrospective | 4 | First- and second-line | Amplification/ Overexpression | IHC 3+ or 2+ confirmed by FISH (breast criteria) | Trastuzumab | Activity | SD 25% | mPFS: 2.5 mo | |
| Limaye SA et al. 2013 [14] | Retrospective | 5 | Adjuvant and first-line | Amplification/ Overexpression | 3+ (strong complete membrane immunoreactivity in >30% of tumor cells) or 2+ (weak to moderate complete membrane immunoreactivity in at least 10% of tumor cells) with a FISH ratio > 2.2 | Paclitaxel, carboplatin, plus trastuzumab | Activity | PR (2), CR (1), PD (2) | mOS: 40 mo | |
| Perissinotti AJ et al., 2013 [15] | Retrospective | 13 | Adjuvant and progressed on previous treatments | Amplification/ Overexpression | IHC 3+ or 2+ (breast criteria) with FISH ratio > 2.0 or an average number of HER2 gene copies/cell of 6 or greater. | Trastuzumab or trastuzumab plus CT | Activity | no response to single-agent; PR (3) with combined treatments | NR | |
| De Block K et al. 2016 [16] | Retrospective | 6 | Progressed on previous treatments | Amplification/ Overexpression | IHC 3+ or 2+ confirmed by FISH (breast criteria) | Trastuzumab plus taxane | Activity | PR (5), SD (1) | mPFS: 10.8 mo | |
| Takahashi H et al., 2019 [17] | Phase II | 57 | Progressed on previous treatments (no antiHER2 agents) | Overexpression/ Amplification | IHC 3+ or gene amplification confirmed by FISH, according to the ASCO/CAP guidelines for breast cancer | Doce plus trastuzumab | ORR | 70.2% | mPFS: 8.9 mo (95% CI, 7.8 to 9.9 months) mOS: 39.7 mo (95% CI, not reached) | |
| Kurzrock R et al., 2019 [18] | Phase II | 15 | Progressed on previous treatments (also anti-HER2 agents) | Amplification/ overexpression and mutation | IHC 3+ according to Breast cancer criteria 2013; or gene amplification with a HER2/CEP17 ratio of >2.0 or HER2 gene copy number > 6.0 by ISH; or HER2 gene copy number gain by NGS or RT-PCR. | Pertuzumab plus trastuzumab | ORR | 63% | mPFS: 8.6 mo mOS: 20.4 mo | |
| Jhaveri KL et al., 2019 [19] | Phase II | 3 SGC: 2 MCPG and 1 SCCPG | Progressed on previous treatments (no antiHER2 agents) | Amplification | ErbB2 gene copy number > 7 by NGS | T-DM1 | ORR | PR 5.6%: 1 MCPG and 1 SCCPG; SD 47% | 6 mo PFS rate: 23.6% [90% CI 14.2% to 39.2%]. | |
| Kawakita et al., 2022 [20] | Retrospective | 111 | Progressed on previous treatments (no antiHER2 agents) | Overexpression/ amplification | IHC 3+ or gene amplification by FISH according to the ASCO/CAP guidelines for breast cancer. | Doce plus trastuzumab | ORR | 72% | mPFS: 9 mo (8–11 months); OS: 38 mo (33–49 months) | |
| Sousa LG et al., 2022 [21] | Retrospective | 17 | First and subsequent line of therapy | Overexpression/ amplification | IHC 3+ or 2+; FISH-positivity based on the breast cancer criteria (ratio > 2.2 or copy number > 6) | Trastuzumab plus CT | ORR | 47% | mPFS: 9.6 mo (95% CI, 4.9–11.6%) | |
