Metabolic Reprogramming of Phospholipid Fatty Acids as a Signature of Lung Cancer Type
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Statement
2.3. Tissue Collection
2.4. Tissue Phospholipid Fatty Acids Extraction
2.5. Phospholipid Composition
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Rebecca, M.E.; Mph, L.S. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bye, A.; Sjøblom, B.; Wentzel-Larsen, T.; Grønberg, B.H.; Baracos, V.E.; Marianne, J.; Aass, N.; Bremnes, R.M.; Fløtten, Ø.; Jordhøy, M. Muscle Mass and Association to Quality of Life in Non- Small Cell Lung Cancer Patients. J. Cachexia Sarcopenia Muscle 2017, 8, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C. Small-Cell Lung Cancer. Nat. Rev. Dis. Prim. 2018, 7, 3. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Stoica, C.; Ferreira, A.K.; Hannan, K.; Bakovic, M. Bilayer Forming Phospholipids as Targets for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 5266. [Google Scholar] [CrossRef] [PubMed]
- Vishwa, R.; Bharathwajchetty, B.; Girisa, S.; Santha, B. Lipid Metabolism and Its Implications in Tumor Cell Plasticity and Drug Resistance: What We Learned Thus Far? Cancer Metastasis Rev. 2024, 43, 293–319. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Expert Opinion on Therapeutic Targets Fatty Acid Synthase (FASN) as a Therapeutic Target in Breast Cancer. Expert Opin. Ther. Targets 2017, 21, 1001–1016. [Google Scholar] [CrossRef]
- Westheim, A.J.F.; Stoffels, L.M.; Dubois, L.J.; van Bergenhenegouwen, J.; van Helvoort, A.; Langen, R.C.J.; Shiri-Sverdlov, R.; Theys, J. The Modulatory Effects of Fatty Acids on Cancer Progression. Biomedicines 2023, 11, 280. [Google Scholar] [CrossRef]
- Ristic-Medic, D.; Vucic, V.; Takic, M.; Karadžic, I.; Glibetic, M. Polyunsaturated Fatty Acids in Health and Disease. J. Serbian Chem. Soc. 2013, 78, 1269–1289. [Google Scholar] [CrossRef]
