The Potential of Human Pulmonary Mesenchymal Stem Cells as Vectors for Radiosensitizing Metallic Nanoparticles: An In Vitro Study †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe3O4@Au Nanoparticles
2.2. Cell Culture
2.3. Effect of Fe3O4@Au Nanoparticles on the Viability of HPMSCs
2.4. Measurement of the Cytotoxic Effect of Fe3O4@Au Nanoparticles
2.5. Cellular Uptake of Fe3O4@Au Nanoparticles
2.6. Measurement of the Expression of Genes by RT-qPCR Analysis (SYBR Green)
2.7. Measurement of ROS Generation
2.8. Quantification of Released Cytokines, Chemokines, and Growth Factor
2.9. Statistical Analysis
3. Results
3.1. Effect of Fe3O4@Au Nanoparticles on the Cell Viability of HPMSCs
3.2. Uptake of Fe3O4@Au Nanoparticles by HPMSCs
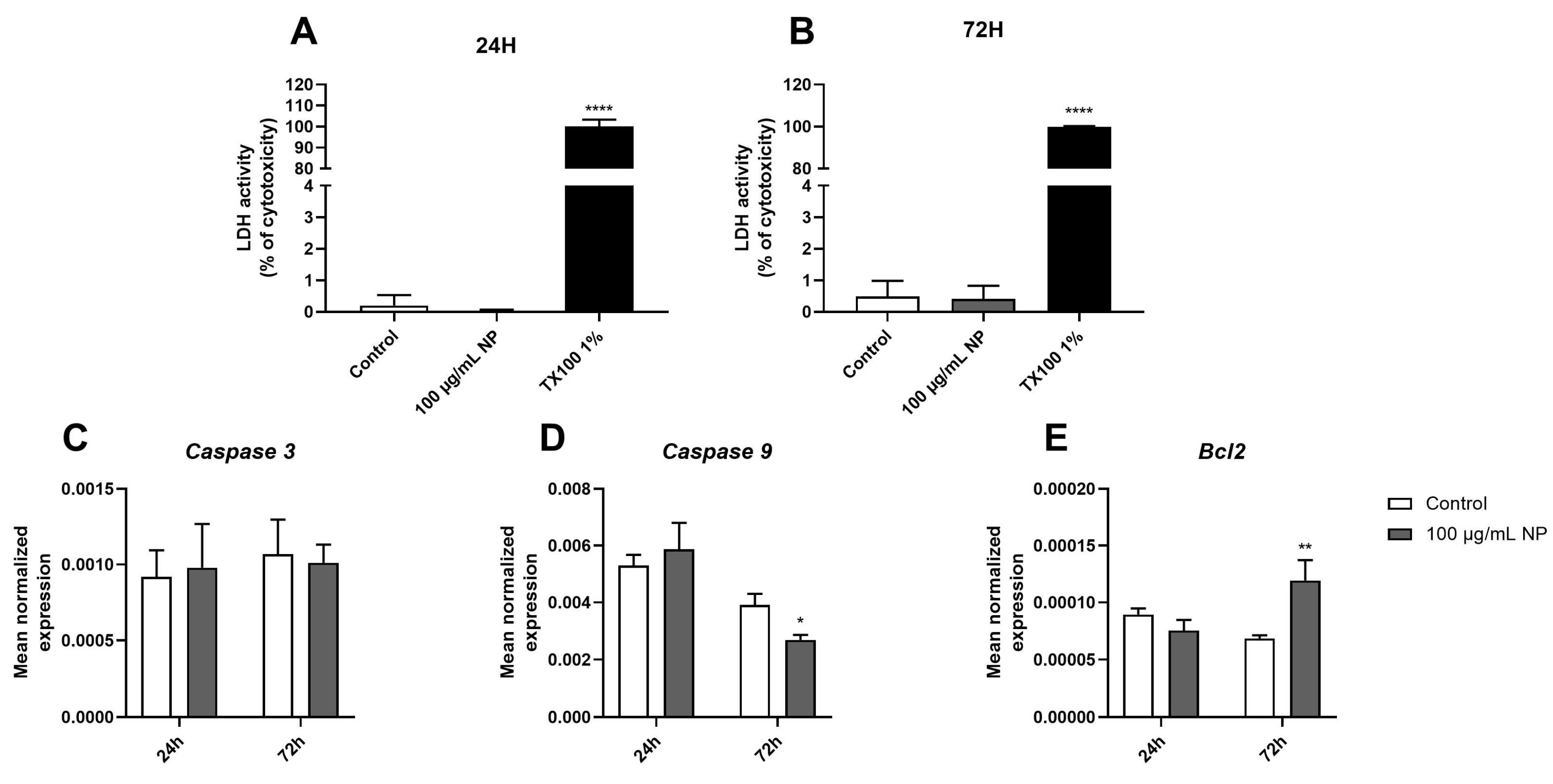
3.3. Effect of Fe3O4@Au Nanoparticles on HPMSC Apoptosis and Necrosis
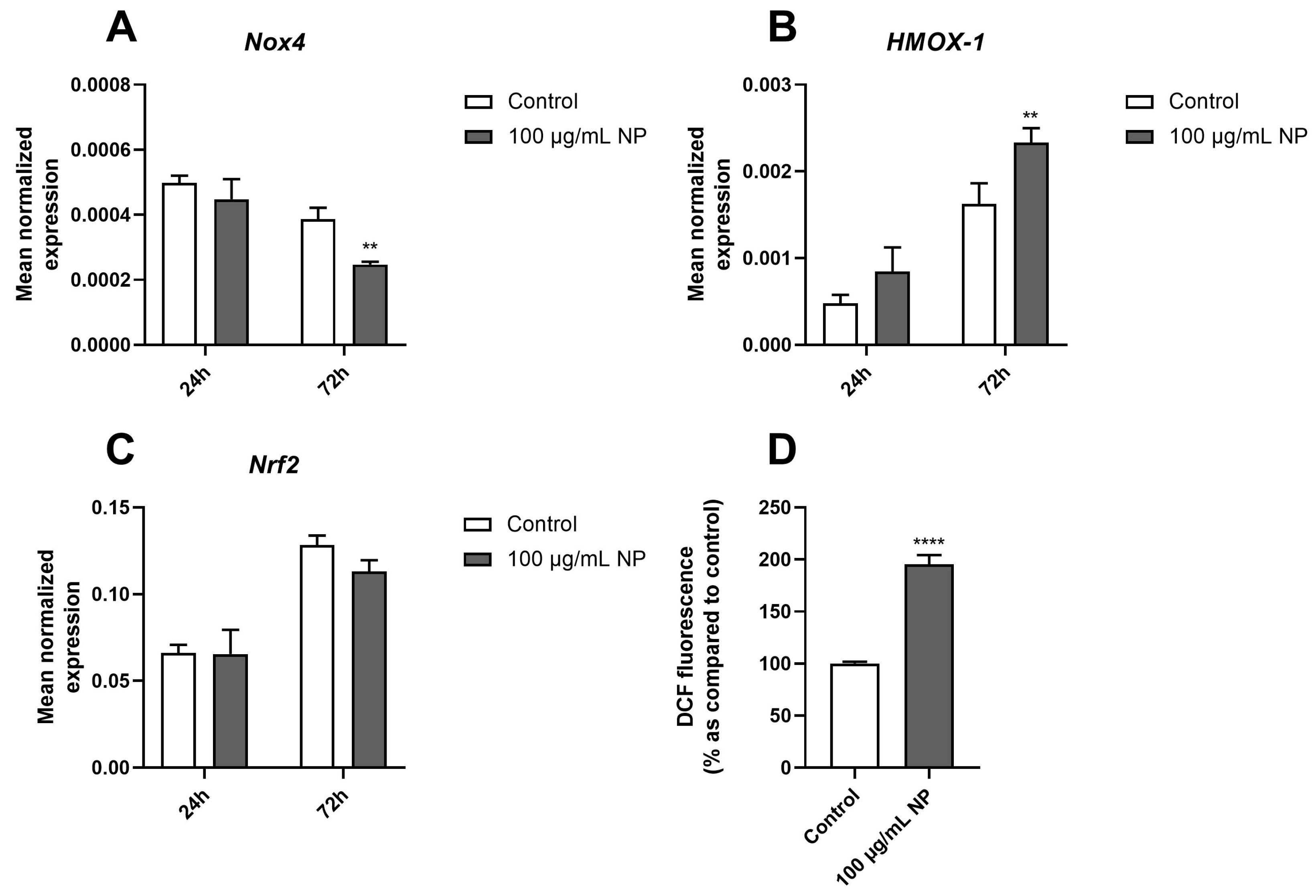
3.4. Effect of Fe3O4@Au Nanoparticles on the Redox Status of HPMSCs
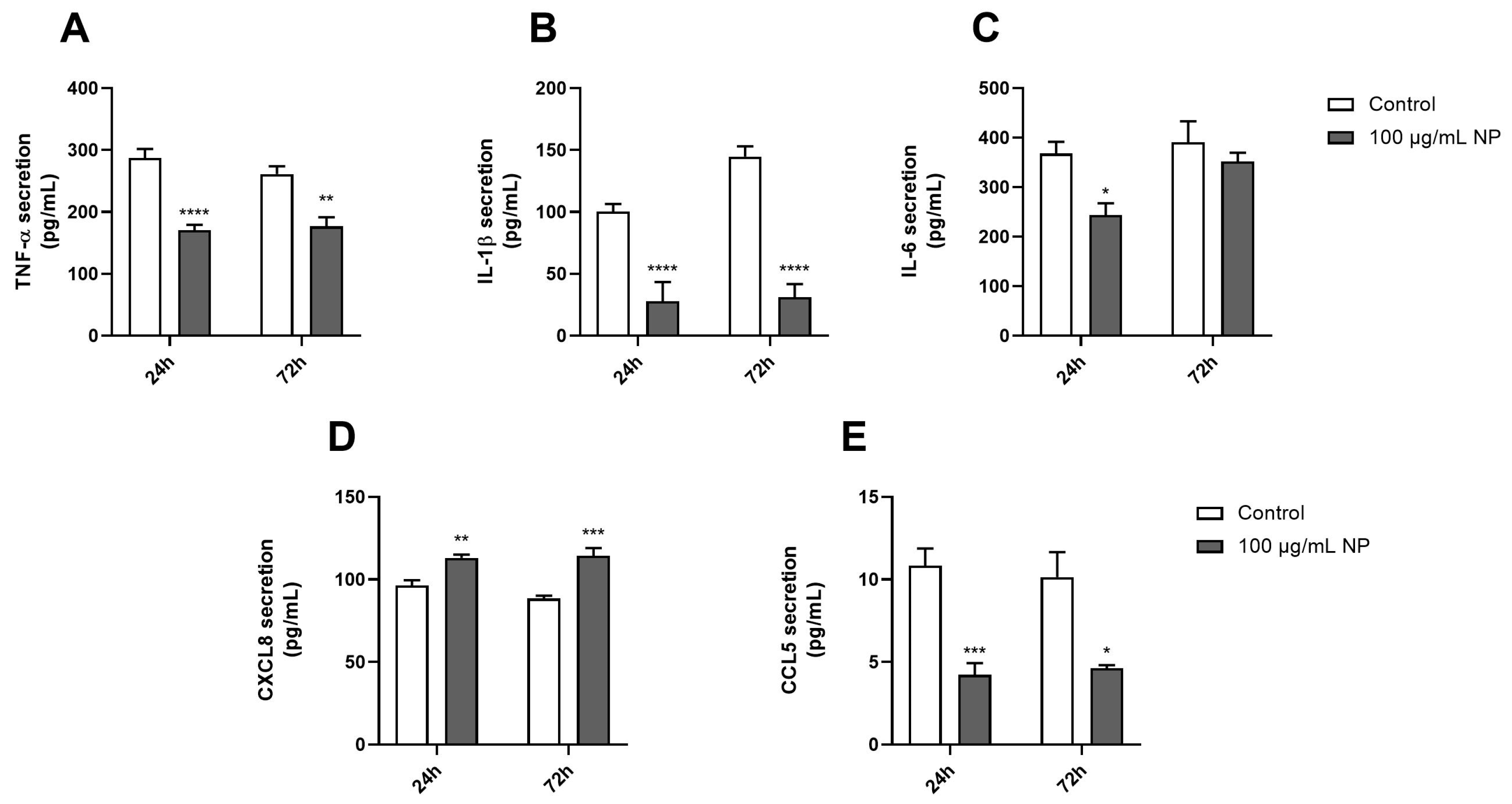
3.5. Effect of Fe3O4@Au Nanoparticles on the Proinflammatory Response of HPMSCs
3.6. Effect of Fe3O4@Au Nanoparticles on the Expression and Production of Protumorigenic Markers on HPMSCs
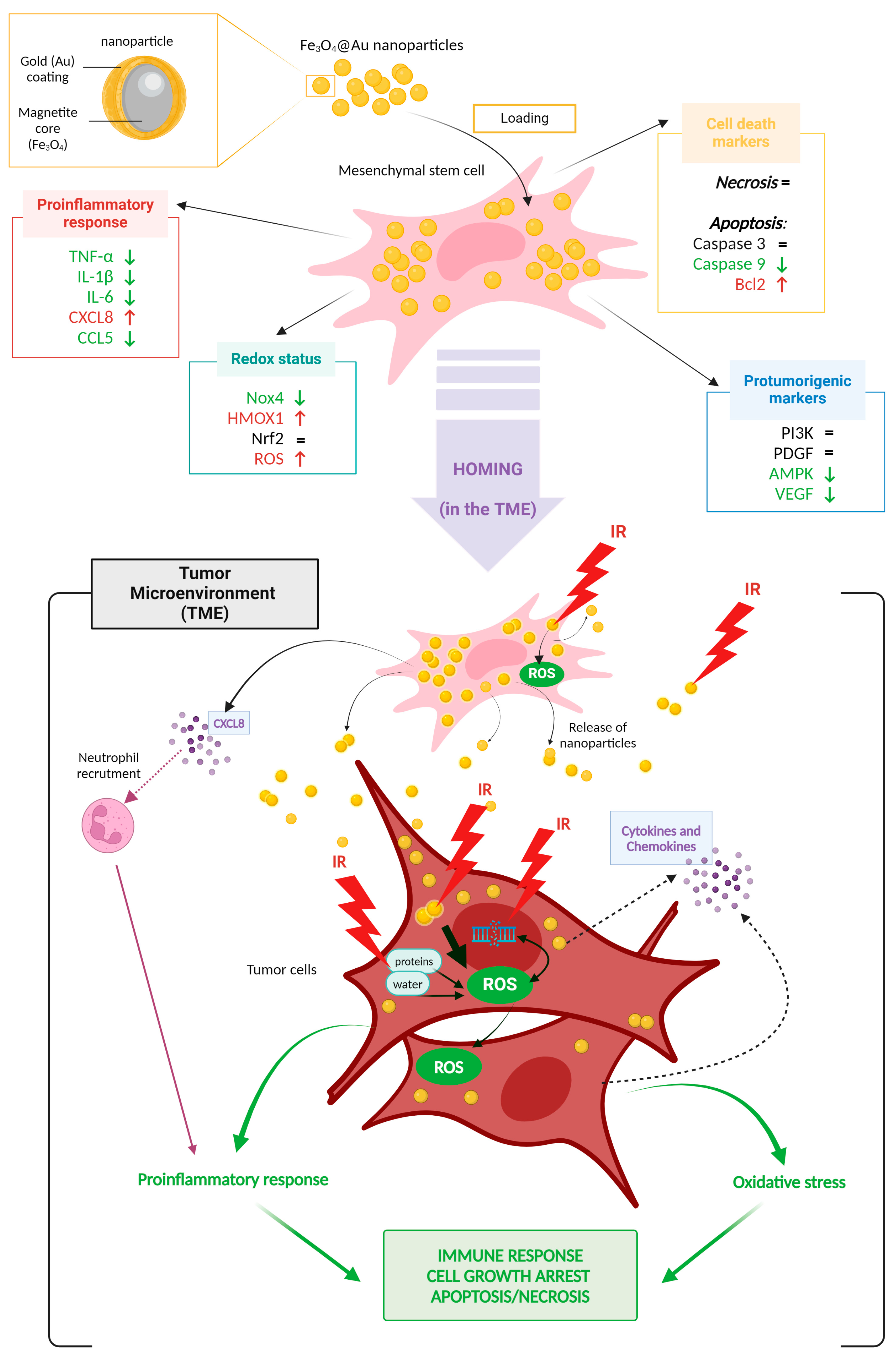
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.A.; Martin, R.F. Cancer Radiotherapy: Understanding the Price of Tumor Eradication. Front. Cell Dev. Biol. 2020, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, H.; Dong, Y.; Luo, X.; Yu, H.; Moore, Z.; Bey, E.A.; Boothman, D.A.; Gao, J. Superparamagnetic Iron Oxide Nanoparticles: Amplifying ROS Stress to Improve Anticancer Drug Efficacy. Theranostics 2013, 3, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of Reactive Oxygen Species: An Emerging Approach for Cancer Therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Brandmaier, A.; Formenti, S.C. The Impact of Radiation Therapy on Innate and Adaptive Tumor Immunity. Semin. Radiat. Oncol. 2020, 30, 139–144. [Google Scholar] [CrossRef]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of Cytokines/Chemokines and Cell Death in UVB-Irradiated Human Keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef]
- Mirjolet, C.; Diallo, I.; Bertaut, A.; Veres, C.; Sargos, P.; Helfre, S.; Sunyach, M.-P.; Truc, G.; Le Pechoux, C.; Paumier, A.; et al. Treatment Related Factors Associated with the Risk of Breast Radio-Induced-Sarcoma. Radiother. Oncol. 2022, 171, 14–21. [Google Scholar] [CrossRef]
- Cohen-Jonathan-Moyal, É.; Vendrely, V.; Motte, L.; Balosso, J.; Thariat, J. Radioresistant Tumours: From Identification to Targeting. Cancer/Radiothérapie 2020, 24, 699–705. [Google Scholar] [CrossRef]
