Therapeutic Opportunities for Biomarkers in Metastatic Spine Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
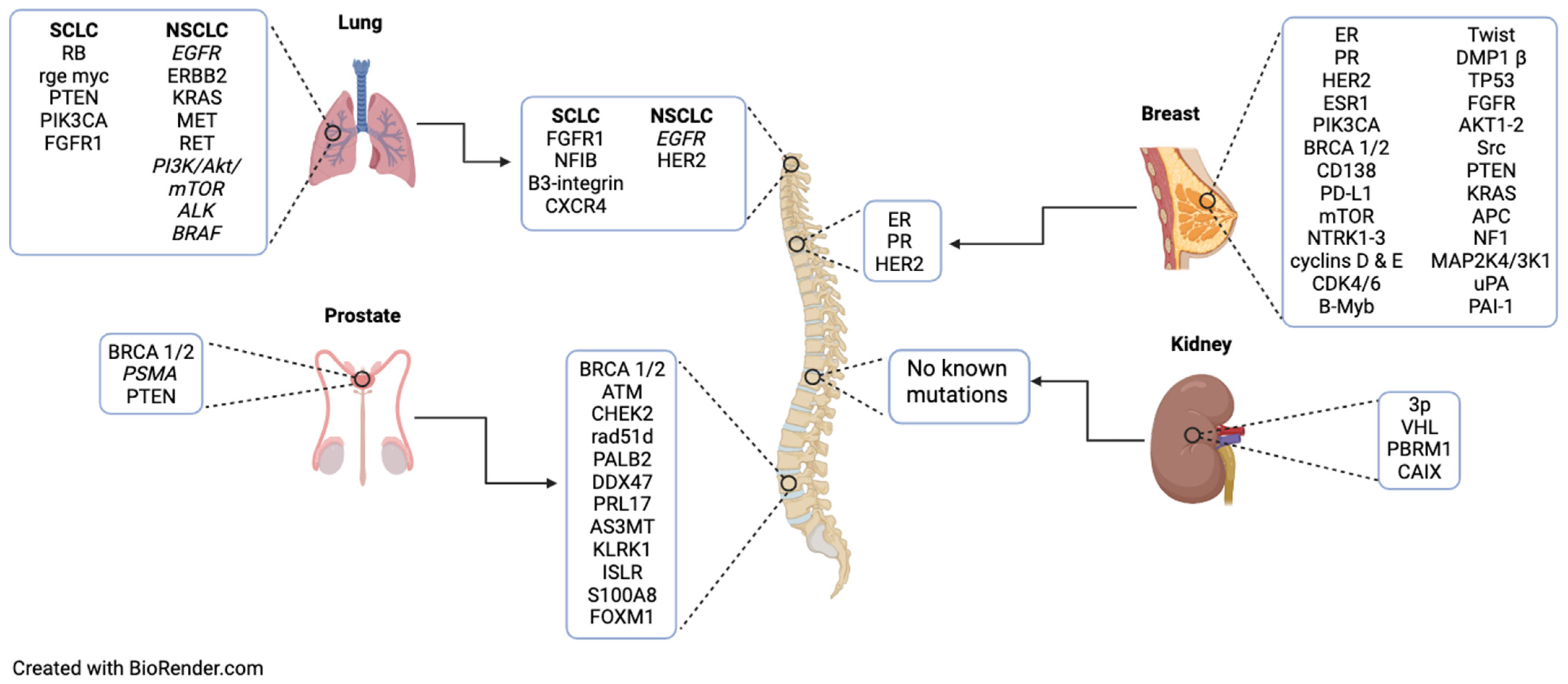
2. Lung Cancer
2.1. Primary: SCLC
2.2. Spinal Metastasis: SCLC
2.3. Primary: NSCLC
2.4. Spinal Metastasis: NSCLC
3. Breast Cancer
3.1. Primary
3.2. Spinal Metastasis
4. Prostate Cancer
4.1. Primary
4.2. Spinal Metastasis
5. Renal Cell Cancer
5.1. Primary
5.2. Metastasis
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fridley, J.S.; Syed, S.; Niu, T.; Leary, O.P.; Gokaslan, Z.L. Presentation of spinal cord and column tumors. Neuro-Oncol. Pract. 2020, 7, i18–i24. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Sun, J.; Hu, L.; Bok, S.; Yallowitz, A.R.; Cung, M.; McCormick, J.; Zheng, L.J.; Debnath, S.; Niu, Y.; Tan, A.Y.; et al. A vertebral skeletal stem cell lineage driving metastasis. Nature 2023, 621, 602–609. [Google Scholar] [CrossRef]
- Dea, N.; Versteeg, A.L.; Sahgal, A.; Verlaan, J.-J.; Charest-Morin, R.; Rhines, L.D.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Lazary, A.; et al. Metastatic Spine Disease: Should Patients With Short Life Expectancy Be Denied Surgical Care? An International Retrospective Cohort Study. Neurosurgery 2019, 87, 303–311. [Google Scholar] [CrossRef]
- Serratrice, N.; Faddoul, J.; Tarabay, B.; Attieh, C.; Chalah, M.A.; Ayache, S.S.; Lahoud, G.N.A. Ten Years After SINS: Role of Surgery and Radiotherapy in the Management of Patients with Vertebral Metastases. Front. Oncol. 2022, 12, 802595. [Google Scholar] [CrossRef]