| Uijen MJM et al., 2022 [22] | Retrospective | 13 | First and second line | Overexpression/ amplification | IHC 3+ (strong expression in >10%) or 2+ with FISH ratio > 2 (breast criteria) | Doce, trastuzumab, and pertuzumab (1st line); T-DM1 (2nd line) | ORR | 1st line: 58% 2nd line: 57% | 1st line: mPFS: 6.9 mo (95% CI 5.3–8.5); mOS: 42.0 mo (95% CI 13.8–70.1). 2nd line: mPFS of 4.4 mo (95% CI 0–18.8). | |
| Lee J et al., 2022 [23] | Phase II | 43 | Progressed on previous treatments | Overexpression/ amplification | IHC 3+ (strong expression in >10%) or 2+ with FISH ratio > 2 (breast criteria) | Doce plus trastuzumab | ORR | 69.8% | mPFS: 7.9 mo (6.3–9.5) mOS: 23.3 (19.9–26.7) | |
| Meric-Bernstam F et al., 2023 [24] | Phase II | 19 | Progressed on previous treatments (also anti-HER2 agents) | Overexpression/ amplification | IHC ≥ 2+ using current ASCO/CAP guidelines for scoring HER2 in gastric cancer | T-DXd | ORR | 42.1% | mPFS: 12.5 mo | |
| HNSCC | Meric-Bernstam F et al., 2024 [24] | Phase II | 4 | Progressed on previous treatments (also anti-HER2 agents) | Overexpression/ amplification | IHC ≥ 2+ using current ASCO/CAP guidelines for scoring HER2 in gastric cancer | T-DXd | ORR | 50% | NR |
| NSCLC | Mazières et al., 2013 [26] | Retrospective | 65 | First and subsequent line of therapy | Mutations | PCR | CT + Trastuzumab/afatinib/ lapatinib/masatinib | OS | RR 50% DCR 80% | PFS: 5.1 mo OS: 40 mo |
| Hyman DM et al., 2018 [27] | Phase II | 26 | First-line or later line | Mutations | NGS | Neratinib | ORR | ORR 3.8% | mPFS: 5.5 mo | |
| Besse B et al., 2014 [28] | Phase II | 27 | Second-line or later-line | Mutations | NGS | Neratinib +/− temsirolimus | ORR | ORR Neratinib 0% vs. Neratinib + Temsirolimus 21% | mPFS: Neratinib 2.9 mo vs. Neratinib + temsirolimus 4.0 mo | |
| Kris MG et al., 2015 [29] | Phase II | 26 | Second line | Mutations | PCR | Dacomitinib | OS | OR 12% | PFS: 3 mo mOS: 9 mo | |
| Peters S et al., 2018 [30] | Retrospective | 28 | Second line | Mutations | PCR | Afatinib | Activity | TTF 2.9 mo ORR 19% DCR 69% | NR | |
| Zhou C et al., 2022 [31] | Phase II | 60 | Second line | Mutations | NGS | Pyrotinib | ORR | ORR 30% | mPFS: 6.9 mo mOS: 14.4 mo | |
| Song Z et al., 2022 [32] | Phase II | 78 | First-line and later line | Mutations | NGS | Pyrotinib | PFS at 6 months | ORR 19.2%, mDoR 9.9 mo | mPFS: 5.6 mo mOS: 10.5 mo | |
| Yang G et al., 2022 [33] | Phase II | 31/33 | Second-line and later line | Mutations | 32 NGS; 1 PCR | Pyrotinib + apatinib | ORR | ORR 51.5% DCR 93.9% mDoR 6.0 mo | mPFS: 6.9 mo mOS: 14.8 mo | |
| Le X et al., 2022 [34] | Phase II | 90 | Second- line and later line | Mutations | NGS | Poziotinib | ORR | ORR 27.8% DCR 70% mDoR 5.1 mo | mPFS: 5.5 mo | |
| Elamin YY et al., 2022 [35] | Phase II | 30 | First-line and later line | Mutations | NGS | Poziotinib | ORR | ORR: 27% RR (8 weeks) 43% DCR 73% | NR | |