- Movsas, B.; Hu, C.; Sloan, J.; Bradley, J.; Komaki, R.; Masters, G.; Kavadi, V.; Narayan, S.; Michalski, J.; Johnson, D.W.; et al. Quality of Life Analysis of a Radiation Dose–Escalation Study of Patients with Non–Small-Cell Lung Cancer: A Secondary Analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol. 2016, 2, 359. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy Toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Araghi, M.; Mannani, R.; Heidarnejad maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent Advances in Non-Small Cell Lung Cancer Targeted Therapy: An Update Review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Ramroth, J.; Cutter, D.J.; Darby, S.C.; Higgins, G.S.; McGale, P.; Partridge, M.; Taylor, C.W. Dose and Fractionation in Radiation Therapy of Curative Intent for Non-Small Cell Lung Cancer: Meta-Analysis of Randomized Trials. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, Y.; Adday, H.D.; Abdel Samad, F.; Qayyum, H.; Mohamed, T. Using Femtosecond Laser Pulses to Explore the Nonlinear Optical Properties of Ag/Au Alloy Nanoparticles Synthesized by Pulsed Laser Ablation in a Liquid. Nanomaterials 2024, 14, 1290. [Google Scholar] [CrossRef] [PubMed]
- Bonamy, C.; Pesnel, S.; Ben Haddada, M.; Gorgette, O.; Schmitt, C.; Morel, A.-L.; Sauvonnet, N. Impact of Green Gold Nanoparticle Coating on Internalization, Trafficking, and Efficiency for Photothermal Therapy of Skin Cancer. ACS Omega 2023, 8, 4092–4105. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Coulter, J.A.; Hounsell, A.R.; Butterworth, K.T.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Cell-Specific Radiosensitization by Gold Nanoparticles at Megavoltage Radiation Energies. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 531–539. [Google Scholar] [CrossRef]
- Tanaka, T.; Shiramoto, S.; Miyashita, M.; Fujishima, Y.; Kaneo, Y. Tumor Targeting Based on the Effect of Enhanced Permeability and Retention (EPR) and the Mechanism of Receptor-Mediated Endocytosis (RME). Int. J. Pharm. 2004, 277, 39–61. [Google Scholar] [CrossRef]
- Ma, P.; Xiao, H.; Yu, C.; Liu, J.; Cheng, Z.; Song, H.; Zhang, X.; Li, C.; Wang, J.; Gu, Z.; et al. Enhanced Cisplatin Chemotherapy by Iron Oxide Nanocarrier-Mediated Generation of Highly Toxic Reactive Oxygen Species. Nano Lett. 2017, 17, 928–937. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef]
- Slama, Y.; Arcambal, A.; Septembre-Malaterre, A.; Morel, A.-L.; Pesnel, S.; Gasque, P. Evaluation of Core-Shell Fe3O4@Au Nanoparticles as Radioenhancer in A549 Cell Lung Cancer Model. Heliyon 2024, 10, e29297. [Google Scholar] [CrossRef]
- Haque, M.; Shakil, M.S.; Mahmud, K.M. The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers 2023, 15, 1892. [Google Scholar] [CrossRef]