- Williams, D.M.; Thirukumaran, C.P.; Oses, J.T.; Mesfin, A.; Moses, J. Complications and Mortality Rates Following Surgical Management of Extradural Spine Tumors in New York State. Spine 2020, 45, 474–482. [Google Scholar] [CrossRef]
- Mesfin, A.; Sciubba, D.M.; Dea, N.; Nater, A.; Bird, J.E.; Quraishi, N.A.; Fisher, C.G.; Shin, J.H.; Fehlings, M.G.; Kumar, N.; et al. Changing the Adverse Event Profile in Metastatic Spine Surgery: An Evidence-Based Approach to Target Wound Complications and Instrumentation Failure. Spine 2016, 41 (Suppl. S20), S262–S270. [Google Scholar] [CrossRef]
- Schilling, A.T.; Ehresman, J.; Huq, S.; Ahmed, A.K.; Lubelski, D.; Cottrill, E.; Pennington, Z.; Shin, J.H.; Sciubba, D.M. Risk Factors for Wound-Related Complications After Surgery for Primary and Metastatic Spine Tumors: A Systematic Review and Meta-Analysis. World Neurosurg. 2020, 141, 467–478.e3. [Google Scholar] [CrossRef]
- Melo, L.M.N.; Lesner, N.P.; Sabatier, M.; Ubellacker, J.M.; Tasdogan, A. Emerging metabolomic tools to study cancer metastasis. Trends Cancer 2022, 8, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, D.; Plass, C. Epigenetic signatures in cancer: Proper controls, current challenges and the potential for clinical translation. Genome Med. 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, A.; Tambaro, F.P.; Dell’aversana, C.; Altucci, L. Cancer epigenetics: Moving forward. PLoS Genet. 2018, 14, e1007362. [Google Scholar] [CrossRef] [PubMed]
- Ziu, E.; Viswanathan, V.K.; Mesfin, F.B. Spinal Metastasis. [Updated 14 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441950/ (accessed on 1 July 2024).
