The 3-Biomarker Classifier—A Novel and Simple Molecular Risk Score Predicting Overall Survival in Patients with Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients and Data Retrieval
2.2. Statistics
- 3-biomarker classifier: high/very high risk vs. low/intermediate risk
- pT-status: T3/4 vs. T1/2
- Grade: G3/4 vs. G1/2
- Nodal status: pN+ vs. pN0
- Localization: right vs. left/transversum
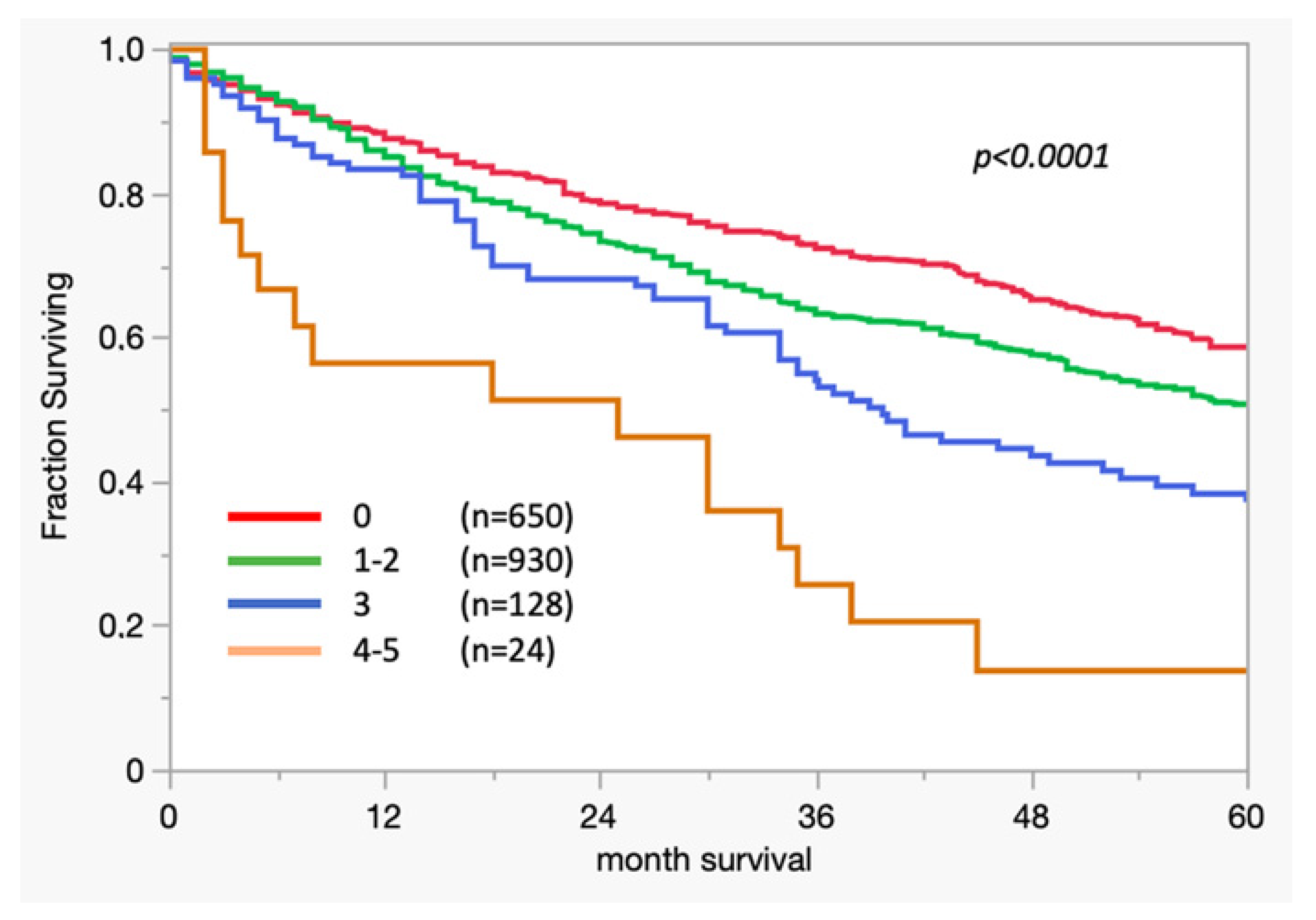
3. Results
3.1. Survival Analysis
3.2. Cox Regression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gill, S.; Loprinzi, C.L.; Sargent, D.J.; Thomé, S.D.; Alberts, S.R.; Haller, D.G.; Benedetti, J.; Francini, G.; Shepherd, L.E.; Francois Seitz, J.; et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J. Clin. Oncol. 2004, 22, 1797–1806. [Google Scholar] [CrossRef]
- Tosi, F.; Magni, E.; Amatu, A.; Mauri, G.; Bencardino, K.; Truini, M.; Veronese, S.; De Carlis, L.; Ferrari, G.; Nichelatti, M.; et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin. Color. Cancer 2017, 16, e153–e163. [Google Scholar] [CrossRef]
- Cunningham, D.; Atkin, W.; Lenz, H.-J.; Lynch, H.T.; Minsky, B.; Nordlinger, B.; Starling, N. Colorectal cancer. Lancet 2010, 375, 1030–1047. [Google Scholar] [CrossRef]
- Hyslop, T.; Waldman, S.A. Molecular staging of node negative patients with colorectal cancer. J. Cancer 2013, 4, 193–199. [Google Scholar] [CrossRef]
- Melling, N.; Simon, R.; Mirlacher, M.; Izbicki, J.R.; Stahl, P.; Terracciano, L.M.; Bokemeyer, C.; Sauter, G.; Marx, A.H. Loss of RNA-binding motif protein 3 expression is associated with right-sided localization and poor prognosis in colorectal cancer. Histopathology 2016, 68, 191–198. [Google Scholar] [CrossRef]
- Melling, N.; Kowitz, C.M.; Simon, R.; Bokemeyer, C.; Terracciano, L.; Sauter, G.; Izbicki, J.R.; Marx, A.H. High Ki67 expression is an independent good prognostic marker in colorectal cancer. J. Clin. Pathol. 2016, 69, 209–214. [Google Scholar] [CrossRef]
- Melling, N.; Grimm, N.; Simon, R.; Stahl, P.; Bokemeyer, C.; Terracciano, L.; Sauter, G.; Izbicki, J.R.; Marx, A.H. Loss of H2Bub1 Expression is Linked to Poor Prognosis in Nodal Negative Colorectal Cancers. Pathol. Oncol. Res. 2016, 22, 95–102. [Google Scholar] [CrossRef]