- Könczöl, M.; Weiss, A.; Stangenberg, E.; Gminski, R.; Garcia-Käufer, M.; Gieré, R.; Merfort, I.; Mersch-Sundermann, V. Cell-Cycle Changes and Oxidative Stress Response to Magnetite in A549 Human Lung Cells. Chem. Res. Toxicol. 2013, 26, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, Y.; Fan, Y.; Cao, L.; Li, J.; Ge, Y.; Tu, W.; Liu, Y.; Cao, X.; Shi, X. Dual-Mode Endogenous and Exogenous Sensitization of Tumor Radiotherapy through Antifouling Dendrimer-Entrapped Gold Nanoparticles. Theranostics 2021, 11, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Haddada, M.B.; Koshel, D.; Yang, Z.; Fu, W.; Spadavecchia, J.; Pesnel, S.; Morel, A.-L. Proof of Concept of Plasmonic Thermal Destruction of Surface Cancers by Gold Nanoparticles Obtained by Green Chemistry. Colloids Surf. B Biointerfaces 2019, 184, 110496. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, W.; Wang, T.; Miao, S.; Lan, S.; Wei, Z.; Meng, Z.; Dai, Q.; Fan, H. Highly Localized, Efficient, and Rapid Photothermal Therapy Using Gold Nanobipyramids for Liver Cancer Cells Triggered by Femtosecond Laser. Sci. Rep. 2023, 13, 3372. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.; Mohamed, W.R.; Elhemely, M.A.; El-Gendy, A.O.; Mohamed, T. Tunable Femtosecond Laser Suppresses the Proliferation of Breast Cancer in Vitro. J. Photochem. Photobiol. B 2023, 240, 112665. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Feng, Q.; Yang, H.; Wang, G.; Huang, L.; Bai, Q.; Zhang, C.; Wang, Y.; Chen, Y.; Cheng, Q.; et al. A Light-Triggered Mesenchymal Stem Cell Delivery System for Photoacoustic Imaging and Chemo-Photothermal Therapy of Triple Negative Breast Cancer. Adv. Sci. 2018, 5, 1800382. [Google Scholar] [CrossRef]
- Pawitan, J.A.; Bui, T.A.; Mubarok, W.; Antarianto, R.D.; Nurhayati, R.W.; Dilogo, I.H.; Oceandy, D. Enhancement of the Therapeutic Capacity of Mesenchymal Stem Cells by Genetic Modification: A Systematic Review. Front. Cell Dev. Biol. 2020, 8, 587776. [Google Scholar] [CrossRef]
- Slama, Y.; Ah-Pine, F.; Khettab, M.; Arcambal, A.; Begue, M.; Dutheil, F.; Gasque, P. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 13511. [Google Scholar] [CrossRef]
- Xiao, J.; Zeng, L.; Ding, S.; Chen, Y.; Zhang, X.; Bian, X.; Tian, G. Tumor-Tropic Adipose-Derived Mesenchymal Stromal Cell Mediated Bi2 Se3 Nano-Radiosensitizers Delivery for Targeted Radiotherapy of Non-Small Cell Lung Cancer. Adv. Healthc. Mater. 2022, 11, 2200143. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Zeng, X.; Guo, W.; Jin, Y.; Wang, S.; Tian, R.; Han, Y.; Guo, L.; Han, J.; et al. Efficient Lung Cancer-Targeted Drug Delivery via a Nanoparticle/MSC System. Acta Pharm. Sin. B 2019, 9, 167–176. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using In Vivo Bioluminescence Imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Drela, K.; Rozycka, J.; Janowski, M.; Lukomska, B. Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse. Stem Cells Dev. 2016, 25, 1513–1531. [Google Scholar] [CrossRef] [PubMed]