- Am, J.; Arranz, S.; Urruticoechea, A.; Ugartemendia, G. Altered Red Blood Cell Membrane Fatty Acid Profile in Cancer Patients. Nutrients 2018, 10, 1853. [Google Scholar] [CrossRef] [PubMed]
- Pakiet, A.; Jędrzejewska, A.; Duzowska, K.; Wacławska, A.; Jabłońska, P.; Zieliński, J.; Mika, A.; Śledziński, T.; Słomińska, E. Serum Fatty Acid Profiles in Breast Cancer Patients Following Treatment. BMC Cancer 2023, 23, 433. [Google Scholar] [CrossRef] [PubMed]
- Masnikosa, R.; Pirić, D.; Post, J.M.; Cvetković, Z.; Petrović, S.; Paunović, M.; Vučić, V.; Bindila, L. Disturbed Plasma Lipidomic Profiles in Females with Diffuse Large B-Cell Lymphoma: A Pilot Study. Cancers 2023, 15, 3653. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Shao, S.; Du, Y.; Zhuang, X.; Wang, X. Plasma Lipidomics Profiling to Identify the Biomarkers of Diagnosis and Radiotherapy Response for Advanced Non-Small-Cell Lung Cancer Patients. J. Lipids 2024, 2024, 6730504. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, L.; Liu, L.; Wei, S.; He, Y.; Chang, J.; Lian, X. Blood Lipids Profile and Lung Cancer Risk in a Meta-Analysis of Prospective Cohort Studies. J. Clin. Lipidol. 2017, 11, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.P.; Forder, A.; Brockley, L.J.; Pewarchuk, M.E.; Telkar, N.; de Araújo, R.P.; Trejo, J.; Benard, K.; Seneda, A.L.; Minutentag, I.W.; et al. Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis. Int. J. Mol. Sci. 2023, 24, 8894. [Google Scholar] [CrossRef]
- Turnic, T.N.; Arsic, A.; Vucic, V.; Petrovic, S. Hydroxymethylglutaryl Coenzyme a Reductase Inhibitors Differentially Modulate Plasma Fatty Acids in Rats With Diet-Induced- Hyperhomocysteinemia: Is ω-3 Fatty Acids Supplementation Necessary? Front. Physiol. 2019, 10, 892. [Google Scholar] [CrossRef]