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Maccauro, G.; Spinelli, M.S.; Mauro, S.; Perisano, C.; Graci, C.; Rosa, M.A. Physiopathology of Spine Metastasis. Int. J. Surg. Oncol. 2011, 2011, e107969. [Google Scholar] [CrossRef]
- Govindan, R.; Page, N.; Morgensztern, D.; Read, W.; Tierney, R.; Vlahiotis, A.; Spitznagel, E.L.; Piccirillo, J. Changing Epidemiology of Small-Cell Lung Cancer in the United States over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J. Clin. Oncol. 2006, 24, 4539–4544. [Google Scholar] [CrossRef]
- Chen, J.; Qi, Y.; Wampfler, J.A.; Jatoi, A.; Garces, Y.I.; Busta, A.J.; Mandrekar, S.J.; Yang, P. Effect of cigarette smoking on quality of life in small cell lung cancer patients. Eur. J. Cancer 2012, 48, 1593–1601. [Google Scholar] [CrossRef]
- Nakazawa, K.; Kurishima, K.; Tamura, T.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in small cell lung cancer. Oncol. Lett. 2012, 4, 617–620. [Google Scholar] [CrossRef]
- Little, C.D.; Nau, M.M.; Carney, D.N.; Gazdar, A.F.; Minna, J.D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature 1983, 306, 194–196. [Google Scholar] [CrossRef]
- Nau, M.M.; Brooks, B.J.; Battey, J.F.; Sausville, E.; Gazdar, A.F.; Kirsch, I.R.; McBride, O.W.; Bertness, V.L.; Hollis, G.F.; Minna, J.D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature 1985, 318, 69–73. [Google Scholar] [CrossRef]
- Cui, M.; Augert, A.; Rongione, M.; Conkrite, K.; Parazzoli, S.; Nikitin, A.Y.; Ingolia, N.; MacPherson, D. PTEN is a potent suppressor of small cell lung cancer. Mol. Cancer Res. 2014, 12, 654–659. [Google Scholar] [CrossRef]
- Ferone, G.; Song, J.Y.; Krijgsman, O.; van der Vliet, J.; Cozijnsen, M.; Semenova, E.A.; Adams, D.J.; Peeper, D.; Berns, A. FGFR1 Oncogenic Activation Reveals an Alternative Cell of Origin of SCLC in Rb1/p53 Mice. Cell Rep. 2020, 30, 3837–3850.e3. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus plat-inum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.G.; Ramalingam, S.S.; Ciuleanu, T.E.; Lee, J.S.; Urban, L.; Caro, R.B.; Park, K.; Sakai, H.; Ohe, Y.; Nishio, M.; et al. First-Line Nivolumab Plus Ipilimumab in Ad-vanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J. Thorac. Oncol. 2022, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, S.; Daigo, Y.; Tsunoda, T.; Yano, S.; Sone, S.; Nakamura, Y. Genome-wide analysis of organ-preferential metastasis of human small cell lung cancer in mice. Mol. Cancer Res. 2003, 1, 485–499. [Google Scholar]
- Semenova, E.A.; Kwon, M.-C.; Monkhorst, K.; Song, J.-Y.; Bhaskaran, R.; Krijgsman, O.; Kuilman, T.; Peters, D.; Buikhuisen, W.A.; Smit, E.F.; et al. Transcription Factor NFIB Is a Driver of Small Cell Lung Cancer Progression in Mice and Marks Metastatic Disease in Patients. Cell Rep. 2016, 16, 631–643. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.P.; Guo, S.; Min, J.; Liu, L.L.; Su, H.C.; Feng, Y.-M.; Zhang, H.-L. Down-regulation of β3-integrin inhibits bone metastasis of small cell lung cancer. Mol. Biol. Rep. 2012, 39, 3029–3035. [Google Scholar] [CrossRef]
- Ma, N.; Pang, H.; Shen, W.; Zhang, F.; Cui, Z.; Wang, J.; Wang, J.; Liu, L.; Zhang, H. Downregulation of CXCR4 by SDF-KDEL in SBC-5 cells inhibits their migration in vitro and organ metastasis in vivo. Int. J. Mol. Med. 2014, 35, 425–432. [Google Scholar] [CrossRef]
- Weeden, C.; Solomon, B.; Asselin-Labat, M.-L. FGFR1 inhibition in lung squamous cell carcinoma: Questions and controversies. Cell Death Discov. 2015, 1, 15049. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, Z.; Zhang, Y.; Xue, D.; Xia, H.; She, J.; Li, F. Integrin β3 Promotes Resistance to EGFR-TKI in Non-Small-Cell Lung Cancer by Upregulating AXL through the YAP Pathway. Cells 2022, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Taromi, S.; Kayser, G.; Catusse, J.; von Elverfeldt, D.; Reichardt, W.; Braun, F.; Weber, W.A.; Zeiser, R.; Burger, M. CXCR4 antagonists suppress small cell lung cancer progression. Oncotarget 2016, 7, 85185–85195. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Li, Q.; Xu, X.; Li, X.; You, X.; Yu, Z. Genetic profile of non-small cell lung cancer (NSCLC): A hospital-based survey in Jinhua. Mol. Genet. Genom. Med. 2020, 8, e1398. [Google Scholar] [CrossRef]
- Myers, D.J.; Wallen, J.M. Lung Adenocarcinoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK519578/ (accessed on 1 July 2024).
