Low-Risk and High-Risk NSMPs: A Prognostic Subclassification of No Specific Molecular Profile Subtype of Endometrial Carcinomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
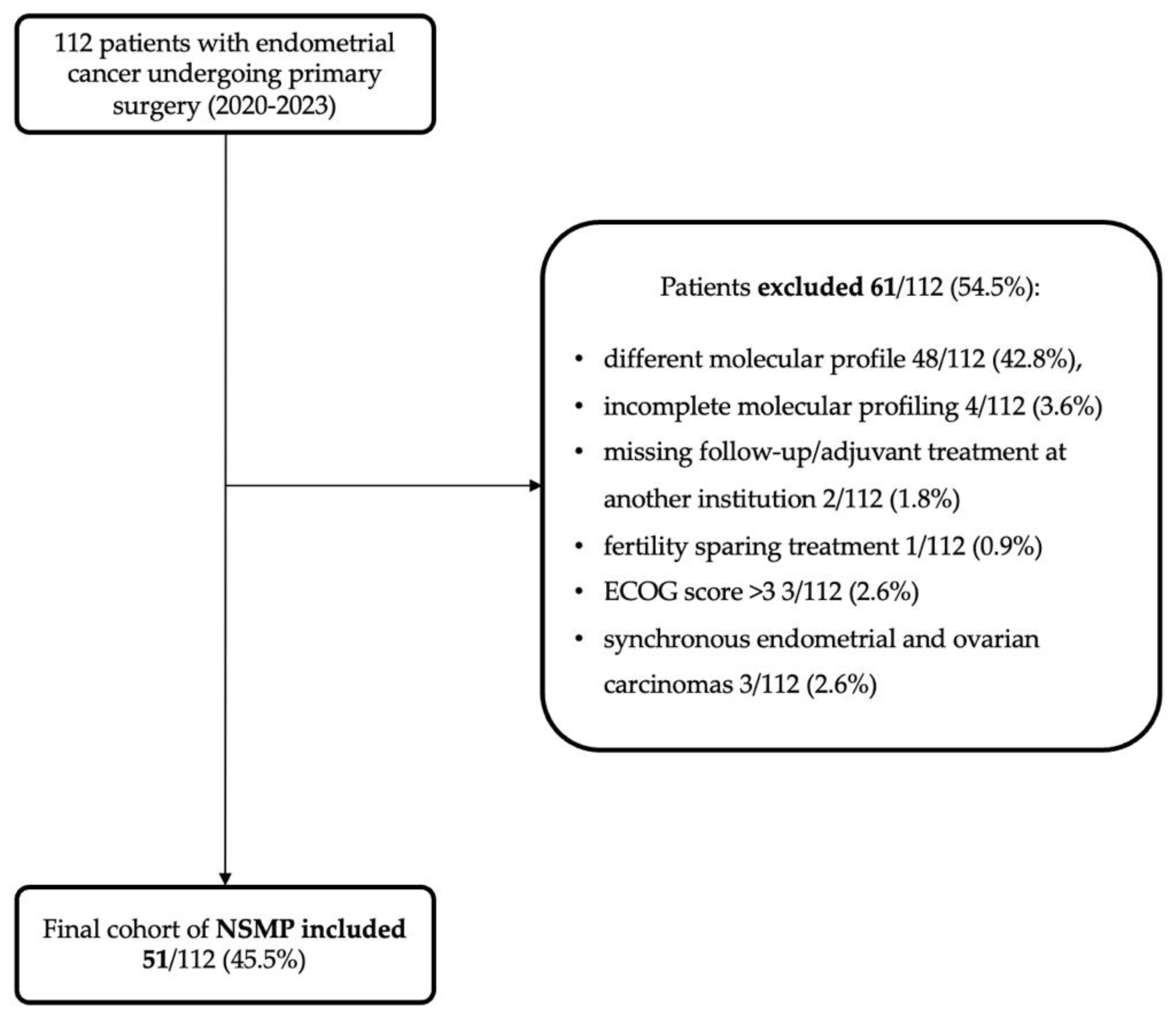
2.1. Study Design and Population
2.2. Data Collection
2.3. Outcomes of the Study
2.4. Immunohistochemistry and Next Generation Sequencing
2.5. Statistical Analysis
3. Results
3.1. Study Population and Histopathological Features
3.2. Low-Risk (LR) vs. High-Risk (HR) NSMPs
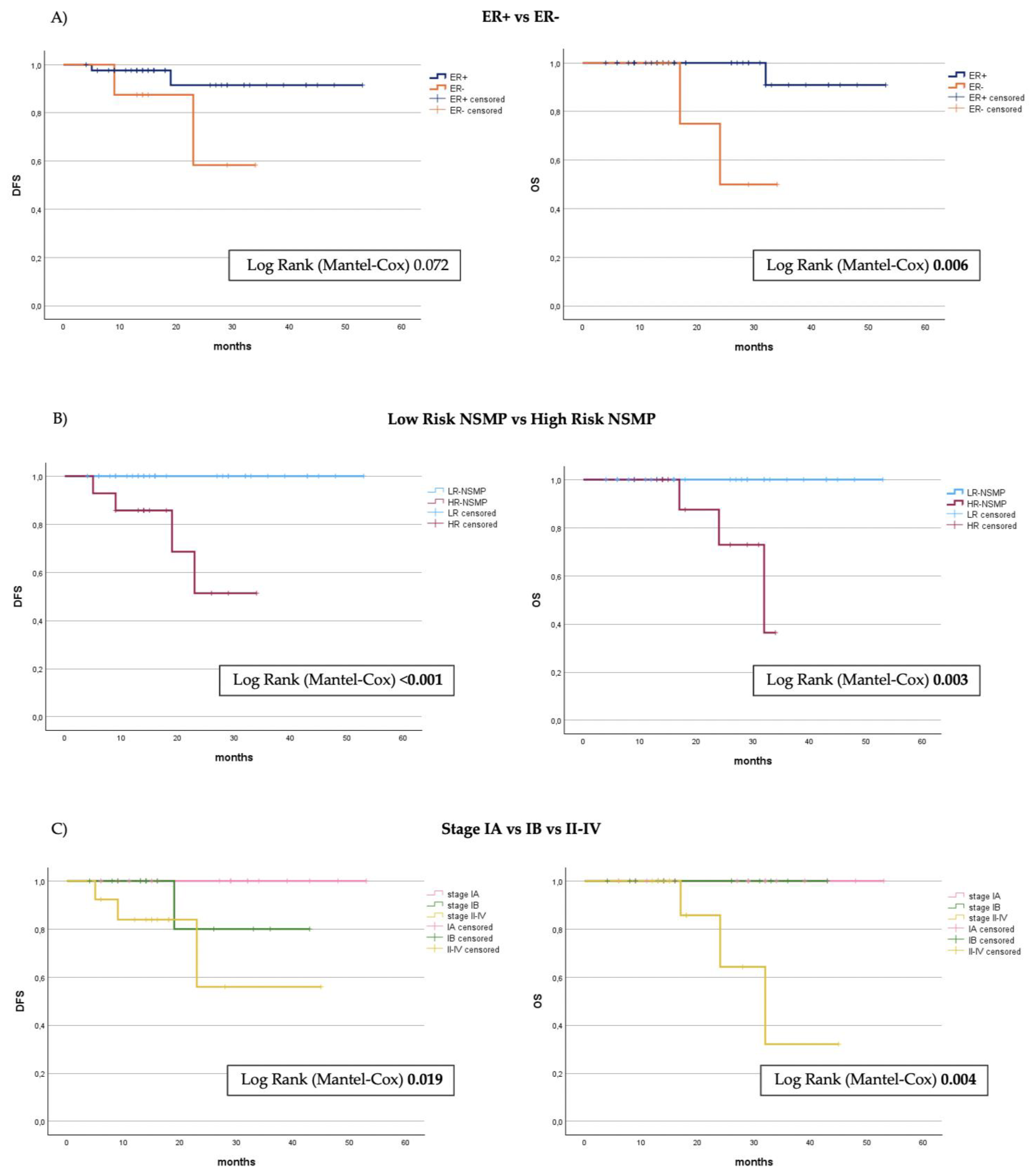
3.3. Survival Analysis
4. Discussion
4.1. Potential Implications for Management of High Risk-NSMP
4.2. Potential Implications for Management of Low-Risk NSMP
4.3. Strengths and Weaknesses of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plotkin, A.; Kuzeljevic, B.; De Villa, V.; Thompson, E.F.; Gilks, C.B.; Clarke, B.A.; Köbel, M.; McAlpine, J.N. Interlaboratory Concordance of ProMisE Molecular Classification of Endometrial Carcinoma Based on Endometrial Biopsy Specimens. Int. J. Gynecol. Pathol. 2020, 39, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Bosse, T.; Nout, R.A.; MacKay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie; et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. 2015, 28, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- McAlpine, J.N.; Chiu, D.S.; Nout, R.A.; Church, D.N.; Schmidt, P.; Lam, S.; Leung, S.; Bellone, S.; Wong, A.; Brucker, S.Y.; et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer 2021, 127, 2409–2422. [Google Scholar] [CrossRef]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Gilks, C.B.; McAlpine, J.N. p53abn Endometrial Cancer: Understanding the most aggressive endometrial cancers in the era of molecular classification. Int. J. Gynecol. Cancer 2021, 31, 907–913. [Google Scholar] [CrossRef]