- Krstic, P.; Vucic, V.; Paunovic, M.; Petrovic, S.; Nedovic, N.; Kostic, S.; Arsic, A. Similar fatty acid status of plasma lipids in postmenopausal women newly diagnosed with breast cancer and those receiving aromatase inhibitor therapy. Vojn. Pregl. 2021, 78, 1140–1145. [Google Scholar] [CrossRef]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Scott, J.S.; Nassar, Z.D.; Swinnen, J.V.; Butler, L.M. Monounsaturated Fatty Acids: Key Regulators of Cell Viability and Intracellular Signaling in Cancer. Mol. Cancer Res. 2022, 20, 1354–1364. [Google Scholar] [CrossRef]
- Zaidi, N.; Lupien, L.; Kuemmerle, N.B.; Kinlaw, W.B.; Swinnen, J.V.; Smans, K. Progress in Lipid Research Lipogenesis and Lipolysis: The Pathways Exploited by the Cancer Cells to Acquire Fatty Acids FaƩy Acids. Prog. Lipid Res. 2013, 52, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, F.; Mosayebi, G.; Montazeri, V.; Darabi, M.; Fayezi, S. Breast Cancer Fatty Acid Composition of Tissue Cultured Breast Carcinoma and the Effect of Stearoyl-CoA Desaturase 1 Inhibition. J. Breast Cancer 2014, 17, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.R.; Hay, N. Trends in Pharmacological Sciences Emerging Targets in Lipid Metabolism for Cancer Therapy. Trends Pharmacol. Sci. 2024, 45, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Y.; Zhou, D.; Li, Z. Significantly Increased Monounsaturated Observed by Mass Spectrometry Imaging. Sci. Rep. 2014, 4, 5959. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, C.; Alessio, A.D.; Iacopino, F.; Proietti, G. Pivotal Role of Human Stearoyl-CoA Desaturases (SCD1 and 5) in Breast Cancer Progression: Oleic Acid-Based Effect of SCD1 on Cell Migration and a Novel pro-Cell Survival Role for SCD5. Oncotarget 2018, 9, 24364–24380. [Google Scholar] [CrossRef]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; De Stanchina, E.; Mathew, R.; White, E.P. Hypoxic and Ras-Transformed Cells Support Growth by Scavenging Unsaturated Fatty Acids from Lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef]
- Hess, D.; Chisholm, J.W.; Igal, R.A. Inhibition of StearoylCoA Desaturase Activity Blocks Cell Cycle Progression and Induces Programmed Cell Death in Lung Cancer Cells. PLoS ONE 2010, 5, e11394. [Google Scholar] [CrossRef]