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef]
- Shaw, A.T.; Engelman, J.A. ALK in Lung Cancer: Past, Present, and Future. J. Clin. Oncol. 2013, 31, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.B.; Otterson, G.A. Agents to treat BRAF-mutant lung cancer. Drugs Context. 2019, 8, 212566. [Google Scholar]
- Knapp, B.J.; Devarakonda, S.; Govindan, R. Bone metastases in non-small cell lung cancer: A narrative review. J. Thorac. Dis. 2022, 14, 1696–1712. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Chiu, C.-H.; Chou, P.-H.; Ma, H.-L.; Wang, J.-P.; Wang, S.-T.; Liu, C.-L.; Chang, M.-C. Functional outcomes and survival after surgical stabilization for inoperable non-small-cell lung cancer with spinal metastasis of the thoracic and lumbar spines: A retrospective comparison between epidermal growth factor receptor-tyrosine kinase inhibitor and platinum-based chemotherapy groups. Spinal Cord. 2020, 58, 194–202. [Google Scholar] [CrossRef]
- Gow, C.-H.; Chang, Y.-L.; Hsu, Y.-C.; Tsai, M.-F.; Wu, C.-T.; Yu, C.-J.; Yang, C.-H.; Lee, Y.-C.; Yang, P.-C.; Shih, J.-Y. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann. Oncol. 2009, 20, 696–702. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Bilani, N.; Zabor, E.C.; Elson, L.; Elimimian, E.B.; Nahleh, Z. Breast Cancer in the United States: A Cross-Sectional Overview. J. Cancer Epidemiol. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; et al. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. JNCI J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations—A mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Götte, M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol. Cancer 2017, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Seale, K.N.; Tkaczuk, K.H.R. Circulating Biomarkers in Breast Cancer. Clin. Breast Cancer 2021, 22, e319–e331. [Google Scholar] [CrossRef]
- Tarighati, E.; Keivan, H.; Mahani, H. A review of prognostic and predictive biomarkers in breast cancer. Clin. Exp. Med. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Tang, S.; Wei, L.; Sun, Y.; Zhou, F.; Zhu, S.; Yang, R.; Huang, Y.; Zhang, H.; Xu, H.; Yang, J. CA153 in Breast Secretions as a Potential Molecular Marker for Diagnosing Breast Cancer: A Meta Analysis. PLoS ONE 2016, 11, e0163030. [Google Scholar] [CrossRef]
- Giridhar, K.V.; Liu, M.C. Available and emerging molecular markers in the clinical management of breast cancer. Expert Rev. Mol. Diagn. 2019, 19, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fry, E.A. Novel Molecular Markers for Breast Cancer. Biomark. Cancer 2016, 8, BIC.S38394-42. [Google Scholar] [CrossRef]
- Goodwin, C.R.; Abu-Bonsrah, N.; Rhines, L.D.; Verlaan, J.J.; Bilsky, M.H.; Laufer, I.; Boriani, S.; Sciubba, D.M.; Bettegowda, C. Molecular Markers and Targeted Therapeutics in Metastatic Tumors of the Spine: Changing the Treatment Paradigms. Spine 2016, 41 (Suppl. S20), S218–S223. [Google Scholar] [CrossRef]