- Eggink, F.A.; Van Gool, I.C.; Leary, A.; Pollock, P.M.; Crosbie, E.J.; Mileshkin, L.; Jordanova, E.S.; Adam, J.; Freeman-Mills, L.; Church, D.N.; et al. Immunological profiling of molecularly classified high-risk endometrial cancers identifies POLE-mutant and microsatellite unstable carcinomas as candidates for checkpoint inhibition. Oncoimmunology 2016, 6, e1264565. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman; et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Momeni-Boroujeni, A.; Nguyen, B.; Vanderbilt, C.M.; Ladanyi, M.; Abu-Rustum, N.R.; Aghajanian, C.; Ellenson, L.H.; Weigelt, B.; Soslow, R.A. Genomic landscape of endometrial carcinomas of no specific molecular profile. Mod. Pathol. 2022, 35, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, L.J.; Visser, N.C.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. Added Value of Estrogen Receptor, Progesterone Receptor, and L1 Cell Adhesion Molecule Expression to Histology-Based Endometrial Carcinoma Recurrence Prediction Models: An ENITEC Collaboration Study. Int. J. Gynecol. Cancer 2018, 28, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, F.K.; Karnezis, A.N.; Kommoss, F.; Talhouk, A.; Taran, F.A.; Staebler, A.; Gilks, C.B.; Huntsman, D.G.; Krämer, B.; Brucker; et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br. J. Cancer 2018, 119, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. Clinicopathologic and Immunohistochemical Correlates of CTNNB1 Mutated Endometrial Endometrioid Carcinoma. Int. J. Gynecol. Pathol. 2020, 39, 119–127. [Google Scholar] [CrossRef]
- Depreeuw, J.; Stelloo, E.; Osse, E.M.; Creutzberg, C.L.; Nout, R.A.; Moisse, M.; Garcia-Dios, D.A.; Dewaele, M.; Willekens, K.; Marine, J.C.; et al. Amplification of 1q32.1 Refines the Molecular Classification of Endometrial Carcinoma. Clin. Cancer Res. 2017, 23, 7232–7241. [Google Scholar] [CrossRef]