- Gupta, A.; Das, D.; Taneja, R. Targeting Dysregulated Lipid Metabolism in Cancer with Pharmacological Inhibitors. Cancers 2024, 16, 1313. [Google Scholar] [CrossRef]
- Arshad, Z.; Rezapour-Firouzi, S.; Ebrahimifar, M.; Jarrahi, M.; Mohammadian, M. Association of Delta-6-Desaturase Expression with Aggressiveness of Cancer, Diabetes Mellitus, and Multiple Sclerosis: A Narrative Review. Asian Pac. J. Cancer Prev. 2019, 20, 1005–1018. [Google Scholar] [CrossRef]
- Hanna, V.S.; Abdel, E.; Hafez, A. Synopsis of Arachidonic Acid Metabolism: A Review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Yang, P.; Cartwright, C.A.; Li, J.I.N.; Wen, S.; Prokhorova, I.N.A.N.; Shureiqi, I.; Troncoso, P.; Navone, N.M.; Newman, R.A.; Kim, J.; et al. Arachidonic Acid Metabolism in Human Prostate Cancer. Int. J. Oncol. 2012, 41, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, I.; Yano, Y.; Mori, M.; Manabe, S.; Fukuo, K. Impact of Serum Eicosapentaenoic Acid/Arachidonic Acid Ratio on Overall Survival in Lung Cancer Patients Treated with Pembrolizumab: A Pilot Study. Sci. Rep. 2024, 14, 1384. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.C.; Walker, A.F. Premenstrual syndrome. Encycl. Hum. Nutr. 1998, 139, 35–42. [Google Scholar] [CrossRef]
- Shah, H.; Pang, L.; Wang, H.; Shu, D.; Qian, S.Y.; Sathish, V. Growth inhibitory and anti-metastatic activity of epithelial cell adhesion molecule targeted three-way junctional delta-5-desaturase siRNA nanoparticle for breast cancer therapy. Nanomedicine 2020, 30, 102298. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Gu, Z.; Shan, K.; Chen, H.; Chen, Y.Q. n-3 Polyunsaturated Fatty Acids and Their Role in Cancer Chemoprevention. Curr. Pharmacol. Rep. 2015, 1, 283–294. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food. Funct. 2021, 12, 30–40. [Google Scholar] [CrossRef]