| Sun S et al., 2022 [36] | Phase II | 70 | First-line | Mutations | NGS | Poziotinib | ORR | ORR 41% DCR 73%, mDoR 5.7 mo | mPFS: 5.6 mo | |
| Mazières J et al., 2016 [37] | Retrospective | 58/101 | Second-line and later line | Mutations | PCR/NGS | T-DM1 | Activity | ORR 50.9% DC 75.5% | PFS: 4.8 weeks OS: 13.3 weeks | |
| Li BT et al., 2020 [38] | Phase II | 49 | First-line and later line | Mutations and/or amplifications | NGS and FISH | T-DM1 | ORR | ORR: Mut: 50% Ampl: 55% Mut + Ampl 50%; mDoR 4.4 mo | PFS: 5.0 mo | |
| Iwama E et al., 2021 [39] | Phase II | 22 | Second-line and later line | Mutations | NGS or PCR | T-DM1 | ORR | ORR 38.1% mDoR 3.5 mo | mPFS: 2.8 mo | |
| Li BT et al., 2022 [40] | Phase II | 91 | Second-line | Mutations | NGS | T-DXd | ORR | ORR 55% mDoR 9.3 mo | mPFS: 8.2 mo mOS: 17.8 mo | |
| Li BT et al., 2022 [40] | Phase II | 91 | Second-line | Overexpression | FISH breast | T-DXd | ORR | ORR 24.5% mDoR 6.0 mo | mOS: 11.3 mo mPFS: 5.4 mo | |
| Goto K et al., 2023 [41] | Phase II | 152 | Second-line | Mutations | NGS | T-DXd | ORR | mDoR 16.8 mo vs. NE; DCR 93.1% vs. 92%; ORR 49.0% vs. 56.0% | NR | |
| Mazieres J et al., 2022 [42] | Phase II | 45 | Second-line | Mutations | NGS | Trastuzumab, pertuzumab, doce | ORR | mDoR 11.0 mo ORR 29% | mPFS: 6.8 mo | |
| Mazieres J et al., 2019 [43] | Retrospective | 29/551 | First-line and later line | Mutations | NGS/Other | ICI | Activity | ORR 7% | mPFS: 2.5 mo | |
| Guisier F et al., 2020 [44] | Retrospective | 23/107 | Second-line and later line | Mutations | NGS | ICI | Activity | ORR 27% RR 27.3% DCR 50% mDoR 15.2 | mPFS: 2.2 mo OS: 20.4 mo | |
| Peters S et al., 2019 [45] | Phase II | 49 | Second-line | Overexpression | IHC breast cancer (3+ vs. 2+) | T-DM1 | ORR | ORR HER3+ vs. HER2+: 20% vs. 0% | HER 3+ vs. HER2: mPFS 2.7 vs. 2.6 mo; mOS 15.3 vs. 12.2 mo | |
| Yang G et al., 2022 [46] | Retrospective | 293 | First-line | Mutations or amplification | NGS | CT vs. CT + ICI vs. CT + AI | Activity | CT: ORR 16.9% DCR 89.2% CT + ICI: ORR 28.9% DCR 80.0% CT + AI: ORR 23.8% DCR 91.3% | CT: mPFS: 4.03 mo mOS: 31.67 mo CT + ICI: mPFS: 5.20 mo CT + AI: mPFS: 5.63 mo mOS: 36.27 mo | |
| Song Z et al., 2022 [47] | Phase II | 27 | First-line and later line | Amplified | NGS | Pyrotinib | PFS | ORR 22.2% | mPFS: 6.3 mo mOS: 12.5 mo |
| Type of Tumor | Author, Year [Ref] | Study Design | N° pts | Treatment Line | Type of HER2 Alteration Evaluated | Definition of HER2 Positivity | Drug | Primary Endpoint | Results | Survival Data |
|---|---|---|---|---|---|---|---|---|---|---|
| Biliary tract cancers | Javle et al., 2022 [60] | Phase II | 29 | Subsequent lines | Amplification/ Overexpression | IHC 2+ or 3+ (breast criteria) | Trastuzumab plus pertuzumab | ORR | 23% | mPFS: 4.0 mo mOS: 10.9 mo |