- Hagenhoff, A.; Bruns, C.J.; Zhao, Y.; von Lüttichau, I.; Niess, H.; Spitzweg, C.; Nelson, P.J. Harnessing Mesenchymal Stem Cell Homing as an Anticancer Therapy. Expert. Opin. Biol. Ther. 2016, 16, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Pavon, L.F.; Sibov, T.T.; de Souza, A.V.; da Cruz, E.F.; Malheiros, S.M.F.; Cabral, F.R.; de Souza, J.G.; Boufleur, P.; de Oliveira, D.M.; de Toledo, S.R.C.; et al. Tropism of Mesenchymal Stem Cell toward CD133+ Stem Cell of Glioblastoma in Vitro and Promote Tumor Proliferation in Vivo. Stem Cell Res. Ther. 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.-L.; Ben Haddada, M.; Nikitenko, S.I.; Chave, T. Synthèse Assistée par Ultrasons de Nanoparticules Constituées d’un Cœur Ferrique Recouvert d’Or. Patent. FR3131850A1, 21 July 2023. [Google Scholar]
- Khoei, S.; Mahdavi, S.R.; Fakhimikabir, H.; Shakeri-Zadeh, A.; Hashemian, A. The Role of Iron Oxide Nanoparticles in the Radiosensitization of Human Prostate Carcinoma Cell Line DU145 at Megavoltage Radiation Energies. Int. J. Radiat. Biol. 2014, 90, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in Magnetic Resonance Imaging: From Simple to Dual Contrast Agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef]
- Moustaoui, H.; Movia, D.; Dupont, N.; Bouchemal, N.; Casale, S.; Djaker, N.; Savarin, P.; Prina-Mello, A.; de la Chapelle, M.L.; Spadavecchia, J. Tunable Design of Gold(III)–Doxorubicin Complex–PEGylated Nanocarrier. The Golden Doxorubicin for Oncological Applications. ACS Appl. Mater. Interfaces 2016, 8, 19946–19957. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin Reductase: A Target for Gold Compounds Acting as Potential Anticancer Drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Rhee, K.-J.; Lee, J.; Eom, Y. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033. [Google Scholar] [CrossRef]
- Szewc, M.; Radzikowska-Bűchner, E.; Wdowiak, P.; Kozak, J.; Kuszta, P.; Niezabitowska, E.; Matysiak, J.; Kubiński, K.; Masłyk, M. MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents—A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives. Int. J. Mol. Sci. 2022, 23, 2745. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Sun, Z.; Wu, Y.; You, W.; Mao, Z.; Wang, W. Mesenchymal Stem Cells Engineered by Nonviral Vectors: A Powerful Tool in Cancer Gene Therapy. Pharmaceutics 2021, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, M.; Yang, Z.; Zeng, C.-Y.; Chen, J.; Xie, Y.; Luo, S.-W.; Zhang, K.-H.; Zhou, S.-F.; Lu, N.-H. Mesenchymal Stem Cell-Based NK4 Gene Therapy in Nude Mice Bearing Gastric Cancer Xenografts. Drug Des. Devel Ther. 2014, 8, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Ruano, D.; López-Martín, J.A.; Moreno, L.; Lassaletta, Á.; Bautista, F.