- Kim, I.; Myung, S.; Do, M.Y.; Ryu, Y.; Kim, M.J.; Do, E.; Park, S.; Yoon, S.M.; Ye, B.D.; Byeon, J.; et al. Western-Style Diets Induce Macrophage Infiltration and Contribute to Colitis-Associated Carcinogenesis. J. Gastroenterol. Hepatol. 2010, 25, 1785–1794. [Google Scholar] [CrossRef]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. Review The Role of Lipids in Cancer Progression and Metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef]
- Hoxha, M.; Zappacosta, B. A Review on the Role of Fatty Acids in Colorectal Cancer Progression. Front. Pharmacol. 2022, 13, 1032806. [Google Scholar] [CrossRef]
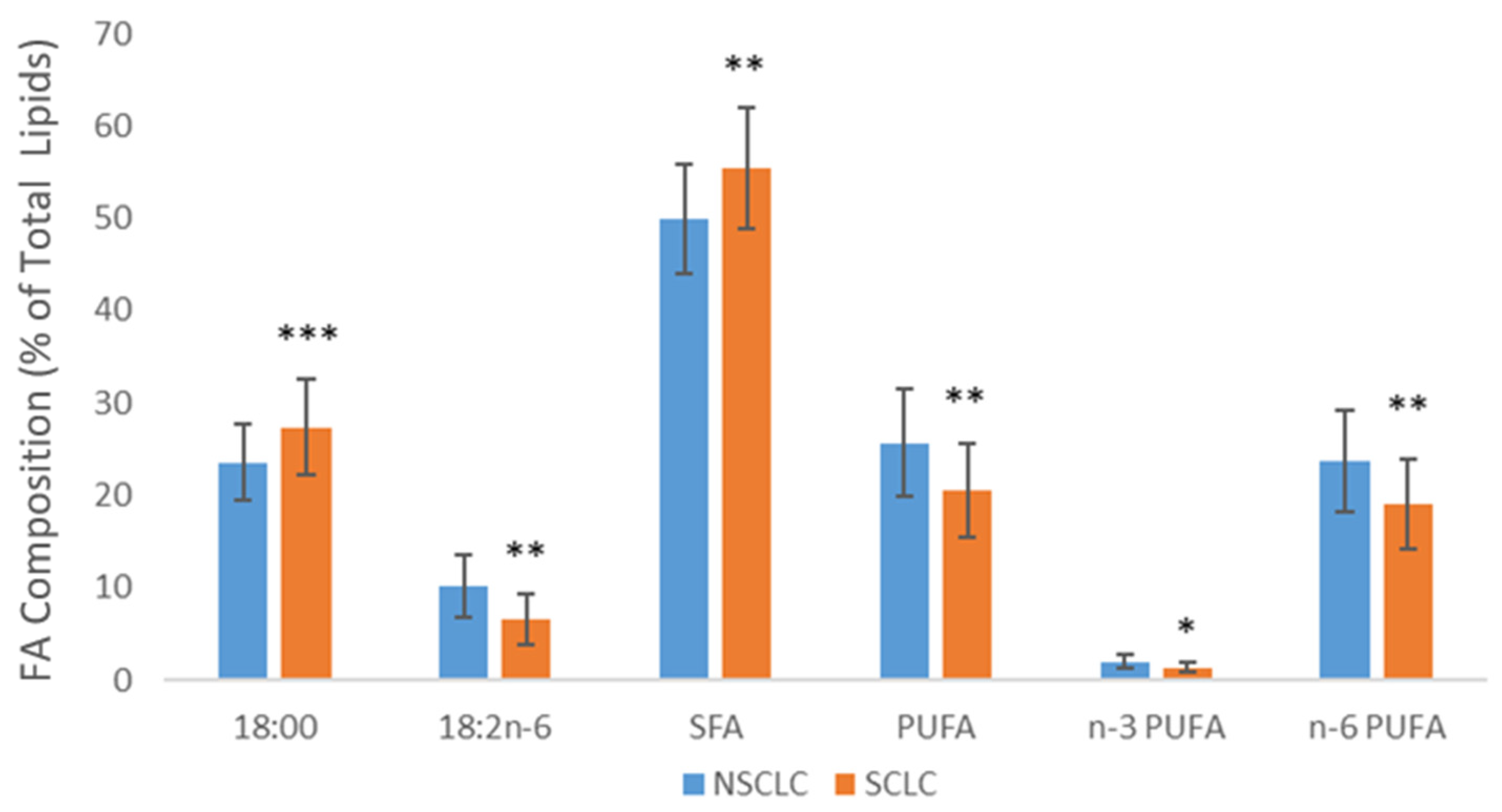
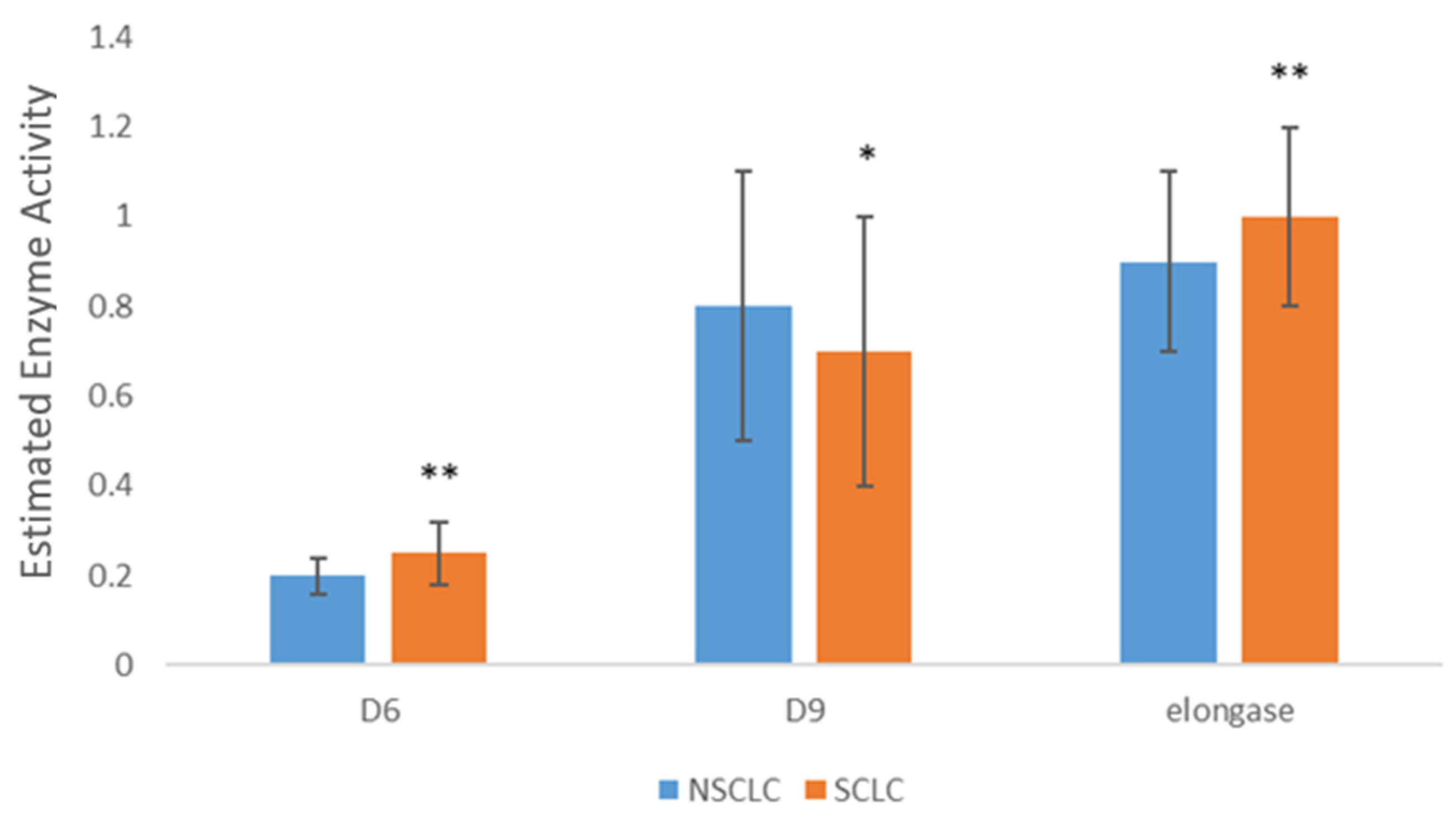
| NSCLC (n = 37) | SCLC (n = 27) | |
|---|---|---|
| TC | 4.62 ± 1.06 | 4.80 ± 1.29 |
| HDL | 1.04 ± 0.37 | 1.01 ± 0.39 |
| LDL | 2.97 ± 0.89 | 3.04 ± 1.30 |
| TG | 1.19 ± 0.42 | 1.65 ± 0.78 ** |
| CRP | 39.7 ± 42.6 | 49.3 ± 54.5 |
| fibrinogen | 5.5 ± 1.3 | 5.1 ± 0.9 |
| Fatty Acids | Control | NSCLC |
|---|---|---|
| 16:0 | 30.2 ± 4.9 | 26.3 ± 4.0 *** |
| 18:0 | 24.6 ± 5.4 | 23.6 ± 4.1 |
| SFA | 54.8 ± 7.0 | 49.9 ± 5.9 *** |
| 16:1n-7 | 1.5 ± 0.9 | 1.5 ± 1.2 |
| 18:1n-7 | 3.0 ± 1.0 | 4.4 ± 1.5 *** |
| 18:1n-9 | 17.4 ± 3.3 | 18.2 ± 4.2 |
| MUFA | 21.9 ± 3.9 | 24.2 ± 5.3 * |
| 18:2n-6 | 10.3 ± 3.2 | 10.2 ± 3.3 |
| 18:3n-6 | ND | ND |
| 20:3n-6 | 1.7 ± 0.7 | 2.0 ± 0.6 * |
| 20:4n-6 | 7.6 ± 2.4 | 9.5 ± 2.3 *** |
| 22:4n-6 | 1.3 ± 0.5 | 1.9 ± 0.7 *** |
| n-6 PUFA | 21.0 ± 5.2 | 23.7 ± 5.5 *** |
| 18:3n-3 | 0.3 ± 0.2 | 0.2 ± 0.1 ** |
| 20:5n-3 | 0.2 ± 0.1 | 0.2 ± 0.2 |
| 22:5n-3 | 0.6 ± 0.2 | 0.6 ± 0.2 ** |
| 22:6n-3 | 1.2 ± 0.8 | 1.2 ± 0.6 |
| n-3 PUFA | 1.9 ± 0.8 | 2.0 ± 0.8 |
| PUFA | 22.9 ± 5.6 | 25.7 ± 5.8 *** |
| n-6/n-3 ratio | 12.2 ± 3.7 | 13.0 ± 4.4 |
| EPA/AA | 0.03 ± 0.02 | 0.02 ± 0.02 |
| Desaturase and Elongase | Control | NSCLC |
|---|---|---|
| Δ5 | 5.1 ± 2.4 | 5.1 ± 1.6 |
| Δ6 | 0.17 ± 0.05 | 0.20 ± 0.04 *** |
| Δ9 | 0.7 ± 0.2 | 0.8 ± 0.3 |
| SCD1 | 0.05 ± 0.03 | 0.05 ± 0.05 |
| SCD2 | 0.75 ± 0.25 | 0.81 ± 0.33 |
| Elongase | 0.8 ± 0.2 | 0.9 ± 0.2 |
| Fatty Acids | Control | SCLC |