- Vermij, L.; Smit, V.; Nout, R.; Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Electronic address: Clinicalguidelines@esmo.org. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- van den Heerik AS, V.M.; Horeweg, N.; Nout, R.A.; Lutgens LC, H.W.; van der Steen-Banasik, E.M.; Westerveld, G.H.; van den Berg, H.A.; Slot, A.; Koppe FL, A.; Kommoss, S.; et al. PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 2002–2007. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee, FIGO Women’s Cancer Committee. FIGO staging of endometrial cancer: 2023. Int. J. Gynaecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Zannoni, G.F.; Santoro, A.; D’Alessandris, N.; Scaglione, G.; Inzani, F.; Angelico, G.; Bragantini, E.; Piermattei, A.; Cianfrini, F.; Bisaro, B.; et al. Biomarker characterization in endometrial cancer in Italy: First survey data analysis. Pathologica 2022, 114, 189–198. [Google Scholar] [CrossRef]
- León-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef]
- Wik, E.; Ræder, M.B.; Krakstad, C.; Trovik, J.; Birkeland, E.; Hoivik, E.A.; Mjos, S.; Werner, H.M.; Mannelqvist, M.; Stefansson, I.M.; et al. Lack of estrogen receptor-α is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin. Cancer Res. 2013, 19, 1094–1105. [Google Scholar] [CrossRef]
- Onoprienko, A.; Hofstetter, G.; Muellauer, L.; Dorittke, T.; Polterauer, S.; Grimm, C.; Bartl, T. Prognostic role of transcription factor ARID1A in patients with endometrial cancer of no specific molecular profile (NSMP) subtype. Int. J. Gynecol. Cancer 2024, 34, 840–846. [Google Scholar] [CrossRef]