| Harding JJ et al., 2023 [61] | Phase II | 25 | Subsequent lines | Mutation | HER2 gene in NGS (MSK-IMPACT) | Neratinib | ORR | 16% | mPFS: 2.8 mo mOS: 5.4 mo | |
| Lee CK et al., 2013 [62] | Phase II | 34 | First-line | Amplification/ Overexpression | IHC 3+ or IHC 2+ and in situ hybridization positive or ERBB2 gene copy number ≥ 6.0 or using NGS | FOLFOX plus trastuzumab | ORR | 29% | mPFS: 5.1 mo mOS: 10.7 mo | |
| Nakamura Y et al., 2023 [63] | Phase II | 30 | Second- and further lines | Amplification/ overexpression | IHC 3+ or IHC 2+ and in situ hybridization positive or ERBB2 gene copy number ≥ 6.0 or using NGS | Tucatinib plus trastuzumab | ORR | 46% | mPFS: 5.5 mo mOS: 15.5 mo | |
| Meric-Bernstam F et al., 2022 [64] | Phase I | 22 | Subsequent lines | Amplification/ overexpression | IHC 3+ or gene amplification confirmed using FISH, according to the ASCO/CAP guidelines for gastroesophageal cancer | Zanidatamab | ORR | 38% | mPFS: 3.5 mo | |
| Harding JJ et al., 2023 [65] | Phase IIB | 80 | Progression on previous gemcitabine-based therapy | Amplification/ overexpression | IHC 3+ or gene amplification confirmed by FISH, according to the ASCO/CAP guidelines for gastroesophageal cancer | Zanidatamab | ORR | 41% | mPFS: 5.5 mo | |
| Ohba A et al., 2022 [66] | Phase II | 24 | Progression on previous gemcitabine-based therapy | Amplification/ overexpression | HER2-positive: IHC 3+ or gene amplification confirmed using FISH; HER2-low expression [HER2-low]: IHC/ISH status of 0/+, 1+/−, 1+/+, or 2+/− | T-DXd | ORR (HER2-positive) | 36% | mPFS: 4.4 mo mOS: 7.1 mo | |
| Meric-Bernstam F et al., 2024 [24] | Phase II | 41 | Progression on ≥2 systemic treatment | Amplification/ overexpression | HER2-overexpressing tumors with IHC 3+/2+ using current ASCO/CAP guidelines for scoring HER2 in gastric cancer | T-DXd | ORR | 27% | mPFS: 4.1 mo | |
| Colorectal carcinoma | Clark et al., 2003 [67] | Phase II | 21 | Second or third line | Overexpression | IHC 2+ (breast criteria) | Trastuzumab plus FLOX | ORR | 24% | mDOR: 4.5 mo (range 2.7–11 mo) |
| Ramanathan et al., 2004 [68] | Phase II | 9 | First or second line | Overexpression/amplification | IHC 3+ or 2+ (breast criteria) confirmed by FISH | Trastuzumab plus irinotecan | ORR | ORR 71% PR (5) | NR | |
| Sartore-Bianchi et al., 2016 [69] | Phase II | 27 | Refractory/late lines | Overexpression and amplification | HERACLES Diagnostic Criteria | Trastuzumab plus lapatinib | ORR | 30% | mPFS: 5.3 mo mOS: 11.5 mo | |
| Meric-Bernstam et al., 2019 [70] | Phase II | 57 | Late lines | Amplification/ overexpression and mutation | FISH/CISH, IHC and/or NGS through local testing and revaluation | Trastuzumab plus pertuzumab | ORR | ORR 32%, PR (17), CR (1) | mPFS: 2.9 mo estimated mOS: 11.5 mo | |
| Sartore-Bianchi et al., 2020 [71], | Phase II | 31 | Second and third lines | Overexpression/ Amplification | HERACLES Diagnostic Criteria | Pertuzumab and T-DM1 | ORR | 9.7% | mPFS: 4.1 mo | |