|---|---|---|
| 16:0 | 28.8 ± 4.5 | 28.0 ± 2.7 |
| 18:0 | 28.4 ± 6.7 | 27.4 ± 5.1 |
| SFA | 57.2 ± 7.8 | 55.4 ± 6.6 |
| 16:1n-7 | 1.3 ± 1.0 | 1.1 ± 0.7 |
| 18:1n-7 | 3.0 ± 0.7 | 4.4 ± 1.2 *** |
| 18:1n-9 | 16.1 ± 3.1 | 18.4 ± 4.0 * |
| MUFA | 20.5 ± 3.4 | 23.9 ± 4.4 ** |
| 18:2n-6 | 10.3 ± 3.5 | 6.6 ± 2.7 *** |
| 18:3n-6 | ND | ND |
| 20:3n-6 | 1.9 ± 0.7 | 1.5 ± 0.5 * |
| 20:4n-6 | 6.7 ± 2.2 | 9.0 ± 2.9 *** |
| 22:4n-6 | 1.2 ± 0.5 | 1.9 ± 1.0 *** |
| n-6 PUFA | 20.1 ± 5.8 | 19.1 ± 4.9 |
| 18:3n-3 | 0.2 ± 0.2 | 0.2 ± 0.1 |
| 20:5n-3 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| 22:5n-3 | 0.6 ± 0.4 | 0.4 ± 0.3 *** |
| 22:6n-3 | 1.1 ± 0.4 | 0.9 ± 0.3 *** |
| n-3 PUFA | 1.9 ± 0.6 | 1.4 ± 0.6 *** |
| PUFA | 22.1 ± 6.1 | 20.5 ± 5.1 † |
| n-6/n-3 ratio | 11.0 ± 3.6 | 15.4 ± 4.4 *** |
| EPA/AA | 0.03 ± 0.03 | 0.02 ± 0.02 * |
| Desaturase and Elongase | Control | SCLC |
|---|---|---|
| Δ5 | 4.0 ± 1.6 | 6.1 ± 2.4 ** |
| Δ6 | 0.18 ± 0.05 | 0.25 ± 0.07 *** |
| Δ9 | 0.6 ± 0.2 | 0.7 ± 0.3 * |
| SCD1 | 0.04 ± 0.02 | 0.04 ± 0.02 † |
| SCD2 | 0.64 ± 0.19 | 0.73 ± 0.30 |
| Elongase | 1.0 ± 0.3 | 1.0 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paunovic, M.; Stojanovic, A.; Pokimica, B.; Martacic, J.D.; Cvetkovic, Z.; Ivanovic, N.; Vucic, V. Metabolic Reprogramming of Phospholipid Fatty Acids as a Signature of Lung Cancer Type. Cancers 2024, 16, 3320. https://doi.org/10.3390/cancers16193320
Paunovic M, Stojanovic A, Pokimica B, Martacic JD, Cvetkovic Z, Ivanovic N, Vucic V. Metabolic Reprogramming of Phospholipid Fatty Acids as a Signature of Lung Cancer Type. Cancers. 2024; 16(19):3320. https://doi.org/10.3390/cancers16193320
Chicago/Turabian StylePaunovic, Marija, Ana Stojanovic, Biljana Pokimica, Jasmina Debeljak Martacic, Zorica Cvetkovic, Nebojsa Ivanovic, and Vesna Vucic. 2024. "Metabolic Reprogramming of Phospholipid Fatty Acids as a Signature of Lung Cancer Type" Cancers 16, no. 19: 3320. https://doi.org/10.3390/cancers16193320







