Revisiting HER2 in Prostate Cancer from an Inclusive Perspective: From Biomarkers to Omics
Abstract
:Simple Summary
Abstract
1. Introduction
2. HER2 in Cancer
3. HER2 and Genetic Ancestry
4. HER2 in Prostate Cancer
5. HER2, Androgen Receptor, and Other Prostate Cancer Biomarkers
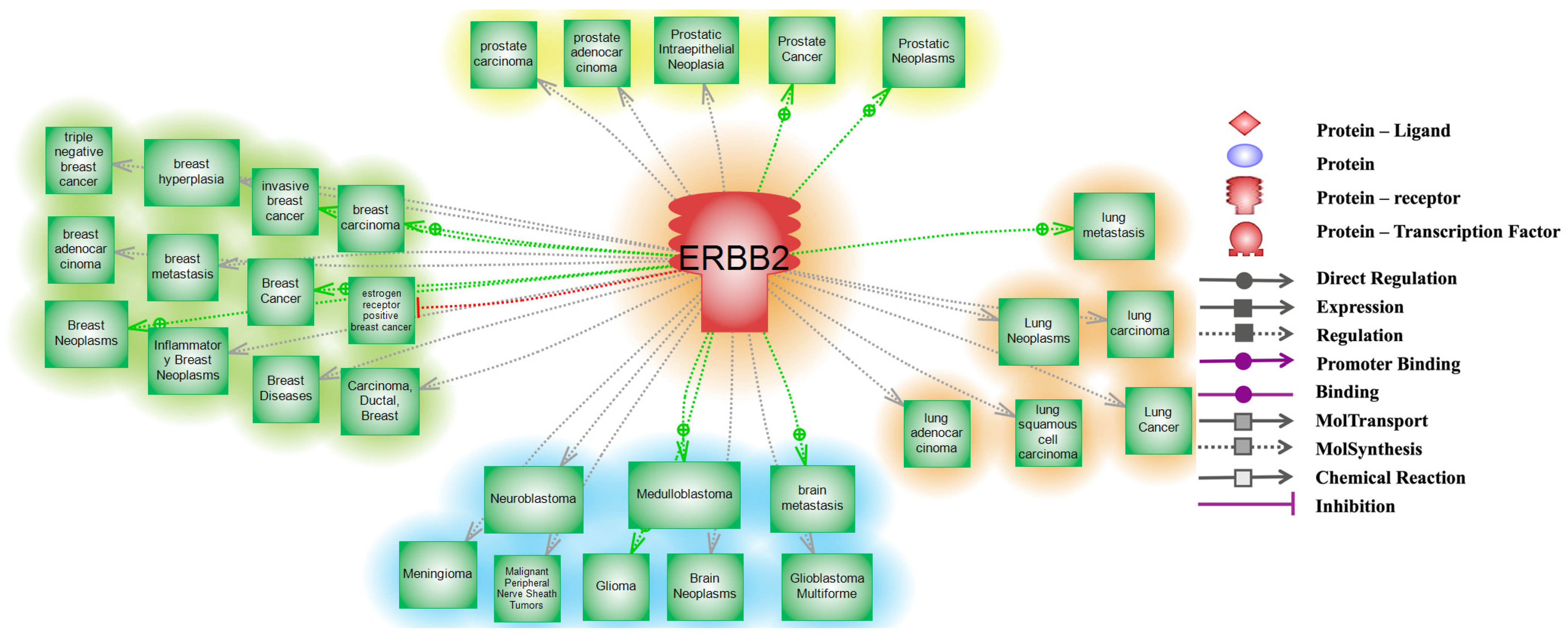
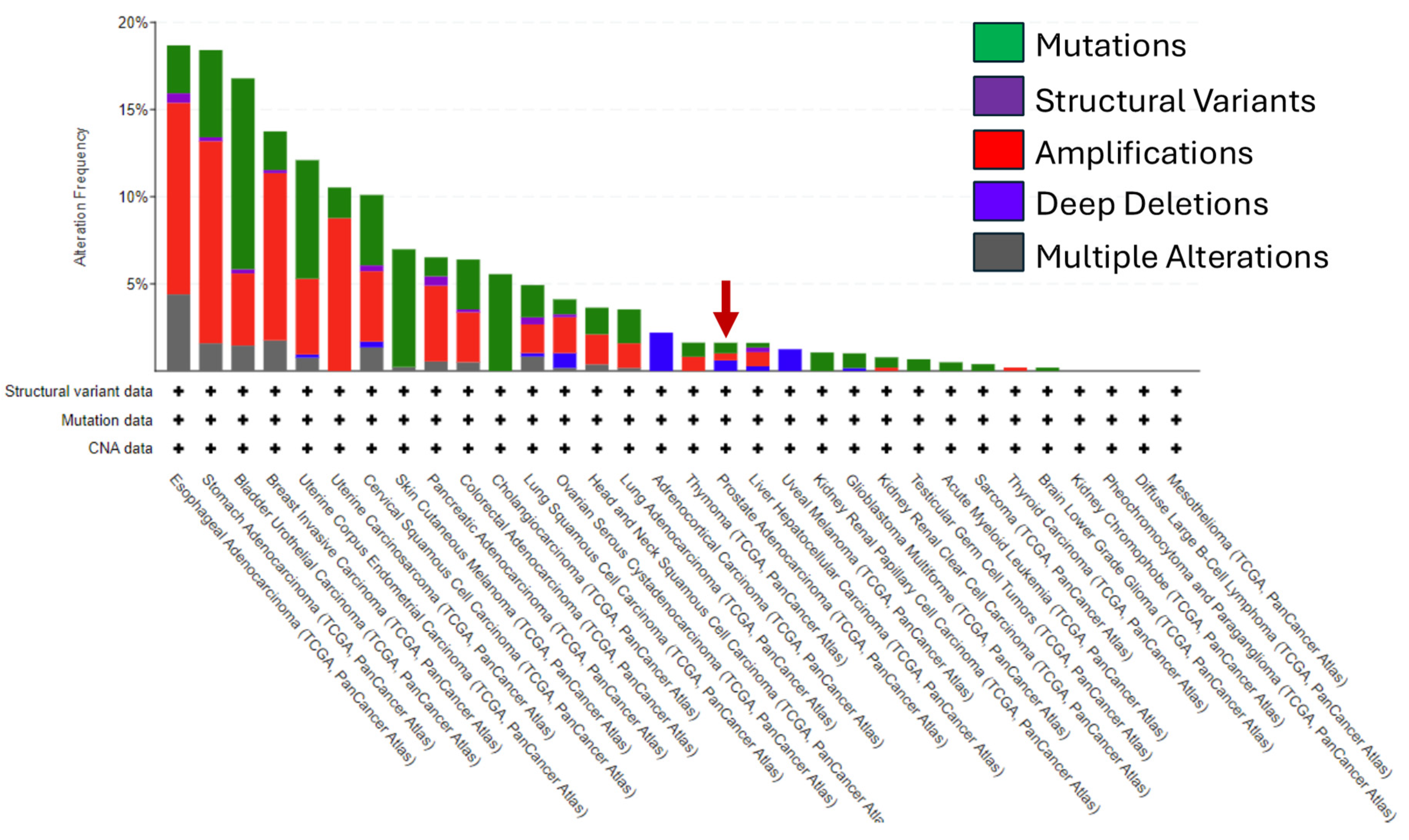
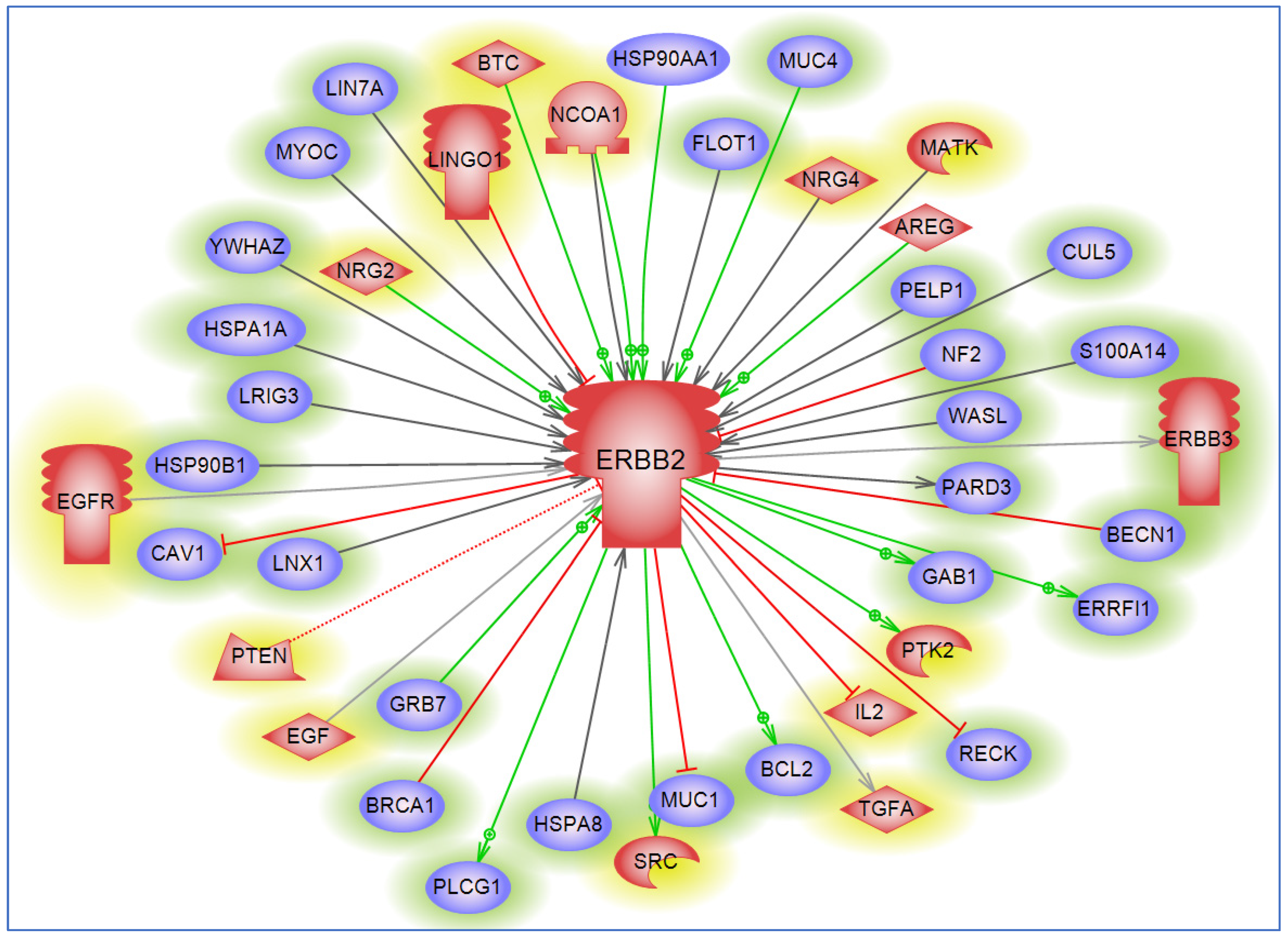
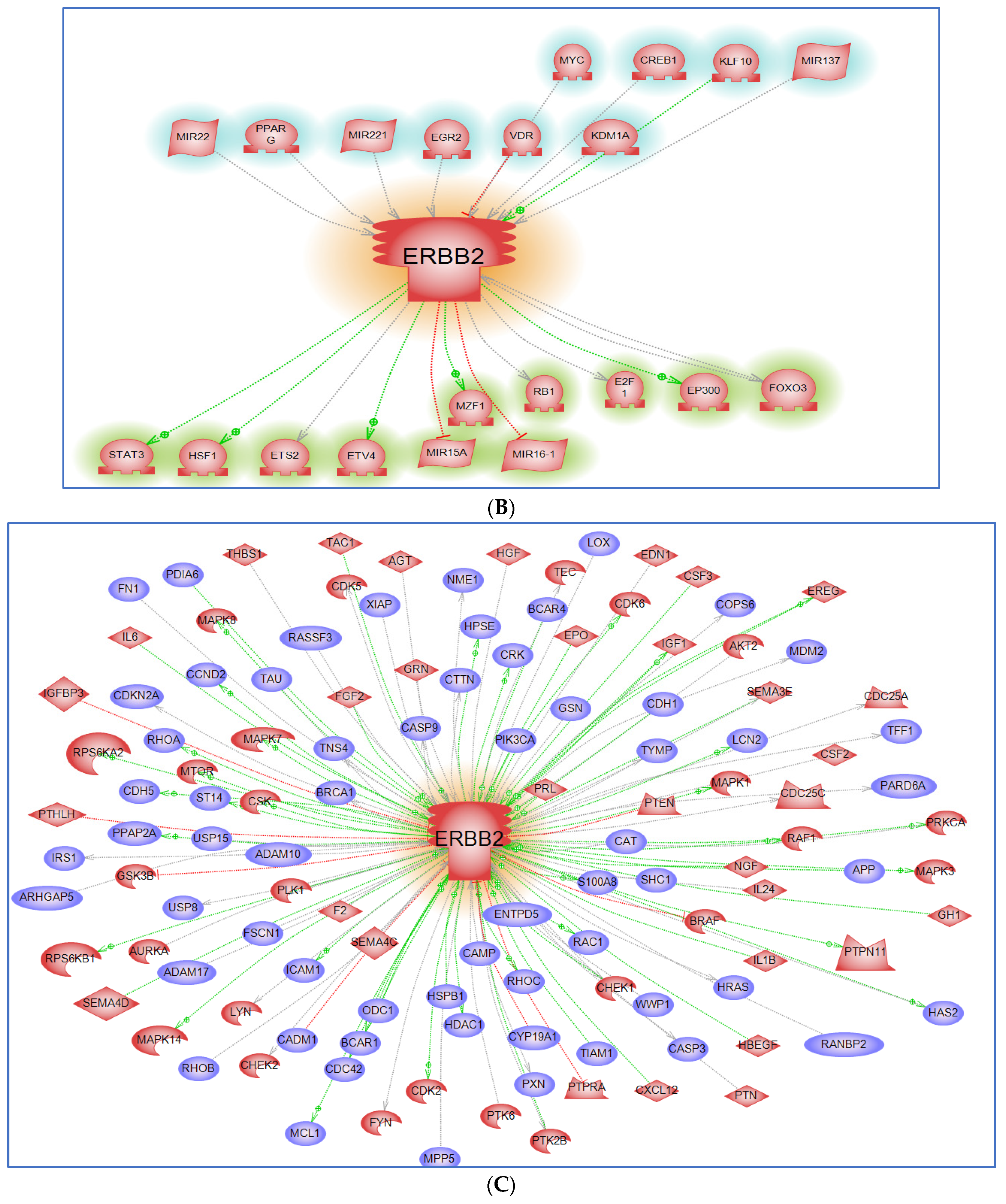
6. Interacting Partners and Networks
7. Lack of Diverse Biospecimens in HER2 Bench Studies
8. Lack of Diversity in HER2 Clinical Trials
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Maywood 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Farha, M.W.; Salami, S.S. Biomarkers for prostate cancer detection and risk stratification. Ther. Adv. Urol. 2022, 14, 17562872221103988. [Google Scholar] [CrossRef]
- Johnson, J.R.; Kittles, R.A. Genetic ancestry and racial differences in prostate tumours. Nat. Rev. Urol. 2022, 19, 133–134. [Google Scholar] [CrossRef]
- Johnson, J.R.; Mavingire, N.; Woods-Burnham, L.; Walker, M.; Lewis, D.; Hooker, S.E.; Galloway, D.; Rivers, B.; Kittles, R.A. The complex interplay of modifiable risk factors affecting prostate cancer disparities in African American men. Nat. Rev. Urol. 2024, 21, 422–432. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Minner, S.; Jessen, B.; Stiedenroth, L.; Burandt, E.; Köllermann, J.; Mirlacher, M.; Erbersdobler, A.; Eichelberg, C.; Fisch, M.; Brümmendorf, T.H.; et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin. Cancer Res. 2010, 16, 1553–1560. [Google Scholar] [CrossRef]
- Isharwal, S.; Miller, M.C.; Epstein, J.I.; Mangold, L.A.; Humphreys, E.; Partin, A.W.; Veltri, R.W. Prognostic value of Her-2/neu and DNA index for progression, metastasis and prostate cancer-specific death in men with long-term follow-up after radical prostatectomy. Int. J. Cancer 2008, 123, 2636–2643. [Google Scholar] [CrossRef]
- Morote, J.; de Torres, I.; Caceres, C.; Vallejo, C.; Schwartz, S., Jr.; Reventos, J. Prognostic value of immunohistochemical expression of the c-erbB-2 oncoprotein in metastasic prostate cancer. Int. J. Cancer 1999, 84, 421–425. [Google Scholar] [CrossRef]
- Nishio, Y.; Yamada, Y.; Kokubo, H.; Nakamura, K.; Aoki, S.; Taki, T.; Honda, N.; Nakagawa, A.; Saga, S.; Hara, K. Prognostic significance of immunohistochemical expression of the HER-2/neu oncoprotein in bone metastatic prostate cancer. Urology 2006, 68, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Estephan, F.; Lap, C.J.; Banagan, J.; Antonio, M.; Liu, S.; Diao, G.; Rozalen, A.Z.; Rajendran, R.; Krasnow, S.; Subrahmanyam, R.; et al. The prevalence and clinical significance of HER2 expression in prostate adenocarcinoma. Ann. Diagn. Pathol. 2023, 67, 152219. [Google Scholar] [CrossRef]
- Rimawi, M.F.; Mayer, I.A.; Forero, A.; Nanda, R.; Goetz, M.P.; Rodriguez, A.A.; Pavlick, A.C.; Wang, T.; Hilsenbeck, S.G.; Gutierrez, C.; et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1726–1731. [Google Scholar] [CrossRef]
- Press, M.F.; Cordon-Cardo, C.; Slamon, D.J. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 1990, 5, 953–962. [Google Scholar]
- Gutierrez, C.; Schiff, R. HER2: Biology, detection, and clinical implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Nikitin, A.; Egorov, S.; Daraselia, N.; Mazo, I. Pathway studio—The analysis and navigation of molecular networks. Bioinformatics 2003, 19, 2155–2157. [Google Scholar] [CrossRef] [PubMed]