| Gupta et al., 2022 [72] | Phase II | 28 | Late lines | Amplification/ overexpression and mutation | NGS | Trastuzumab plus pertuzumab | ORR | 14% | mPFS: 17.2 wks | |
| Yoshino et al., 2019 [73] | Phase II | 19 | Refractory | Amplification on tissue and ctDNA mutations | Evaluation on tissue (IHC and ISH) and in ctDNA using NGS (criteria not otherwise specified) | Trastuzumab plus pertuzumab | ORR | Tissue positive group: ORR 35%, CR (1), PR (5). ctDNA-positive group: ORR 33%, CR (1), PR (4). | mPFS: 4.0 mo | |
| Siena et al., 2021 [74] | Phase II | 78 | Third-line | Overexpression/ amplification | IHC and ISH (criteria not specified) Cohort A—53 (IHC 3+ or IHC2+ ISH-positive) | T-DXd | ORR | ORR 45.3% DCR 83.0% | mPFS 6.3 mo mOS 15.5 mo | |
| Strickler et al., 2023 [75] | Phase II | 117 | Later lines/Refractory | Overexpression/ amplification | IHC 3+, IHC 2+ (breast criteria) and FISH/CISH amplified or amplification by NGS | Tucatinib plus trastuzumab | ORR | ORR 38·1%, CR (3), PR (29) | mPFS: 8.2 mo mOS: 24.1 mo | |
| Chang et al., 2022 [76] | Phase II | 16 | Third line or beyond | Overexpression/ amplification | IHC (HERACLES Diagnostic Criteria), FISH or NGS | Trastuzumab plus pyrotinib | ORR | ORR: 50% all ORR: 57% in RAS wild type | mPFS: 7.53 mo mOS: 16.8 mo |
| Type of Tumor | Author, Year [Ref] | Study Design | N° pts | Treatment Line | Type of HER2 Alteration Evaluated | Definition of HER2 Positivity | Drug | Primary Endpoint | Results | Survival Data |
|---|---|---|---|---|---|---|---|---|---|---|
| Urothelial carcinoma | Hussain MH et al., 2008 [96] | Phase II | 44 | First line | Overexpression, amplification, serum HER-2/neu-ECD level | IHC 2+ or 3+ (breast criteria), serum HER-2/neu-ECD ≥ 16 ng/mL | Trastuzumab, carboplatin, paclitaxel, gemcitabine | Cardiac toxicity | 22.7% | PFS: 9.3 mo (95% CI, 6.7 to 10.2 mo) OS: 14.1 mo (95% CI, 11.5 to 17.1 mo) |
| Wülfing C et al., 2009 [97] | Phase II | 59 | Second line | Overexpression | IHC 2+ or 3+ (breast criteria) | Lapatinib | ORR > 10% | 2% | TTP: 8.6 wk (95% CI, 8.0 wk to 11.3 wk) OS: 17.9 wk (95% CI, 13.1 wk to 30.3 wk) | |
| Galsky MD et al., 2012 [88] | Phase II | 9 | Second line | Amplification | FISH ratio ≥ 2 | Lapatinib | 12 wk-ORR | ORR 0 | NR | |
| Oudard S et al., 2015 [98] | Phase II | 61 | First line | Amplification/ overexpression | IHC 3+ or gene amplification confirmed using FISH (breast criteria) | Platinum, gemcitabine ± trastuzumab | PFS | PFS 8.2 mo vs. 10.2 mo | mOS: 15.7 mo vs. 14.1 mo, (p = 0.684) | |
| Choudhury NJ et al., 2017 [99] | Phase II | 23 | Second and subsequent lines of therapy | Overexpression/amplification/ mutation/copy number alteration | IHC 3+ or 2+ confirmed using FISH (breast criteria)/ Mutation using NGS/ Copy number ≥ 3.5 by NGS | Afatinib | 3 mo PFS | 21% pts met 3 mo-PFS | mOS: 5.3 mo mPFS: 1.4 mo | |