- Banin Hirata, B.K.; Oda, J.M.M.; Losi Guembarovski, R.; Ariza, C.B.; Oliveira, C.E.C.D.; Watanabe, M.A.E. Molecular Markers for Breast Cancer: Prediction on Tumor Behavior. Dis. Mark. 2014, 2014, 513158. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, A.; Pérez-Ruiz, E.; Jiménez, B.; Ribelles, N.; Márquez, A.; García-Ríos, I.; Conejo, E.A. Targeted therapy of metastatic breast cancer. Clin. Transl. Oncol. 2009, 11, 643–650. [Google Scholar] [CrossRef]
- Arnedos, M.; Vicier, C.; Loi, S.; Lefebvre, C.; Michiels, S.; Bonnefoi, H.; Andre, F. Precision medicine for metastatic breast can-cer—Limitations and solutions. Nat. Rev. Clin. Oncol. 2015, 12, 693–704. [Google Scholar] [CrossRef]
- Yamamoto-Ibusuki, M.; Arnedos, M.; André, F. Targeted therapies for ER+/HER2- metastatic breast cancer. BMC Med. 2015, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- De Paula Costa Monteiro, I.; Madureira, P.; De Vasconscelos, A.; Humberto Pozza, D.; Andrade De Mello, R. Targeting HER family in HER2-positive metastatic breast cancer: Potential biomarkers and novel targeted therapies. Pharmacogenomics 2015, 16, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; McGowan, P.M.; Harbeck, N.; Thomssen, C.; Schmitt, M. uPA and PAI-1 as biomarkers in breast cancer: Validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, E.; Karavasilis, V.; Kostadima, L.; Ignatiadis, M.; Fountzilas, G.; Pavlidis, N. Metastatic breast carcinoma confined to bone: Portrait of a clinical entity. Cancer 2004, 101, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Rubens, R. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Shehadi, J.A.; Sciubba, D.M.; Suk, I.; Suki, D.; Maldaun, M.V.C.; McCutcheon, I.E.; Nader, R.; Theriault, R.; Rhines, L.D.; Gokaslan, Z.L. Surgical treatment strategies and outcome in patients with breast cancer metastatic to the spine: A review of 87 patients. Eur. Spine J. 2007, 16, 1179–1192. [Google Scholar] [CrossRef]
- Ju, D.G.; Yurter, A.; Gokaslan, Z.L.; Sciubba, D.M. Diagnosis and surgical management of breast cancer metastatic to the spine. World J. Clin. Oncol. 2014, 5, 263–271. [Google Scholar] [CrossRef]
- Amelot, A.; Terrier, L.-M.; Cristini, J.; Buffenoir, K.; Pascal-Moussellard, H.; Carpentier, A.; Bonaccorsi, R.; Le Nail, L.-R.; Mathon, B. Survival in breast cancer patients with spine metastases: Prognostic assessment involving molecular markers. Eur. J. Surg. Oncol. EJSO 2019, 46, 1021–1027. [Google Scholar] [CrossRef]
- Adler, D.; Pepke, W.; Akbar, M. Operative Treatment of Metastatic Breast Cancer in the Spine with Regard to Molecular Pheno-Types. JCMT [Internet]. 2019 May 31 [cited 23 August 2023]. 2019. Available online: https://jcmtjournal.com/article/view/3098 (accessed on 1 July 2024).