- Marsh, D.J.; Ma, Y.; Dickson, K.A. Histone Monoubiquitination in Chromatin Remodelling: Focus on the Histone H2B Interactome and Cancer. Cancers 2020, 12, 3462. [Google Scholar] [CrossRef]
- Sadeghi, L.; Siggens, L.; Svensson, J.P.; Ekwall, K. Centromeric histone H2B monoubiquitination promotes noncoding transcription and chromatin integrity. Nat. Struct. Mol. Biol. 2014, 21, 236–243. [Google Scholar] [CrossRef]
- Jonsson, L.; Hedner, C.; Gaber, A.; Korkocic, D.; Nodin, B.; Uhlén, M.; Eberhard, J.; Jirström, K. High expression of RNA-binding motif protein 3 in esophageal and gastric adenocarcinoma correlates with intestinal metaplasia-associated tumours and independently predicts a reduced risk of recurrence and death. Biomark. Res. 2014, 2, 11. [Google Scholar] [CrossRef]
- Jonsson, L.; Bergman, J.; Nodin, B.; Manjer, J.; Pontén, F.; Uhlén, M.; Jirström, K. Low RBM3 protein expression correlates with tumour progression and poor prognosis in malignant melanoma: An analysis of 215 cases from the Malmo Diet and Cancer Study. J. Transl. Med. 2011, 9, 114. [Google Scholar] [CrossRef]
- Jögi, A.; Brennan, D.J.; Rydén, L.; Magnusson, K.; Fernö, M.; Stål, O.; Borgquist, S.; Uhlen, M.; Landberg, G.; Påhlman, S.; et al. Nuclear expression of the RNA-binding protein RBM3 is associated with an improved clinical outcome in breast cancer. Mod. Pathol. 2009, 22, 1564–1574. [Google Scholar] [CrossRef]
- Siesing, C.; Sorbye, H.; Dragomir, A.; Pfeiffer, P.; Qvortrup, C.; Pontén, F.; Jirström, K.; Glimelius, B.; Eberhard, J. High RBM3 expression is associated with an improved survival and oxaliplatin response in patients with metastatic colorectal cancer. PLoS ONE 2017, 12, e0182512. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Bubendorf, L.; Tapia, C.; Gasser, T.C.; Casella, R.; Grunder, B.; Moch, H.; Mihatsch, M.J.; Sauter, G. Ki67 labeling index in core needle biopsies independently predicts tumor-specific survival in prostate cancer. Hum. Pathol. 1998, 29, 949–954. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Jiang, F.; Wang, L.; Xiao, Q.; Han, F.; Chen, J.; Yuan, S.; Wei, J.; Larsson, S.C.; et al. Identification of novel protein biomarkers and drug targets for colorectal cancer by integrating human plasma proteome with genome. Genome Med. 2023, 15, 75. [Google Scholar] [CrossRef]