| Powles T et al., 2017 [100] | Phase III | 232 | Second and subsequent lines of therapy | Overexpression/ Amplification | IHC 3+ and 2+ confirmed by FISH (breast criteria) | Lapatinib vs. PBO | PFS | mPFS 4.5 mo (lapatinib) (95% CI, 10.5 mo to 5.4 mo) vs. 5.1 mo (95% CI, 3.0 mo to 5.8 mo) (PBO) | OS 12.6 (95% CI, 9.0 to 16.2) and 12.0 (95% CI, 10.5 to 14.9) | |
| Michaelson MD, 2017 [101] | Phase I/II | 66 | Second and subsequent lines of therapy | Overexpression | IHC 2+ or 3+ (breast criteria) | Paclitaxel, radiotherapy ± trastuzumab | Toxicity | AEs in 35% of trastuzumab-treated pts; 30% in non trastuzumab-treated pts | NR | |
| Hyman DM et al., 2018 [27] | Phase II | 16 | Second and subsequent lines of therapy | Mutation | Mutation by NGS | Neratinib | ORR | ORR 0 | mPFS: 1.8 mo | |
| Hainsworth et al., 2018 [102] | Phase IIa | 9 | Second and subsequent lines of therapy | Overexpression/ Amplification | IHC 3+ or amplification using FISH (breast criteria) Activating mutation by NGS | Trastuzumab, pertuzumab | ORR | ORR 33.3% | NR | |
| Banerji U et al., 2019 [103] | Phase I | 16 | Second and subsequent lines of therapy | Overexpression | IHC 1+, 2+, or 3+ (breast criteria) | Trastuzumab-duocarmazine | Safety and recommended dose | safe profile; recommended dose: 1·2 mg/kg | PFS 3.5 mo | |
| Xu Y et al., 2021 [104] | Phase I | 4 | Second and subsequent lines of therapy | Overexpression | IHC 2+ or 3+ (breast criteria) regardless the presence/absence of amplification by FISH | RC48-ADC | Safety and MTD | Safety profile; MTD NR | NR | |
| De Vries EGE et al., 2023 [105] | Phase II | 13 | Second and subsequent lines of therapy | Overexpression | IHC 3+ in ≥30% tumor cells | T-DM1 | BOR | PR 38.5% | PFS: 2.2 mo OS: 7 mo | |
| Meric-Bernstam F et al., 2024 [24] | Phase II | 22 | Second and subsequent lines of therapy | Overexpression | IHC 2+ or 3+ (breast criteria) | T-DXd | ORR | ORR 39% | PFS: 12.8 mo OS: 7 mo | |
| Sheng X et al., 2024 [106] | Phase II | 107 | Second and subsequent lines of therapy | Overexpression/ amplification | IHC 3+ and 2+ confirmed using FISH (breast criteria) | RC48-ADC | ORR | ORR 50.5% | PFS: 5.9 mo OS: 14.2 mo | |
| Font A et al., 2024 [107] | Phase II | 34 | Second and subsequent lines of therapy | Amplification | Amplification by FISH (breast criteria) | Afatinib | 6 mo-PFS | 6 mo-PFS 12% | OS: 30 wk | |
| Prostate cancer | Morris et al., 2002 [109] | Phase II | 23 | After androgen deprivation therapy ± radiotherapy | Overexpression | IHC 3+ and 2+ (breast criteria) | Trastuzumab ± paclitaxel | Efficacy of trastuzumab monotherapy | ORR 0 | NR |
| Lara PN Jr et al., 2004 [110] | Phase II | 4 | After androgen deprivation therapy | Overexpression/amplification | IHC 3+ and 2+ (breast cancer) confirmed using FISH (HER2 ratio > 2) | Trastuzumab or Doce.; non-responders: trastuzumab/Doce | ORR | ORR 0 | PFS: 7 mo | |