- Yogi, V.; Pareek, A.; Singh, O.; Ghori, H.; Tiwari, V.; Redhu, P. Bone metastases incidence and its correlation with hormonal and human epidermal growth factor receptor 2 neu receptors in breast cancer. J. Cancer Res. Ther. 2019, 15, 971–975. [Google Scholar] [CrossRef]
- Lin, J.; Goldstein, L.; Nesbit, A.; Chen, M.Y. Influence of Hormone Receptor Status on Spinal Metastatic Lesions in Patients with Breast Cancer. World Neurosurg. 2015, 85, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bian, C.; Liang, Y.; Jiang, L.; Qian, C.; Dong, J. CX3CL1: A potential chemokine widely involved in the process spinal me-tastases. Oncotarget 2017, 8, 15213–15219. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Ni, Y.-B.; Chan, S.-K.; Shao, M.-M.; Kwok, Y.-K.; Chan, K.-W.; Tan, P.H.; Tse, G.M. CX3CL1 expression is associated with poor outcome in breast cancer patients. Breast Cancer Res. Treat. 2013, 140, 495–504. [Google Scholar] [CrossRef]
- Posdzich, P.; Darr, C.; Hilser, T.; Wahl, M.; Herrmann, K.; Hadaschik, B.; Grünwald, V. Metastatic Prostate Cancer—A Review of Current Treatment Options and Promising New Approaches. Cancers 2023, 15, 461. [Google Scholar] [CrossRef]
- Drzymalski, D.M.; Oh, W.K.; Werner, L.; Regan, M.M.; Kantoff, P.; Tuli, S. Predictors of survival in patients with prostate cancer and spinal metastasis. J. Neurosurg. Spine 2010, 13, 789–794. [Google Scholar] [CrossRef]
- Choudhury, A.D.; Eeles, R.; Freedland, S.J.; Isaacs, W.B.; Pomerantz, M.M.; Schalken, J.A.; Tammela, T.L.; Visakorpi, T. The Role of Genetic Markers in the Management of Prostate Cancer. Eur. Urol. 2012, 62, 577–587. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Deluce, J.E.; Cardenas, L.; Lalani, A.-K.; Vareki, S.M.; Fernandes, R. Emerging Biomarker-Guided Therapies in Prostate Cancer. Curr. Oncol. 2022, 29, 5054–5076. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Parnis, F.; et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2021, 398, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Madan, E.; Yee, M.; Zhang, H. Progress of molecular targeted therapies for prostate cancers. Biochim. Biophys. Acta BBA Rev. Cancer 2012, 1825, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Toren, P.; Zoubeidi, A. Targeting the PI3K/Akt pathway in prostate cancer: Challenges and opportunities (Review). Int. J. Oncol. 2014, 45, 1793–1801. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Ratta, R.; Gafanov, R.; Facchini, G.; Piulats, J.M.; Kramer, G.; Flaig, T.W.; Chandana, S.R.; Li, B.; Burgents, J.; et al. KEYNOTE-921: Phase III Study of Pembrolizumab Plus Docetaxel for Metastatic Castration-Resistant Prostate Cancer. Future Oncol. 2021, 17, 3291–3299. [Google Scholar] [CrossRef]
- Graff, J.N.; Liang, L.W.; Kim, J.; Stenzl, A. KEYNOTE-641: A Phase III study of pembrolizumab plus enzalutamide for metastatic castration-resistant prostate cancer. Future Oncol. 2021, 17, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Rathi, N.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N.; Swami, U. Evolving Role of Immunotherapy in Metastatic Castration Refractory Prostate Cancer. Drugs 2020, 81, 191–206. [Google Scholar] [CrossRef]
- Cordes, L.M.; Gulley, J.L.; Madan, R.A. The evolving role of immunotherapy in prostate cancer. Curr. Opin. Oncol. 2016, 28, 232–240. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.V.; Giaze, T.R.; Chin, K.Y.; Mohamed, N.; Ima-Nirwana, S. Prostate Cancer and Bone Metastases: The Un-derlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Hu, P.; Fan, M.; Song, D.; Yin, H.; Yan, P.; Xian, S.; Li, Z.; Guo, J.; et al. Identification of Bone Metastatic and Prognostic Alternative Splicing Sig-natures in Prostate Adenocarcinoma. Biochem. Genet. 2023, 61, 2242–2259. [Google Scholar] [CrossRef]