- De Leo, A.; de Biase, D.; Lenzi, J.; Barbero, G.; Turchetti, D.; Grillini, M.; Ravegnini, G.; Angelini, S.; Zamagni, C.; Coluccelli, S.; et al. ARID1A and CTNNB1/β-Catenin Molecular Status Affects the Clinicopathologic Features and Prognosis of Endometrial Carcinoma: Implications for an Improved Surrogate Molecular Classification. Cancers 2021, 13, 950. [Google Scholar] [CrossRef]
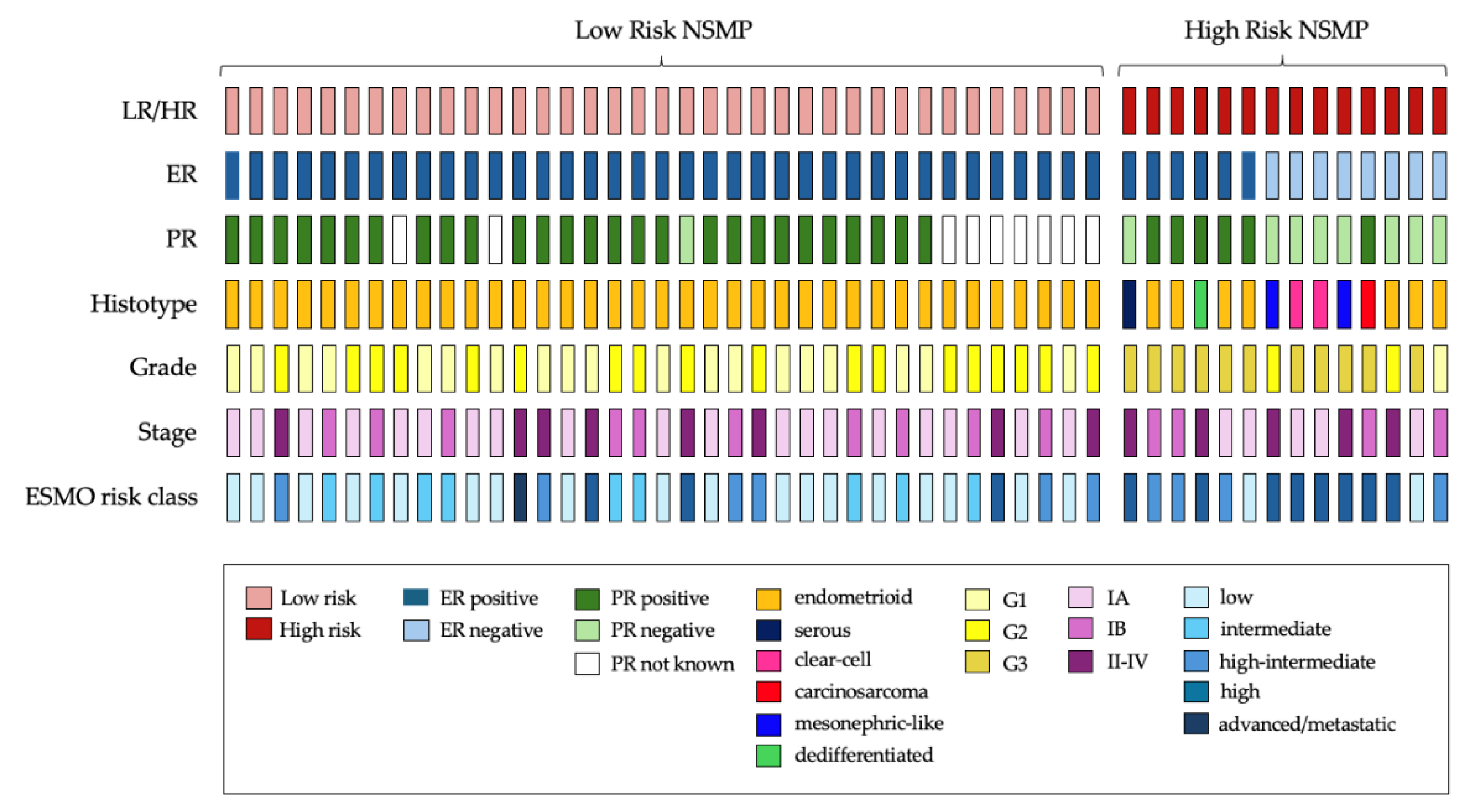

| Total Cohort | Low-Risk | High-Risk | p 1 | |
|---|---|---|---|---|
| Total | 51 (100) | 37 (72.5) | 14 (27.5) | |
| Age(y), median (IQR) | 64 (56.5–75) | 67 (57.5–73.8) | 61 (56–73) | 0.597 |
| Age, categorized | ||||
| ≤60 | 19 (37.3) | 12 (32.4) | 7 (50.0) | 0.251 |
| >60 | 32 (62.7) | 25 (67.6) | 7 (50.0) | |
| BMI(kg/m2), median (IQR) | 28 (24–33) | 29 (25.7–36) | 24 (23–25) | 0.001 |
| Type of Surgery | ||||
| Nodal assessment | 44 (86.3) | 32 (86.5) | 12 (85.7) | 0.470 |
| No nodal assessment | 7 (13.7) | 5 (13.5) | 2 (14.3) | |
| Grade | ||||
| Low Grade (G1–G2) | 40 (78.4) | 37 (100) | 3 (21.4) | <0.001 |
| High Grade (G3) | 11 (21.6) | 0 (0) | 11 (78.6) | |
| Histological Type | ||||
| Endometrioid | 44 (86.3) | 37 (100) | 7 (50) | <0.001 |
| Non endometrioid | 7 (13.7) | 0 (0) | 7 (50) | |
| LVSI | ||||
| Absent | 41 (80.4) | 32 (86.5) | 9 (64.3) | |
| Focal | 2 (3.9) | 0 (0) | 2 (14.3) | 0.042 |
| Substantial | 8 (15.7) | 5 (13.5) | 3 (21.4) | |
| Myometrial Invasion | ||||
| Absent | 2 (3.9) | 2 (5.4) | 0 (0) | 0.379 |
| Present | 49 (96.1) | 35 (94.6) | 14 (100) | |
| Cervical Invasion | ||||
| Absent | 43 (84.3) | 31 (83.8) | 12 (85.7) | 0.867 |
| Present | 8 (15.7) | 6 (16.2) | 2 (14.3) | |
| Vaginal Invasion | ||||
| Absent | 49 (96.1) | 36 (97.3) | 13 (92.8) | 0.470 |
| Present | 2 (3.9) | 1 (2.7) | 1 (7.2) | |
| ER status | ||||
| Positive | 43 (84.3) | 37 (100) | 6 (42.9) | <0.001 |
| Negative | 8 (15.7) | 0 (0) | 8 (57.1) | |
| PR status | ||||
| Positive | 33 (78.6) | 27 (96.4) | 6 (42.9) | |
| Negative | 9 (21.4) | 1 (3.6) | 8 (57.1) | <0.001 |