| Ziada A et al., 2004 [111] | Phase II | 18 | After androgen deprivation therapy | Overexpression/amplification | IHC 3+ and 2+ confirmed using FISH (breast criteria) | Trastuzumab | Efficacy; toxicity | SD 2/18, well-tolerated therapy | NR | |
| EnC | Lesly KK et al., 2013. [112] | Phase II | 30 | Second line and later lines | Protein overexpression | IHC | Lapatinib | PFS, OS | mPFS 1.82 mo | mOS: 7.33 mo |
| EnC + OC | Hainsworth JD et al., 2018 [102] | Phase II | 7/230 (EnC) 8/230 (OC) | Second line and later lines | Amplification/overexpression/mutations | IHC, FISH, NGS | Pertuzumab plus trastuzumab | ORR | EnC: ORR 0% OC: ORR 13% | NR |
| OC | Yang Y et al., 2018. [113] | Retrospective | 80 | Second line and later lines | NR | NR | Trastuzumab vs. trastuzumab plus abraxane | PR | PR 44.2% vs. 45.9% mo | OS 7%vs 7.3% mo |
| EnC, serous Histotype | Fader AN et al., 2018 [114] | Phase II | 61 | Second line and later lines | Protein overexpression and amplification | IHC + FISH | Carboplatin and paclitaxel +/− trastuzumab | mPFS | mPFS: 12.9 mo vs. 8.0 mo | See results |
| EnC + OC + CeC | Destiny-Pan Tumor Trial [115] | Phase II | ongoing | Second line and later lines | Protein overexpression | IHC | T-DXd | ORR | ORR EnC: 85% ORR CeC: 75% ORR OC: 45% | NR |
| EnC | Moustapha H et al., 2021 [116] | Phase II | 28 | Second line and later lines | Protein overexpression/amplifications/ mutations | NR | Pertuzumab plus trastuzumab | ORR | DCR: 37% ORR 7.1% | OS: 53.4% (1 year) mPFS 28.1 wks |
| EnC + OC + CeC | Meric-Bernstam F et al., 2024 [24] | Phase II | EnC 40 out 267; OC 40 out 267; CeC 40 out 267 | Third line and later lines | Protein overexpression | IHC | T-DXd | ORR | EnC: 84.6% CeC 75% OC 63.6% | EnC: PFS: 11.1 mo CeC and OC: NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaquarini, E.; Grillo, F.; Gervaso, L.; Arpa, G.; Fazio, N.; Vanoli, A.; Parente, P. Prognostic and Predictive Roles of HER2 Status in Non-Breast and Non-Gastroesophageal Carcinomas. Cancers 2024, 16, 3145. https://doi.org/10.3390/cancers16183145
Quaquarini E, Grillo F, Gervaso L, Arpa G, Fazio N, Vanoli A, Parente P. Prognostic and Predictive Roles of HER2 Status in Non-Breast and Non-Gastroesophageal Carcinomas. Cancers. 2024; 16(18):3145. https://doi.org/10.3390/cancers16183145
Chicago/Turabian StyleQuaquarini, Erica, Federica Grillo, Lorenzo Gervaso, Giovanni Arpa, Nicola Fazio, Alessandro Vanoli, and Paola Parente. 2024. "Prognostic and Predictive Roles of HER2 Status in Non-Breast and Non-Gastroesophageal Carcinomas" Cancers 16, no. 18: 3145. https://doi.org/10.3390/cancers16183145
APA StyleQuaquarini, E., Grillo, F., Gervaso, L., Arpa, G., Fazio, N., Vanoli, A., & Parente, P. (2024). Prognostic and Predictive Roles of HER2 Status in Non-Breast and Non-Gastroesophageal Carcinomas. Cancers, 16(18), 3145. https://doi.org/10.3390/cancers16183145