- Liang, Z.-T.; Li, J.-K.; Li, J.; Tang, H.; Guo, C.-F.; Zhang, H.-Q. PECAM1 plays a role in the pathogenesis and treatment of bone metastases. Front. Genet. 2023, 14, 1151651. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Hu, X.; Duan, Y.; Li, W.; Qian, J.; Chen, J. A Novel Overall Survival Prediction Signature Based on Comprehensive Research in Prostate Cancer Bone Metastases. Front. Med. 2022, 9, 815541. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Li, M.; Zhang, X.; Xian, S.; Zhang, J.; Yin, H.; Liu, Y.; Fan, M.; Li, Z.; Zhu, X.; et al. Construction of Bone Metastasis-Specific Regulation Network Based on Prognostic Stemness-Related Signatures in Prostate Cancer. Dis. Mark. 2022, 2022, 1–27. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Lin, S.C.; Kao, C.Y.; Lee, H.J.; Creighton, C.J.; Ittmann, M.M.; Tsai, S.J.; Tsai, S.Y.; Tsai, M.-J. Dysregulation of miRNAs-COUP-TFII-FOXM1-CENPF axis contributes to the metastasis of prostate cancer. Nat. Commun. 2016, 7, 11418. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sullivan, P.Z.; Niu, T.; Abinader, J.F.; Syed, S.; Sampath, P.; Telfeian, A.; Fridley, J.; Klinge, P.; Camara, J.; Oyelese, A.; et al. Evolution of surgical treatment of metastatic spine tumors. J. Neuro-Oncol. 2022, 157, 277–283. [Google Scholar] [CrossRef]
- Williamson, S.R.; Gill, A.J.; Argani, P.; Chen, Y.B.; Egevad, L.; Kristiansen, G.; Grignon, D.J.; Hes, O. Report from the International Society of Urological Pathology (ISUP) Consultation Conference On Molecular Pathology Of Urogenital Cancers. III. Molecular Pathology of Kidney Cancer. Am. J. Surg. Pathol. 2020, 44, e47–e65. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- Amaro, F.; Carvalho, M.; Bastos, M.d.L.; de Pinho, P.G.; Pinto, J. Metabolic signature biomarkers for predicting the recurrence of urological cancers. Clin. Chim. Acta 2023, 549, 117553. [Google Scholar] [CrossRef]
- Escudier, B. Emerging immunotherapies for renal cell carcinoma. Ann. Oncol. 2012, 23, viii35–viii40. [Google Scholar] [CrossRef] [PubMed]
- Vano, Y.-A.; Tartour, E.; Fournier, L.S.; Beuselinck, B.; Mejean, A.; Oudard, S. Prognostic factors in patients with advanced renal cell carcinoma treated with VEGF-targeted agents. Expert Rev. Anticancer Ther. 2014, 14, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Mickley, A.; Kovaleva, O.; Kzhyshkowska, J.; Gratchev, A. Molecular and immunologic markers of kidney cancer-potential appli-cations in predictive, preventive and personalized medicine. EPMA J. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Murakami, H.; Kawahara, N.; Tomita, K.; Tsuchiya, H. Surgical Metastasectomy in the Spine: A Review Article. Oncologist 2021, 26, e1833–e1843. [Google Scholar] [CrossRef]
- Ngo, T.C.; Wood, C.G.; Karam, J.A. Biomarkers of renal cell carcinoma. Urol. Oncol. 2014, 32, 243–251. [Google Scholar] [CrossRef]
- Pal, S.K.; Li, S.M.; Wu, X.; Qin, H.; Kortylewski, M.; Hsu, J.; Carmichael, C.; Frankel, P. Stool Bacteriomic Profiling in Patients with Metastatic Renal Cell Carcinoma Receiving Vascular Endothelial Growth Factor-Tyrosine Kinase Inhibitors. Clin. Cancer Res. 2015, 21, 5286–5293. [Google Scholar] [CrossRef]
- Salgia, N.J.; Bergerot, P.G.; Maia, M.C.; Dizman, N.; Hsu, J.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti–PD-1 Immune Checkpoint Inhibitors. Eur. Urol. 2020, 78, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Uei, H.; Oshima, M.; Ajiro, Y. Scoring system for prediction of metastatic spine tumor prognosis. World J. Orthop. 2014, 5, 262–271. [Google Scholar] [CrossRef]