- Guan, B.; Xu, M.; Zheng, R.; Guan, G.; Xu, B. Novel biomarkers to predict treatment response and prognosis in locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. BMC Cancer 2023, 23, 1099. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Grady, W.M.; Markowitz, S.D.; Nielsen, H.J.; Batra, S.K.; Lampe, P.D. Biomarkers for Early Detection of Colorectal Cancer: The Early Detection Research Network, a Framework for Clinical Translation. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, J.; Yang, W.; Ye, W.C. CircRNAs in colorectal cancer: Potential biomarkers and therapeutic targets. Cell Death Dis. 2023, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Waldman, S.A.; Hyslop, T.; Schulz, S.; Barkun, A.; Nielsen, K.; Haaf, J.; Bonaccorso, C.; Li, Y.; Weinberg, D.S. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA 2009, 301, 745–752. [Google Scholar] [CrossRef]
- Mejia, A.; Schulz, S.; Hyslop, T.; Weinberg, D.S.; Waldman, S.A. Molecular staging estimates occult tumor burden in colorectal cancer. Adv. Clin. Chem. 2010, 52, 19–39. [Google Scholar]
- Wang, S.; Guan, X.; Ma, M.; Zhuang, M.; Ma, T.; Liu, Z.; Chen, H.; Jiang, Z.; Chen, Y.; Wang, G.; et al. Reconsidering the prognostic significance of tumour deposit count in the TNM staging system for colorectal cancer. Sci. Rep. 2020, 10, 89. [Google Scholar] [CrossRef]
- Ahluwalia, P.; Mondal, A.K.; Bloomer, C.; Fulzele, S.; Jones, K.; Ananth, S.; Gahlay, G.K.; Heneidi, S.; Rojiani, A.M.; Kota, V.; et al. Identification and Clinical Validation of a Novel 4 Gene-Signature with Prognostic Utility in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 3818. [Google Scholar] [CrossRef]
- Gao, G.; Shi, X.; Long, Y.; Yao, Z.; Shen, J.; Shen, L. The prognostic and clinicopathological significance of RBM3 in the survival of patients with tumor: A Prisma-compliant meta-analysis. Medicine 2020, 99, e20002. [Google Scholar] [CrossRef]
- Sugai, T.; Yamada, N.; Osakabe, M.; Hashimoto, M.; Uesugi, N.; Eizuka, M.; Tanaka, Y.; Sugimoto, R.; Yanagawa, N.; Matsumoto, T. Microenvironmental markers are correlated with lymph node metastasis in invasive submucosal colorectal cancer. Histopathology 2021, 79, 584–598. [Google Scholar] [CrossRef]
- Tarcic, O.; Pateras, I.S.; Cooks, T.; Shema, E.; Kanterman, J.; Ashkenazi, H.; Boocholez, H.; Hubert, A.; Rotkopf, R.; Baniyash, M.; et al. RNF20 Links Histone H2B Ubiquitylation with Inflammation and Inflammation-Associated Cancer. Cell Rep. 2016, 14, 1462–1476. [Google Scholar] [CrossRef]