| Unknown | ||||
| FIGO Stage 2009 | ||||
| IA | 24 (47.0) | 19 (51.4) | 5 (35.7) | |
| IB | 14 (27.5) | 10 (27) | 4 (28.6) | 0.514 |
| II-IV | 13 (25.5) | 8 (21.6) | 5 (35.7) | |
| Risk Class (ESGO/ESTRO/ESP 2020) | ||||
| Low | 20 (39.2) | 18 (48.7) | 2 (14.3) | |
| Intermediate | 9 (17.6) | 9 (24.3) | 0 (0) | |
| Intermediate–High | 10 (19.6) | 6 (16.2) | 4 (28.6) | <0.001 |
| High | 11 (21.6) | 3 (8.1) | 8 (57.1) | |
| Advanced | 1 (2) | 1 (2.7) | 0 (0) | |
| Adjuvant Treatment | ||||
| None | 25 (49.0) | 21 (56.7) | 4 (28.5) | |
| VBRT only | 11 (21.6) | 9 (24.3) | 2 (14.2) | |
| EBRT ± VBRT | 7 (13.7) | 5 (13.5) | 2 (14.2) | 0.010 |
| CTRT ± VBRT | 8 (15.5) | 2 (5.4) | 6 (42.8) | |
| CT only | 0 | 0 | 0 | |
| Survival | ||||
| Recurrence | 4 (7.8) | 0 (0) | 4 (28.6) | <0.001 |
| Died of Disease | 3 (5.9) | 0 (0) | 3 (21.4) | 0.004 |
| Risk Group | Description |
|---|---|
| Low risk | Stage IA (G1–G2) with endometrioid type (dMMR and NSMP) and no or focal LVSI |
| Stage I/II POLEmut cancer; for stage III POLEmut cancers | |
| Intermediate | Stage IA G3 with endometrioid type (dMMR and NSMP) and no or focal LVSI |
| Stage IA non-endometrioid type (serous, clear-cell, undifferentiated carcinoma, carcinosarcoma, mixed) and/or p53-abn cancers without myometrial invasion and no or focal LVSI | |
| Stage IB (G1–G2) with endometrioid type (dMMR and NSMP) and no or focal LVSI | |
| Stage II G1 endometrioid type (dMMR and NSMP) and no or focal LVSI | |
| High-intermediate | High–intermediate risk Stage I endometrioid type (dMMR and NSMP) any grade and any depth of invasion with substantial LVSI |
| Stage IB G3 with endometrioid type (dMMR and NSMP) regardless of LVSI | |
| Stage II G1 endometrioid type (dMMR and NSMP) with substantial LVSI | |
| Stage II G2–G3 endometrioid type (dMMR and NSMP) | |