- Devaux, S.; Cizkova, D.; Quanico, J.; Franck, J.; Nataf, S.; Pays, L.; Hauberg-Lotte, L.; Maass, P.; Kobarg, J.H.; Kobeissy, F.; et al. Proteomic Analysis of the Spatio-temporal Based Molecular Kinetics of Acute Spinal Cord Injury Identifies a Time- and Segment-specific Window for Effective Tissue Repair. Mol. Cell. Proteom. 2016, 15, 2641–2670. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- David, S.Y.; Chan, C.P.T.; Wyn, G.; Lewis, W.G. Systematic Review and Meta-analysis of the Influence of HER2 Expression and Amplification in Operable Oesophageal Cancer. J. Gastrointest. Surg. 2012, 16, 1821–1829. [Google Scholar]
- Huang, G.; Chantry, A.; Epstein, R.J. Overexpression of ErbB2 impairs ligand-dependent downregulation of epidermal growth factor receptors via a post-transcriptional mechanism. J. Cell. Biochem. 1999, 74, 23–30. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef] [PubMed]
- Hines, L.M.; Risendal, B.; Byers, T.; Mengshol, S.; Lowery, J.; Singh, M. Ethnic disparities in breast tumor phenotypic subtypes in Hispanic and non-Hispanic white women. J. Womens Health 2011, 20, 1543–1550. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef]
- Ménard, S.; Tagliabue, E.; Campiglio, M.; Pupa, S.M. Role of HER2 gene overexpression in breast carcinoma. J. Cell Physiol. 2000, 182, 150–162. [Google Scholar] [CrossRef]
- Slamon, D.J. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Giordano, S.H.; Franzoi, M.A.B.; Temin, S.; Anders, C.K.; Chandarlapaty, S.; Crews, J.R.; Kirshner, J.J.; Krop, I.E.; Lin, N.U.; Morikawa, A. Systemic therapy for advanced human epidermal growth factor receptor 2–positive breast cancer: ASCO guideline update. J. Clin. Oncol. 2022, 40, 2612–2635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ding, N.; Xu, X.; Zhang, Y.; Ye, M.; Li, C.; Hu, J. Clinical outcomes of patients with HER2-mutant advanced lung cancer: Chemotherapies versus HER2-directed therapies. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936090. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Raghav, K.P.S.; Moasser, M.M. Molecular Pathways and Mechanisms of HER2 in Cancer Therapy. Clin. Cancer Res. 2023, 29, 2351–2361. [Google Scholar] [CrossRef]
- Batai, K.; Hooker, S.; Kittles, R.A. Leveraging genetic ancestry to study health disparities. Am. J. Phys. Anthr. 2020, 175, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Shriver, M.D.; Kittles, R.A. Genetic ancestry and the search for personalized genetic histories. Nat. Rev. Genet. 2004, 5, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Jackson, F.L. The bioanthropological context of disease. Am. J. Kidney Dis. 1993, 21, 10–14. [Google Scholar] [CrossRef]
- Kittles, R.A.; Young, D.; Weinrich, S.; Hudson, J.; Argyropoulos, G.; Ukoli, F.; Adams-Campbell, L.; Dunston, G.M. Extent of linkage disequilibrium between the androgen receptor gene CAG and GGC repeats in human populations: Implications for prostate cancer risk. Hum. Genet. 2001, 109, 253–261. [Google Scholar] [CrossRef]
- Grizzle, W.E.; Kittles, R.A.; Rais-Bahrami, S.; Shah, E.; Adams, G.W.; DeGuenther, M.S.; Kolettis, P.N.; Nix, J.W.; Bryant, J.E.; Chinsky, R.; et al. Self-Identified African Americans and prostate cancer risk: West African genetic ancestry is associated with prostate cancer diagnosis and with higher Gleason sum on biopsy. Cancer Med. 2019, 8, 6915–6922. [Google Scholar] [CrossRef]