- Mahar, A.L.; Compton, C.; Halabi, S.; Hess, K.R.; Weiser, M.R.; Groome, P.A. Personalizing prognosis in colorectal cancer: A systematic review of the quality and nature of clinical prognostic tools for survival outcomes. J. Surg. Oncol. 2017, 116, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef] [PubMed]

| Clinical/Pathological Features | n Available | |
|---|---|---|
| Gender | Female | 898 |
| Male | 893 | |
| Age | Mean: 69 (29–96) | |
| Tumor grade | G1 | 33 |
| G2 | 1497 | |
| G3 | 242 | |
| Tumor stage | pT1 | 80 |
| pT2 | 283 | |
| pT3 | 1143 | |
| pT4 | 269 | |
| Nodal status | pN0 | 909 |
| pN1 | 448 | |
| pN2 | 392 | |
| Tumor type | Tubular carcinoma | 1264 |
| Mucinous carcinoma | 119 | |
| Others | 22 | |
| Localization | Right colon | 355 |
| Transverse colon | 134 | |
| Left colon | 418 | |
| Rectum | 482 | |
| Total number of patients (n evaluable) | 1791 | |
| 3-Biomarker Classifier Risk | ||||||
|---|---|---|---|---|---|---|
| Parameter | n Evaluable | Low (%) | Intermediate (%) | High (%) | Very High (%) | p Value |
| All cancers | 2212 | 993 (44.9) | 1061 (48.0) | 130 (5.9) | 28 (1.2) | |
| Tumor stage | <0.0001 | |||||
| pT1 | 80 | 47 (58.8) | 31 (38.8) | 2 (2.5) | 0 (0.0) | |
| pT2 | 283 | 126 (44.5) | 141 (49.8) | 16 (5.7) | 0 (0.0) | |
| pT3 | 1143 | 428 (37.4) | 623 (54.5) | 81 (7.1) | 11 (1.0) | |
| pT4 | 269 | 74 (27.5) | 154 (57.2) | 28 (10.4) | 13 (4.8) | |
| Lymph node metastasis | 0.2559 | |||||
| pN0 | 909 | 353 (38.8) | 492 (54.1) | 58 (6.4) | 6 (0.7) | |
| pN1 | 448 | 168 (37.5) | 237 (52.9) | 35 (7.8) | 8 (1.8) | |
| pN2 | 392 | 142 (36.2) | 208 (53.1) | 32 (8.2) | 10 (2.6) | |
| Grading | 0.0065 | |||||
| G1 | 33 | 20 (60.6) | 13 (39.4) | 0 (0.0) | 0 (0.0) | |
| G2 | 1497 | 550 (36.7) | 821 (54.8) | 109 (7.3) | 16 (1.1) | |
| G3 | 242 | 105 (43.4) | 114 (47.1) | 17 (7.0) | 6 (2.5) | |
| Tumor localization | 0.2130 | |||||
| Right | 355 | 132 (37.2) | 184 (51.8) | 29 (8.2) | 10 (2.8) | |
| Transverse | 134 | 42 (31.3) | 72 (53.7) | 16 (11.9) | 4 (3.0) | |
| Left | 418 | 148 (35.4) | 240 (57.4) | 25 (6.0) | 5 (1.2) | |
| Rectum | 482 | 173 (35.9) | 267 (55.4) | 37 (7.7) | 5 (1.0) | |
| Histological type | 0.1805 | |||||