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020, 28, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Nethi, S.K.; Rathi, S.; Layek, B.; Prabha, S. Engineered Mesenchymal Stem Cells for Targeting Solid Tumors: Therapeutic Potential beyond Regenerative Therapy. J. Pharmacol. Exp. Ther. 2019, 370, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Allabun, S.; Ojo, S.; Alqahtani, M.S.; Shukla, P.K.; Abbas, M.; Wechtaisong, C.; Almohiy, H.M. Enhanced Drug Delivery System Using Mesenchymal Stem Cells and Membrane-Coated Nanoparticles. Molecules 2023, 28, 2130. [Google Scholar] [CrossRef]
- Accomasso, L.; Gallina, C.; Turinetto, V.; Giachino, C. Stem Cell Tracking with Nanoparticles for Regenerative Medicine Purposes: An Overview. Stem Cells Int. 2015, 2016, e7920358. [Google Scholar] [CrossRef]
- Schäfer, R.; Kehlbach, R.; Wiskirchen, J.; Bantleon, R.; Pintaske, J.; Brehm, B.R.; Gerber, A.; Wolburg, H.; Claussen, C.D.; Northoff, H. Transferrin Receptor Upregulation: In Vitro Labeling of Rat Mesenchymal Stem Cells with Superparamagnetic Iron Oxide. Radiology 2007, 244, 514–523. [Google Scholar] [CrossRef]
- Mader, E.K.; Butler, G.; Dowdy, S.C.; Mariani, A.; Knutson, K.L.; Federspiel, M.J.; Russell, S.J.; Galanis, E.; Dietz, A.B.; Peng, K.-W. Optimizing Patient Derived Mesenchymal Stem Cells as Virus Carriers for a Phase I Clinical Trial in Ovarian Cancer. J. Transl. Med. 2013, 11, 20. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.-H. A Green Chemistry Approach for Synthesizing Biocompatible Gold Nanoparticles. Nanoscale Res. Lett. 2014, 9, 248. [Google Scholar] [CrossRef]
- Radomska, A.; Leszczyszyn, J.; Radomski, M.W. The Nanopharmacology and Nanotoxicology of Nanomaterials: New Opportunities and Challenges. Adv. Clin. Exp. Med. 2016, 25, 151–162. [Google Scholar] [CrossRef]
- Shiji, R.; Joseph, M.M.; Unnikrishnan, B.S.; Preethi, G.U.; Sreelekha, T.T. Fluorescent Gold Nanoclusters as a Powerful Tool for Sensing Applications in Cancer Management. In Advances in Biomaterials for Biomedical Applications; Tripathi, A., Melo, J.S., Eds.; Advanced Structured Materials; Springer: Singapore, 2017; Volume 66, pp. 385–428. ISBN 978-981-10-3327-8. [Google Scholar]
- Rajkumar, S.; Prabaharan, M. Chapter 29—Theranostic Application of Fe3O4–Au Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Micro and Nano Technologies; Woodhead Publishing: Sawston, UK, 2019; pp. 607–623. ISBN 978-0-12-814134-2. [Google Scholar]
- Watanabe, M.; Yoneda, M.; Morohashi, A.; Hori, Y.; Okamoto, D.; Sato, A.; Kurioka, D.; Nittami, T.; Hirokawa, Y.; Shiraishi, T.; et al. Effects of Fe3O4 Magnetic Nanoparticles on A549 Cells. Int. J. Mol. Sci. 2013, 14, 15546–15560. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver Nanoparticles: Evaluation of DNA Damage, Toxicity and Functional Impairment in Human Mesenchymal Stem Cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, X.; Kienzle, A.; Müller, W.E.G.; Feng, Q. In Vitro Uptake of Silver Nanoparticles and Their Toxicity in Human Mesenchymal Stem Cells Derived from Bone Marrow. J. Nanosci. Nanotechnol. 2016, 16, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Sreekanth, P.J.; Varma, H.K.; Mohanan, P.V. Zinc Oxide Nanoparticles Induced Oxidative Stress in Mouse Bone Marrow Mesenchymal Stem Cells. Toxicol. Mech. Methods 2014, 24, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, L.; Cao, G. Acute Toxicity Test of CuO Nanoparticles Using Human Mesenchymal Stem Cells. Toxicol. Mech. Methods 2014, 24, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.C.; Paciotti, G.F.; Libutti, S.K. Colloidal Gold: A Novel Nanoparticle for Targeted Cancer Therapeutics. In Cancer Nanotechnology: Methods and Protocols; Grobmyer, S.R., Moudgil, B.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 375–384. ISBN 978-1-60761-609-2. [Google Scholar]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Shameli, K.; Miyake, M.; Teow, S.-Y.; Peh, S.-C.; Mohamad, S.E.; Mohd Taib, S.H. Green Fabrication of Biologically Active Magnetic Core-Shell Fe3O4/Au Nanoparticles and Their Potential Anticancer Effect. Mater. Sci. Eng. C 2019, 96, 51–57. [Google Scholar] [CrossRef]
- Zanella, D.; Bossi, E.; Gornati, R.; Bastos, C.; Faria, N.; Bernardini, G. Iron Oxide Nanoparticles Can Cross Plasma Membranes. Sci. Rep. 2017, 7, 11413. [Google Scholar] [CrossRef]
- Babaye Abdollahi, B.; Ghorbani, M.; Hamishehkar, H.; Malekzadeh, R.; Farajollahi, A. Synthesis and Characterization of Actively HER-2 Targeted Fe3O4@Au Nanoparticles for Molecular Radiosensitization of Breast Cancer. Bioimpacts 2023, 13, 17–29. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, M.; Wu, J.; Li, C.; Shen, D.; Yang, D.; Li, L.; Ge, M.; Chang, Z.; Dong, W. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. Uptake and Intracellular Distribution of Silver Nanoparticles in Human Mesenchymal Stem Cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Yi, J.; Chung, K.-H.; Ryu, D.-Y.; Choi, J.; Park, K. Oxidative Stress and Apoptosis Induced by Titanium Dioxide Nanoparticles in Cultured BEAS-2B Cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Enea, M.; Pereira, E.; Peixoto de Almeida, M.; Araújo, A.M.; Bastos, M.d.L.; Carmo, H. Gold Nanoparticles Induce Oxidative Stress and Apoptosis in Human Kidney Cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, L.; Tang, H.; He, X.; Ye, G.; Shi, F.; Kang, M.; Chen, H.; Li, Y. Silver Nanoparticles Induced Oxidative Stress and Mitochondrial Injuries Mediated Autophagy in HC11 Cells Through Akt/AMPK/mTOR Pathway. Biol. Trace Elem. Res. 2021, 199, 1062–1073. [Google Scholar] [CrossRef]