- Alpantaki, K.; Ioannidis, A.; Raptis, K.; Spartalis, E.; Koutserimpas, C. Surgery for spinal metastatic tumors: Prognostication systems in clinical practice (Review). Mol. Clin. Oncol. 2020, 12, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Martin, A.; Reiner, A.S.; Laufer, I.; Schmitt, A.; Bilsky, M.H. Clinical reliability of genomic data obtained from spinal metastatic tumor samples. Neuro-Oncology 2022, 24, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Díaz, B.; García-Botello, D.R.; Wegman-Ostrosky, T.; Reyes-Soto, G.; Ortiz-Sánchez, E.; Herrera-Montalvo, L.A. Tumor microenvironment differences between primary tumor and brain metastases. J. Transl. Med. 2020, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Romasanta, L.A.; Arana, E.; Kovacs, F.M.; Royuela, A. The Management of Metastatic Spinal Cord Compression in Routine Clinical Practice. Cancers 2023, 15, 2821. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.T.; Baker, J.F.; Lenehan, B. The Role of Prognostic Scoring Systems in Assessing Surgical Candidacy for Patients With Vertebral Metastasis: A Narrative Review. Glob. Spine J. 2018, 8, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef]
| Citation | Study Type | Biomarker/ Target | Primary Lesion Type Targeted | Drug Trialed |
|---|---|---|---|---|
| Paz-Ares et al., 2019 [24] | Phase 3 Trial | PD-L1, CTLA-4 | Lung (SCLC) | Durvalumab, tremelimumab |
| Horn et al., 2018 [25] | Phase 3 Trial | PD-L1 | Lung (SCLC) | Atezolimab |
| Paz-Ares et al., 2022 [26] | Phase 3 Trial | PD-L1 | Lung (NSCLC) | Nivolumab, ipilimumab |
| Fisher et al., 1998 [48] | Phase 1 Trial | Estrogen receptors | Breast | Tamoxifen |
| André et al., 2019 [51] | Phase 3 Trial | PIK3CA (in hormone receptor positive, HER2-negative patients) | Breast | Alpelisib |
| Robson et al., 2017 [52] | Phase 3 Trial | PARP (in BRCA mutation and HER2-negative patients) | Breast | Olaparib |
| Schmid et al., 2018 [54] | Phase 3 Trial | PD-L1 (in triple-negative patients) | Breast | Atezolizumab |
| de Bono et al., 2020 [80] | Phase 3 Trial | PARP | Prostate | Olaparib |
| Sartor et al., 2021 [82] | Phase 3 Trial | PSMA | Prostate | Lutetium-177-PSMA-617 |
| Hofman et al., 2021 [83] | Phase 2 Trial | PSMA | Prostate | [177Lu]Lu-PSMA-617 |
| Sweeney et al., 2021 [84] | Phase 3 Trial | PI3K/AKT | Prostate | Ipatasertib |
| Petyrlak et al., 2021 [87] | Phase 3 Trial | PD-1 | Prostate | Pembrolizumab |
| Graff et al., 2021 [88] | Phase 3 Trial | PD-1 | Prostate | Pembrolizumab |
| Kantoff et al., 2010 [91] | Phase 3 Trial | T-cells | Prostate | Sipuleucel-T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeder, C.; Campilan, B.; Leary, O.P.; Arditi, J.; Michles, M.J.; De La Garza Ramos, R.; Akinduro, O.O.; Gokaslan, Z.L.; Martinez Moreno, M.; Sullivan, P.L.Z. Therapeutic Opportunities for Biomarkers in Metastatic Spine Tumors. Cancers 2024, 16, 3152. https://doi.org/10.3390/cancers16183152
Schroeder C, Campilan B, Leary OP, Arditi J, Michles MJ, De La Garza Ramos R, Akinduro OO, Gokaslan ZL, Martinez Moreno M, Sullivan PLZ. Therapeutic Opportunities for Biomarkers in Metastatic Spine Tumors. Cancers. 2024; 16(18):3152. https://doi.org/10.3390/cancers16183152
Chicago/Turabian StyleSchroeder, Christian, Beatrice Campilan, Owen P. Leary, Jonathan Arditi, Madison J. Michles, Rafael De La Garza Ramos, Oluwaseun O. Akinduro, Ziya L. Gokaslan, Margot Martinez Moreno, and Patricia L. Zadnik Sullivan. 2024. "Therapeutic Opportunities for Biomarkers in Metastatic Spine Tumors" Cancers 16, no. 18: 3152. https://doi.org/10.3390/cancers16183152