- Zakharia, F.; Basu, A.; Absher, D.; Assimes, T.L.; Go, A.S.; Hlatky, M.A.; Iribarren, C.; Knowles, J.W.; Li, J.; Narasimhan, B.; et al. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009, 10, R141. [Google Scholar] [CrossRef]
- Lovejoy, P.E. Transformations in Slavery: A History of Slavery in Africa, 2nd ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Curtin, P.D. The Atlantic Slave Trade; University of Wisconsin Press: Milwaukie, WI, USA, 1969. [Google Scholar]
- Eltis, D. The volume and structure of the transatlantic slave trade: A reassessment. William Mary Q. 2001, 58, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gomez, S.J.; Fejerman, L.; Zabaleta, J. Breast Cancer in Latinas: A Focus on Intrinsic Subtypes Distribution. Cancer Epidemiol. Biomark. Prev. 2018, 27, 3–10. [Google Scholar] [CrossRef]
- Rey-Vargas, L.; Sanabria-Salas, M.C.; Fejerman, L.; Serrano-Gomez, S.J. Risk Factors for Triple-Negative Breast Cancer among Latina Women. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1771–1783. [Google Scholar] [CrossRef]
- Rais-Bahrami, S.; Zhu, Y. Disparities in prostate cancer diagnosis and management: Recognizing that disparities exist at all junctures along the prostate cancer journey. Prostate Cancer Prostatic Dis. 2023, 26, 441–442. [Google Scholar] [CrossRef]
- John, E.M.; Koo, J.; Phipps, A.I.; Longacre, T.A.; Kurian, A.W.; Ingles, S.A.; Wu, A.H.; Hines, L.M. Reproductive characteristics, menopausal status, race and ethnicity, and risk of breast cancer subtypes defined by ER, PR and HER2 status: The Breast Cancer Etiology in Minorities study. Breast Cancer Res. 2024, 26, 88. [Google Scholar] [CrossRef] [PubMed]
- Rey-Vargas, L.; Bejarano-Rivera, L.M.; Mejia-Henao, J.C.; Sua, L.F.; Bastidas-Andrade, J.F.; Ossa, C.A.; Gutierrez-Castaneda, L.D.; Fejerman, L.; Sanabria-Salas, M.C.; Serrano-Gomez, S.J. Association of genetic ancestry with HER2, GRB7 AND estrogen receptor expression among Colombian women with breast cancer. Front. Oncol. 2022, 12, 989761. [Google Scholar] [CrossRef]
- Miyashita, M.; Bell, J.S.K.; Wenric, S.; Karaesmen, E.; Rhead, B.; Kase, M.; Kaneva, K.; De La Vega, F.M.; Zheng, Y.; Yoshimatsu, T.F.; et al. Molecular profiling of a real-world breast cancer cohort with genetically inferred ancestries reveals actionable tumor biology differences between European ancestry and African ancestry patient populations. Breast Cancer Res. 2023, 25, 58. [Google Scholar] [CrossRef]
- Purrington, K.S.; Hastert, T.A.; Madhav, K.C.; Nair, M.; Snider, N.; Ruterbusch, J.J.; Schwartz, A.G.; Stoffel, E.M.; Peters, E.S.; Rozek, L.S. The role of area-level socioeconomic disadvantage in racial disparities in cancer incidence in metropolitan Detroit. Cancer Med. 2023, 12, 14623–14635. [Google Scholar] [CrossRef]
- Mori, J.O.; White, J.; Elhussin, I.; Duduyemi, B.M.; Karanam, B.; Yates, C.; Wang, H. Molecular and pathological subtypes related to prostate cancer disparities and disease outcomes in African American and European American patients. Front. Oncol. 2022, 12, 928357. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J.; Dyson, G.; Land, S.; Ruterbusch, J.; Bock, C.H.; Lenk, S.; Herawi, M.; Everson, R.; Giroux, C.N.; Schwartz, A.G.; et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol. Biomark. Prev. 2013, 22, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Batai, K.; Murphy, A.B.; Nonn, L.; Kittles, R.A. Vitamin D and Immune Response: Implications for Prostate Cancer in African Americans. Front. Immunol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Wallace, T.A.; Prueitt, R.L.; Yi, M.; Howe, T.M.; Gillespie, J.W.; Yfantis, H.G.; Stephens, R.M.; Caporaso, N.E.; Loffredo, C.A.; Ambs, S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008, 68, 927–936. [Google Scholar] [CrossRef]