- Yu, S.; Mu, Y.; Zhang, X.; Li, J.; Lee, C.; Wang, H. Molecular Mechanisms Underlying Titanium Dioxide Nanoparticles (TiO2NP) Induced Autophagy in Mesenchymal Stem Cells (MSC). J. Toxicol. Environ. Health Part A 2019, 82, 997–1008. [Google Scholar] [CrossRef]
- He, W.; Zheng, Y.; Feng, Q.; Elkhooly, T.A.; Liu, X.; Yang, X.; Wang, Y.; Xie, Y. Silver Nanoparticles Stimulate Osteogenesis of Human Mesenchymal Stem Cells through Activation of Autophagy. Nanomedicine 2020, 15, 337–353. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and Their Substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Domen, J.; Cheshier, S.H.; Weissman, I.L. The Role of Apoptosis in the Regulation of Hematopoietic Stem Cells. J. Exp. Med. 2000, 191, 253–264. [Google Scholar] [CrossRef]
- Song, W.-J.; Jeong, M.-S.; Choi, D.-M.; Kim, K.-N.; Wie, M.-B. Zinc Oxide Nanoparticles Induce Autophagy and Apoptosis via Oxidative Injury and Pro-Inflammatory Cytokines in Primary Astrocyte Cultures. Nanomaterials 2019, 9, 1043. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Vowden, R.; Hughes, L.; Qi, D.; Francis, W.; Perino, G.; Pink, R.; Xiao, J.; Li, B.; et al. Combination of Cobalt, Chromium and Titanium Nanoparticles Increases Cytotoxicity in Vitro and pro-Inflammatory Cytokines in Vivo. J. Orthop. Transl. 2023, 38, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Pal, R.; Ali, M.; Singh, L.M.; Shahidur Rahman, D.; Kumar Ghosh, S.; Sengupta, M. Immunomodulatory Properties of Silver Nanoparticles Contribute to Anticancer Strategy for Murine Fibrosarcoma. Cell Mol. Immunol. 2016, 13, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Echalar, B.; Dostalova, D.; Palacka, K.; Javorkova, E.; Hermankova, B.; Cervena, T.; Zajicova, A.; Holan, V.; Rossner, P. Effects of Antimicrobial Metal Nanoparticles on Characteristics and Function Properties of Mouse Mesenchymal Stem Cells. Toxicol. Vitr. 2023, 87, 105536. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Bhagat, S.; Paul, S.; Katz, J.P.; Sengupta, D.; Bhargava, D. Neutrophils in Cancer and Potential Therapeutic Strategies Using Neutrophil-Derived Exosomes. Vaccines 2023, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, D.P.; Correia, B.F.; Salvador, R.; de Sousa, N.; Jacinto, A.; Braga, S.; Cabral, M.G. Circulating Low Density Neutrophils of Breast Cancer Patients Are Associated with Their Worse Prognosis Due to the Impairment of T Cell Responses. Oncotarget 2021, 12, 2388–2403. [Google Scholar] [CrossRef]
- Bartneck, M.; Keul, H.A.; Zwadlo-Klarwasser, G.; Groll, J. Phagocytosis Independent Extracellular Nanoparticle Clearance by Human Immune Cells. Nano Lett. 2010, 10, 59–63. [Google Scholar] [CrossRef]
- Yang, H.; Marion, T.N.; Liu, Y.; Zhang, L.; Cao, X.; Hu, H.; Zhao, Y.; Herrmann, M. Nanomaterial Exposure Induced Neutrophil Extracellular Traps: A New Target in Inflammation and Innate Immunity. J. Immunol. Res. 2019, 2019, 3560180. [Google Scholar] [CrossRef]