| High risk | All stages and all histologies with p53-abn and myometrial invasion |
| All stages with serous or undifferentiated carcinoma including carcinosarcoma with myometrial invasion | |
| All stage III and IVA with no residual tumor, regardless of histology and regardless of molecular subtype |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, M.; Spagnol, G.; Vezzaro, T.; Bigardi, S.; De Tommasi, O.; Facchetti, E.; Tripepi, M.; Costeniero, D.; Munerol, C.; Maggino, T.; et al. Low-Risk and High-Risk NSMPs: A Prognostic Subclassification of No Specific Molecular Profile Subtype of Endometrial Carcinomas. Cancers 2024, 16, 3221. https://doi.org/10.3390/cancers16183221
Marchetti M, Spagnol G, Vezzaro T, Bigardi S, De Tommasi O, Facchetti E, Tripepi M, Costeniero D, Munerol C, Maggino T, et al. Low-Risk and High-Risk NSMPs: A Prognostic Subclassification of No Specific Molecular Profile Subtype of Endometrial Carcinomas. Cancers. 2024; 16(18):3221. https://doi.org/10.3390/cancers16183221
Chicago/Turabian StyleMarchetti, Matteo, Giulia Spagnol, Tommaso Vezzaro, Sofia Bigardi, Orazio De Tommasi, Emma Facchetti, Marta Tripepi, Diletta Costeniero, Chiara Munerol, Tiziano Maggino, and et al. 2024. "Low-Risk and High-Risk NSMPs: A Prognostic Subclassification of No Specific Molecular Profile Subtype of Endometrial Carcinomas" Cancers 16, no. 18: 3221. https://doi.org/10.3390/cancers16183221
APA StyleMarchetti, M., Spagnol, G., Vezzaro, T., Bigardi, S., De Tommasi, O., Facchetti, E., Tripepi, M., Costeniero, D., Munerol, C., Maggino, T., D’Antona, D., Tozzi, R., Saccardi, C., & Noventa, M. (2024). Low-Risk and High-Risk NSMPs: A Prognostic Subclassification of No Specific Molecular Profile Subtype of Endometrial Carcinomas. Cancers, 16(18), 3221. https://doi.org/10.3390/cancers16183221