- Reams, R.R.; Agrawal, D.; Davis, M.B.; Yoder, S.; Odedina, F.T.; Kumar, N.; Higginbotham, J.M.; Akinremi, T.; Suther, S.; Soliman, K.F. Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: A pilot project study. Infect. Agent. Cancer 2009, 4 (Suppl. 1), S3. [Google Scholar] [CrossRef]
- Savinainen, K.J.; Saramäki, O.R.; Linja, M.J.; Bratt, O.; Tammela, T.L.; Isola, J.J.; Visakorpi, T. Expression and gene copy number analysis of ERBB2 oncogene in prostate cancer. Am. J. Pathol. 2002, 160, 339–345. [Google Scholar] [CrossRef]
- Baltaci, S. Her-2/neu oncogene expression in prostate carcinoma: Evaluation of gene amplification by FISH method. Turk. J. Pathol. 2008, 24, 76–83. [Google Scholar]
- Bubendorf, L.; Kononen, J.; Koivisto, P.; Schraml, P.; Moch, H.; Gasser, T.C.; Willi, N.; Mihatsch, M.J.; Sauter, G.; Kallioniemi, O.P. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999, 59, 803–806. [Google Scholar] [PubMed]
- Ibrahim, G.K.; MacDonald, J.A.; Kerns, B.J.; Ibrahim, S.N.; Humphrey, P.A.; Robertson, C.N. Differential immunoreactivity of her-2/neu oncoprotein in prostatic tissues. Surg. Oncol. 1992, 1, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.J.; Kurnot, R.A.; Sesterhenn, I.A.; Chang, E.H.; Moul, J.W. Expression of the c-erbB-2 (HER-2/neu) oncoprotein in human prostatic carcinoma. J. Urol. 1993, 150, 1427–1433. [Google Scholar] [CrossRef]
- Jorda, M.; Morales, A.; Ghorab, Z.; Fernandez, G.; Nadji, M.; Block, N. Her2 expression in prostatic cancer: A comparison with mammary carcinoma. J. Urol. 2002, 168, 1412–1414. [Google Scholar] [CrossRef]
- Gu, K.; Mes-Masson, A.M.; Gauthier, J.; Saad, F. Overexpression of her-2/neu in human prostate cancer and benign hyperplasia. Cancer Lett. 1996, 99, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Reese, D.M.; Small, E.J.; Magrane, G.; Waldman, F.M.; Chew, K.; Sudilovsky, D. HER2 protein expression and gene amplification in androgen-independent prostate cancer. Am. J. Clin. Pathol. 2001, 116, 234–239. [Google Scholar] [CrossRef]
- Domingo-Domenech, J.; Fernandez, P.L.; Filella, X.; Martinez-Fernandez, A.; Molina, R.; Fernandez, E.; Alcaraz, A.; Codony, J.; Gascon, P.; Mellado, B. Serum HER2 extracellular domain predicts an aggressive clinical outcome and biological PSA response in hormone-independent prostate cancer patients treated with docetaxel. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2008, 19, 269–275. [Google Scholar] [CrossRef]
- Al Olama, A.A.; Kote-Jarai, Z.; Berndt, S.I.; Conti, D.V.; Schumacher, F.; Han, Y.; Benlloch, S.; Hazelett, D.J.; Wang, Z.; Saunders, E.; et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat. Genet. 2014, 46, 1103–1109. [Google Scholar] [CrossRef]
- Plum, P.S.; Gebauer, F.; Kramer, M.; Alakus, H.; Berlth, F.; Chon, S.H.; Schiffmann, L.; Zander, T.; Buttner, R.; Holscher, A.H.; et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer 2019, 19, 38. [Google Scholar] [CrossRef]