- Ouchi, N.; Shibata, R.; Walsh, K. AMP-Activated Protein Kinase Signaling Stimulates VEGF Expression and Angiogenesis in Skeletal Muscle. Circ. Res. 2005, 96, 838–846. [Google Scholar] [CrossRef]
- Reihill, J.A.; Ewart, M.-A.; Hardie, D.G.; Salt, I.P. AMP-Activated Protein Kinase Mediates VEGF-Stimulated Endothelial NO Production. Biochem. Biophys. Res. Commun. 2007, 354, 1084–1088. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Y.; Liu, Q.; He, J.; Xiang, P.; Wang, D.; Hu, X.; Chen, J.; Zhu, W.; Yu, H. Growth Differentiation Factor 11 Promotes Differentiation of MSCs into Endothelial-like Cells for Angiogenesis. J. Cell Mol. Med. 2020, 24, 8703–8717. [Google Scholar] [CrossRef]



| Target Gene | Forward | Reverse | |
|---|---|---|---|
| Apoptotic markers | Bcl2 | TTCCTGCATCTCATGCCAAG | CTGGGAGGAGAAGATGCCC |
| Caspase 3 | TGGAACCAAAGATCATACATGGAA | TTCCCTGAGGTTTGCTGCAT | |
| Caspase 9 | TGCGTGGTGGTCATTCTCTC | ATGGTCTTTCTGCTCCCCAC | |
| Redox markers | HMOX-1 | AAGACTGCGTTCCTGCTCAA | GGGGGCAGAATCTTGCACT |
| Nox4 | TCGCCAACGAAGGGGTTAAA | GACACAATCTAGCCCCAACA | |
| Nrf2 | GCTATGGAGACACACTACTTGG | CCAGGACTTCAGGCAATTCT | |
| Protumorigenic markers | AMPK | TGTCACAGGCATATGGTGGTC | GGGCCTGCATACAATCTTCC |
| PDGF | TCCTGTCTCTCTGCTGCTAC | ATCAAAGGAGCGGATCGAGT | |
| PI3K | TCTTTGTGCAACCTACGTGA | AGCCATTCATTCCACCTGGG | |
| VEGF | ACAACAAATGTGAATGCAGACCA | GAGGCTCCAGGGCATTAGAC | |
| Housekeeping gene | GAPDH | TGCGTCGCCAGCCGAG | AGTTAAAAGCAGCCCTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcambal, A.; Septembre-Malaterre, A.; Pesnel, S.; Morel, A.-L.; Gasque, P.; Begue, M.; Slama, Y. The Potential of Human Pulmonary Mesenchymal Stem Cells as Vectors for Radiosensitizing Metallic Nanoparticles: An In Vitro Study. Cancers 2024, 16, 3239. https://doi.org/10.3390/cancers16183239
Arcambal A, Septembre-Malaterre A, Pesnel S, Morel A-L, Gasque P, Begue M, Slama Y. The Potential of Human Pulmonary Mesenchymal Stem Cells as Vectors for Radiosensitizing Metallic Nanoparticles: An In Vitro Study. Cancers. 2024; 16(18):3239. https://doi.org/10.3390/cancers16183239
Chicago/Turabian StyleArcambal, Angélique, Axelle Septembre-Malaterre, Sabrina Pesnel, Anne-Laure Morel, Philippe Gasque, Mickael Begue, and Youssef Slama. 2024. "The Potential of Human Pulmonary Mesenchymal Stem Cells as Vectors for Radiosensitizing Metallic Nanoparticles: An In Vitro Study" Cancers 16, no. 18: 3239. https://doi.org/10.3390/cancers16183239
APA StyleArcambal, A., Septembre-Malaterre, A., Pesnel, S., Morel, A.-L., Gasque, P., Begue, M., & Slama, Y. (2024). The Potential of Human Pulmonary Mesenchymal Stem Cells as Vectors for Radiosensitizing Metallic Nanoparticles: An In Vitro Study. Cancers, 16(18), 3239. https://doi.org/10.3390/cancers16183239