| Adenocarcinoma | 1264 | 442 (35.0) | 704 (55.7) | 96 (7.6) | 21 (1.7) | |
| Mucinous | 119 | 53 (44.5) | 58 (48.7) | 8 (6.7) | 0 (0.0) | |
| Others | 22 | 9 (40.9) | 10 (45.5) | 2 (9.1) | 1 (4.5) | |
| Peritumoral lymphocytes | 0.1453 | |||||
| Absent | 787 | 297 (37.7) | 427 (54.3) | 52 (6.6) | 11 (1.4) | |
| Present | 602 | 198 (32.9) | 339 (56.3) | 54 (9.0) | 11 (1.8) | |
| Vascular invasion | 0.0022 | |||||
| No | 783 | 299 (38.2) | 428 (54.7) | 50 (6.4) | 6 (0.8) | |
| Yes | 605 | 196 (32.4) | 337 (55.7) | 56 (9.3) | 16 (2.6) | |
| 3-Biomarker-Classifier | Tumor Status | Grading | Nodal Status | Tumor Localization | |
|---|---|---|---|---|---|
| High/Very High Risk vs. Low/Intermediate Risk | T3/4 vs. T1/2 | G3/4 vs. G1/2 | pN+ vs. pN0 | Right vs. Left/Transversum | |
| Hazard Ratio | 1.45 | 2.23 | 1.32 | 2.49 | 1.00 |
| p-value | 0.0016 | <0.001 | 0.0126 | <0.001 | 0.9618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melling, N.; Fard-Aghaie, M.H.; Hube-Magg, C.; Kluth, M.; Simon, R.; Tachezy, M.; Ghadban, T.; Reeh, M.; Izbicki, J.R.; Sauter, G.; et al. The 3-Biomarker Classifier—A Novel and Simple Molecular Risk Score Predicting Overall Survival in Patients with Colorectal Cancer. Cancers 2024, 16, 3223. https://doi.org/10.3390/cancers16183223
Melling N, Fard-Aghaie MH, Hube-Magg C, Kluth M, Simon R, Tachezy M, Ghadban T, Reeh M, Izbicki JR, Sauter G, et al. The 3-Biomarker Classifier—A Novel and Simple Molecular Risk Score Predicting Overall Survival in Patients with Colorectal Cancer. Cancers. 2024; 16(18):3223. https://doi.org/10.3390/cancers16183223
Chicago/Turabian StyleMelling, Nathaniel, Mohammad H. Fard-Aghaie, Claudia Hube-Magg, Martina Kluth, Ronald Simon, Michael Tachezy, Tarik Ghadban, Matthias Reeh, Jakob R. Izbicki, Guido Sauter, and et al. 2024. "The 3-Biomarker Classifier—A Novel and Simple Molecular Risk Score Predicting Overall Survival in Patients with Colorectal Cancer" Cancers 16, no. 18: 3223. https://doi.org/10.3390/cancers16183223
APA StyleMelling, N., Fard-Aghaie, M. H., Hube-Magg, C., Kluth, M., Simon, R., Tachezy, M., Ghadban, T., Reeh, M., Izbicki, J. R., Sauter, G., & Grupp, K. (2024). The 3-Biomarker Classifier—A Novel and Simple Molecular Risk Score Predicting Overall Survival in Patients with Colorectal Cancer. Cancers, 16(18), 3223. https://doi.org/10.3390/cancers16183223