- Sharifi, N.; Salmaninejad, A.; Ferdosi, S.; Bajestani, A.N.; Khaleghiyan, M.; Estiar, M.A.; Jamali, M.; Nowroozi, M.R.; Shakoori, A. HER2 gene amplification in patients with prostate cancer: Evaluating a CISH-based method. Oncol. Lett. 2016, 12, 4651–4658. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Gandour-Edwards, R.; Lara, P.N., Jr.; de Vere White, R.; LaSalle, J.M. Detection of low level HER-2/neu gene amplification in prostate cancer by fluorescence in situ hybridization. Cancer J. 2001, 7, 395–403. [Google Scholar] [PubMed]
- Ross, J.S.; Sheehan, C.; Hayner-Buchan, A.M.; Ambros, R.A.; Kallakury, B.V.; Kaufman, R.; Fisher, H.A.; Muraca, P.J. HER-2/neu gene amplification status in prostate cancer by fluorescence in situ hybridization. Hum. Pathol. 1997, 28, 827–833. [Google Scholar] [CrossRef]
- Ross, J.S.; Sheehan, C.E.; Hayner-Buchan, A.M.; Ambros, R.A.; Kallakury, B.V.; Kaufman, R.P., Jr.; Fisher, H.A.; Rifkin, M.D.; Muraca, P.J. Prognostic significance of HER-2/neu gene amplification status by fluorescence in situ hybridization of prostate carcinoma. Cancer 1997, 79, 2162–2170. [Google Scholar] [CrossRef]
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016, 375, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.H.; Hong, M.E.; Jung, Y.Y.; Lee, C.H.; Lee, T.J.; Park, E.S.; Kim, M.K.; Yoo, J.H.; Lee, S.W. Correlation of AR, EGFR, and HER2 Expression Levels in Prostate Cancer: Immunohistochemical Analysis and Chromogenic In Situ Hybridization. Cancer Res. Treat. 2012, 44, 50–56. [Google Scholar] [CrossRef]
- Shi, Y.; Brands, F.H.; Chatterjee, S.; Feng, A.C.; Groshen, S.; Schewe, J.; Lieskovsky, G.; Cote, R.J. Her-2/neu expression in prostate cancer: High level of expression associated with exposure to hormone therapy and androgen independent disease. J. Urol. 2001, 166, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.T.; Boyd, J.C.; Frierson, H.F., Jr. Loss of heterozygosity at 13q14 and 13q21 in high grade, high stage prostate cancer. Prostate 2001, 49, 166–171. [Google Scholar] [CrossRef]
- Hanna, W.M.; Rüschoff, J.; Bilous, M.; Coudry, R.A.; Dowsett, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 in situ hybridization in breast cancer: Clinical implications of polysomy 17 and genetic heterogeneity. Mod. Pathol. 2014, 27, 4–18. [Google Scholar] [CrossRef]
- Porzycki, P.; Ciszkowicz, E. Modern biomarkers in prostate cancer diagnosis. Cent. Eur. J. Urol. 2020, 73, 300–306. [Google Scholar] [CrossRef]
- Cary, K.C.; Cooperberg, M.R. Biomarkers in prostate cancer surveillance and screening: Past, present, and future. Ther. Adv. Urol. 2013, 5, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Adamaki, M.; Zoumpourlis, V. Prostate Cancer Biomarkers: From diagnosis to prognosis and precision-guided therapeutics. Pharmacol. Ther. 2021, 228, 107932. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Akamatsu, S.; Tsukahara, S.; Nagakawa, S.; Matsumoto, T.; Eto, M. Androgen receptor mutations for precision medicine in prostate cancer. Endocr. Relat. Cancer 2022, 29, R143–R155. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; Girelli, G.; Demichelis, F. Genomic Correlates to the Newly Proposed Grading Prognostic Groups for Prostate Cancer. Eur. Urol. 2016, 69, 557–560. [Google Scholar] [CrossRef]
- Posdzich, P.; Darr, C.; Hilser, T.; Wahl, M.; Herrmann, K.; Hadaschik, B. Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches. Cancers 2023, 15, 461. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Xia, M.; Guo, Z.; Hu, Z. The Role of PARP Inhibitors in the Treatment of Prostate Cancer: Recent Advances in Clinical Trials. Biomolecules 2021, 11, 722. [Google Scholar] [CrossRef]
- Taylor, A.K.; Kosoff, D.; Emamekhoo, H.; Lang, J.M.; Kyriakopoulos, C.E. PARP inhibitors in metastatic prostate cancer. Front. Oncol. 2023, 13, 1159557. [Google Scholar] [CrossRef]
- Rossini, A.; Giussani, M.; Ripamonti, F.; Aiello, P.; Regondi, V.; Balsari, A.; Triulzi, T.; Tagliabue, E. Combined targeting of EGFR and HER2 against prostate cancer stem cells. Cancer Biol. Ther. 2020, 21, 463–475. [Google Scholar] [CrossRef]
- Maillet, D.; Allioli, N.; Plesa, A.; Decaussin-Petrucci, M.; Tartas, S.; Sajous, C.; Ruffion, A.; Crouzet, S.; Freyer, G.; Vlaeminck-Guillem, V. Her2 Expression in Circulating Tumor Cells Is Associated with Poor Outcomes in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 6014. [Google Scholar] [CrossRef]
- Veeramani, S.; Chou, Y.-W.; Lin, F.C.; Muniyan, S.; Lin, F.-F.; Kumar, S.; Xie, Y.; Lele, S.M.; Tu, Y.; Lin, M.-F. Reactive oxygen species induced by p66Shc longevity protein mediate nongenomic androgen action via tyrosine phosphorylation signaling to enhance tumorigenicity of prostate cancer cells. Free Radic. Biol. Med. 2012, 53, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Lucci, M.A.; Orlandi, R.; Triulzi, T.; Tagliabue, E.; Balsari, A.; Villa-Moruzzi, E. Expression profile of tyrosine phosphatases in HER2 breast cancer cells and tumors. Cell Oncol. 2010, 32, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; O’Byrne, K.J.; Cox, J.; Krammer, B.; Gatter, K.C.; Harris, A.L. bcl-2 and c-erbB-2 proteins are involved in the regulation of VEGF and of thymidine phosphorylase angiogenic activity in non-small-cell lung cancer. Clin. Exp. Metastasis 1999, 17, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Warden, C.; Chen, S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 149, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Patel, R.; Singh, L.B.; Nixon, C.; Seywright, M.; Barnetson, R.J.; Brunton, V.G.; Muller, W.J.; Edwards, J.; Sansom, O.J.; et al. HER2 overcomes PTEN (loss)-induced senescence to cause aggressive prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 16392–16397. [Google Scholar] [CrossRef]
- Bishop, J.L.; Thaper, D.; Zoubeidi, A. The Multifaceted Roles of STAT3 Signaling in the Progression of Prostate Cancer. Cancers 2014, 6, 829–859. [Google Scholar] [CrossRef]
- Miller, D.R.; Ingersoll, M.A.; Lin, M.F. ErbB-2 signaling in advanced prostate cancer progression and potential therapy. Endocr. Relat. Cancer 2019, 26, R195–R209. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Li, Y.M.; Pan, Y.; Wei, Y.; Cheng, X.; Zhou, B.P.; Tan, M.; Zhou, X.; Xia, W.; Hortobagyi, G.N.; Yu, D.; et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 2004, 6, 459–469. [Google Scholar] [CrossRef]
- Chinni, S.R.; Yamamoto, H.; Dong, Z.; Sabbota, A.; Bonfil, R.D.; Cher, M.L. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol. Cancer Res. 2008, 6, 446–457. [Google Scholar] [CrossRef]
- Pienta, K.J.; Abate-Shen, C.; Agus, D.B.; Attar, R.M.; Chung, L.W.; Greenberg, N.M.; Hahn, W.C.; Isaacs, J.T.; Navone, N.M.; Peehl, D.M.; et al. The current state of preclinical prostate cancer animal models. Prostate 2008, 68, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines—Part 1. J. Urol. 2005, 173, 342–359. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Shappell, S.B.; Thomas, G.V.; Roberts, R.L.; Herbert, R.; Ittmann, M.M.; Rubin, M.A.; Humphrey, P.A.; Sundberg, J.P.; Rozengurt, N.; Barrios, R.; et al. Prostate pathology of genetically engineered mice: Definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004, 64, 2270–2305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of organoid culture in cancer research. Cancer Med. 2023, 12, 14375–14386. [Google Scholar] [CrossRef] [PubMed]
- Hooker, S.E.; Woods-Burnham, L.; Bathina, M.; Lloyd, S.; Gorjala, P.; Mitra, R.; Nonn, L.; Kimbro, K.S.; Kittles, R.A. Genetic Ancestry Analysis Reveals Misclassification of Commonly Used Cancer Cell Lines. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1003. [Google Scholar] [CrossRef]
- Patierno, B.M.; Foo, W.C.; Allen, T.; Somarelli, J.A.; Ware, K.E.; Gupta, S.; Wise, S.; Wise, J.P., Sr.; Qin, X.; Zhang, D.; et al. Characterization of a castrate-resistant prostate cancer xenograft derived from a patient of West African ancestry. Prostate Cancer Prostatic Dis. 2022, 25, 513–523. [Google Scholar] [CrossRef]
- Palanisamy, N.; Yang, J.; Shepherd, P.D.A.; Li-Ning-Tapia, E.M.; Labanca, E.; Manyam, G.C.; Ravoori, M.K.; Kundra, V.; Araujo, J.C.; Efstathiou, E.; et al. The MD Anderson Prostate Cancer Patient-derived Xenograft Series (MDA PCa PDX) Captures the Molecular Landscape of Prostate Cancer and Facilitates Marker-driven Therapy Development. Clin. Cancer Res. 2020, 26, 4933–4946. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Kobori, G.; Goto, T.; Akamatsu, S.; Terada, N.; Kobayashi, T.; Tanaka, Y.; Jung, G.; Kamba, T.; Ogawa, O.; et al. An original patient-derived xenograft of prostate cancer with cyst formation. Prostate 2016, 76, 994–1003. [Google Scholar] [CrossRef]
- Kimura, T.; Kiyota, H.; Nakata, D.; Masaki, T.; Kusaka, M.; Egawa, S. A novel androgen-dependent prostate cancer xenograft model derived from skin metastasis of a Japanese patient. Prostate 2009, 69, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Okasho, K.; Mizuno, K.; Fukui, T.; Lin, Y.Y.; Kamiyama, Y.; Sunada, T.; Li, X.; Kimura, H.; Sumiyoshi, T.; Goto, T.; et al. Establishment and characterization of a novel treatment-related neuroendocrine prostate cancer cell line KUCaP13. Cancer Sci. 2021, 112, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Asadzadeh Aghdaei, H.; Baghaei, K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J. Transl. Med. 2022, 20, 206. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Taylor, R.A.; Toivanen, R.; Pedersen, J.; Norden, S.; Pook, D.W.; Frydenberg, M.; Papargiris, M.M.; Niranjan, B.; Richards, M.G.; et al. A preclinical xenograft model of prostate cancer using human tumors. Nat. Protoc. 2013, 8, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.B.; van Weerden, W.M.; Erkens-Schulze, S.; de Ridder, C.M.; Bangma, C.H.; Trapman, J.; Jenster, G. The human PC346 xenograft and cell line panel: A model system for prostate cancer progression. Eur. Urol. 2006, 49, 245–257. [Google Scholar] [CrossRef]
- Karkampouna, S.; La Manna, F.; Benjak, A.; Kiener, M.; De Menna, M.; Zoni, E.; Grosjean, J.; Klima, I.; Garofoli, A.; Bolis, M.; et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat. Commun. 2021, 12, 1117. [Google Scholar] [CrossRef]
- Monterosso, M.E.; Futrega, K.; Lott, W.B.; Vela, I.; Williams, E.D.; Doran, M.R. Using the Microwell-mesh to culture microtissues in vitro and as a carrier to implant microtissues in vivo into mice. Sci. Rep. 2021, 11, 5118. [Google Scholar] [CrossRef]
- Sablatura, L.K.; Bircsak, K.M.; Shepherd, P.; Bathina, M.; Queiroz, K.; Farach-Carson, M.C.; Kittles, R.A.; Constantinou, P.E.; Saleh, A.; Navone, N.M.; et al. A 3D Perfusable Platform for In Vitro Culture of Patient Derived Xenografts. Adv. Heal. Mater. 2023, 12, e2201434. [Google Scholar] [CrossRef]
- Morris, M.J.; Reuter, V.E.; Kelly, W.K.; Slovin, S.F.; Kenneson, K.; Verbel, D.; Osman, I.; Scher, H.I. HER-2 profiling and targeting in prostate carcinoma. Cancer 2002, 94, 980–986. [Google Scholar] [CrossRef]
- Ziada, A.; Barqawi, A.; Glode, L.M.; Varella-Garcia, M.; Crighton, F.; Majeski, S.; Rosenblum, M.; Kane, M.; Chen, L.; Crawford, E.D. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate 2004, 60, 332–337. [Google Scholar] [CrossRef]
- Lara, P.N., Jr.; Chee, K.G.; Longmate, J.; Ruel, C.; Meyers, F.J.; Gray, C.R.; Edwards, R.G.; Gumerlock, P.H.; Twardowski, P.; Doroshow, J.H.; et al. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: Final results from the California Cancer Consortium Screening and Phase II Trial. Cancer 2004, 100, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, Y.H.; Kolesar, J.; Huang, W.; Dipaola, R.; Pins, M.; Carducci, M.; Stein, M.; Bubley, G.J.; Wilding, G. Eastern Cooperative Oncology Group Phase II Trial of lapatinib in men with biochemically relapsed, androgen dependent prostate cancer. Urol. Oncol. 2013, 31, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Bradbury, I.; Eidtmann, H.; Di Cosimo, S.; de Azambuja, E.; Aura, C.; Gomez, H.; Dinh, P.; Fauria, K.; Van Dooren, V.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet 2012, 379, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Sartor, A.O.; Armstrong, A.J.; Ahaghotu, C.; McLeod, D.G.; Cooperberg, M.R.; Penson, D.F.; Higano, C.S. Overall survival (OS) of African-American (AA) and Caucasian (CAU) men who received sipuleucel-T for metastatic castration-resistant prostate cancer (mCRPC): Final PROCEED analysis. J. Clin. Oncol. 2019, 37, 5035. [Google Scholar] [CrossRef]
- Halabi, S.; Dutta, S.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Thompson, I.M., Jr.; Chi, K.N.; Araujo, J.C.; Logothetis, C.; Quinn, D.I.; et al. Overall Survival of Black and White Men With Metastatic Castration-Resistant Prostate Cancer Treated with Docetaxel. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 403–410. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavingire, N.; Moore, J.C.; Johnson, J.R.; Dwead, A.M.; Cropp, C.D.; Mechref, Y.; Kobeissy, F.; Rais-Bahrami, S.; Woods-Burnham, L. Revisiting HER2 in Prostate Cancer from an Inclusive Perspective: From Biomarkers to Omics. Cancers 2024, 16, 3262. https://doi.org/10.3390/cancers16193262
Mavingire N, Moore JC, Johnson JR, Dwead AM, Cropp CD, Mechref Y, Kobeissy F, Rais-Bahrami S, Woods-Burnham L. Revisiting HER2 in Prostate Cancer from an Inclusive Perspective: From Biomarkers to Omics. Cancers. 2024; 16(19):3262. https://doi.org/10.3390/cancers16193262
Chicago/Turabian StyleMavingire, Nicole, Janelle C. Moore, Jabril R. Johnson, Abdulrahman M. Dwead, Cheryl D. Cropp, Yehia Mechref, Firas Kobeissy, Soroush Rais-Bahrami, and Leanne Woods-Burnham. 2024. "Revisiting HER2 in Prostate Cancer from an Inclusive Perspective: From Biomarkers to Omics" Cancers 16, no. 19: 3262. https://doi.org/10.3390/cancers16193262
APA StyleMavingire, N., Moore, J. C., Johnson, J. R., Dwead, A. M., Cropp, C. D., Mechref, Y., Kobeissy, F., Rais-Bahrami, S., & Woods-Burnham, L. (2024). Revisiting HER2 in Prostate Cancer from an Inclusive Perspective: From Biomarkers to Omics. Cancers, 16(19), 3262. https://doi.org/10.3390/cancers16193262









