Safety Evaluation of Carbon Dots in UM-UC-5 and A549 Cells for Biomedical Applications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CDs
2.2. Characterization of CDs
2.3. Fluorescence Quantum Yield (QYPL) Calculations
2.4. CDs Toxicity Assays
2.4.1. Cells, Medium, and Compounds
2.4.2. Cell Viability Assays
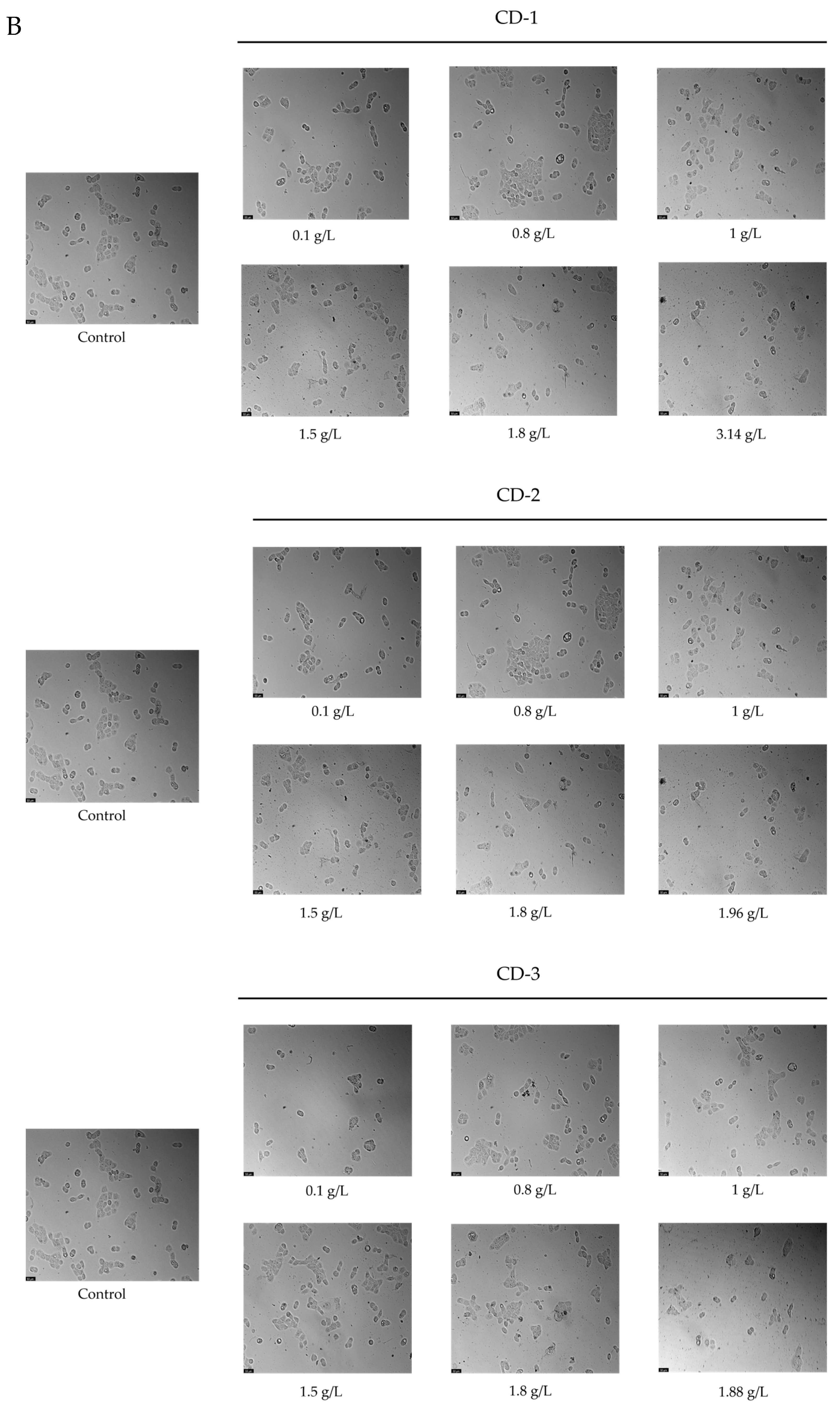
2.4.3. Morphological Analysis
2.4.4. Synergistic Effect Analysis
2.4.5. Statistical Analysis
3. Results and Discussion
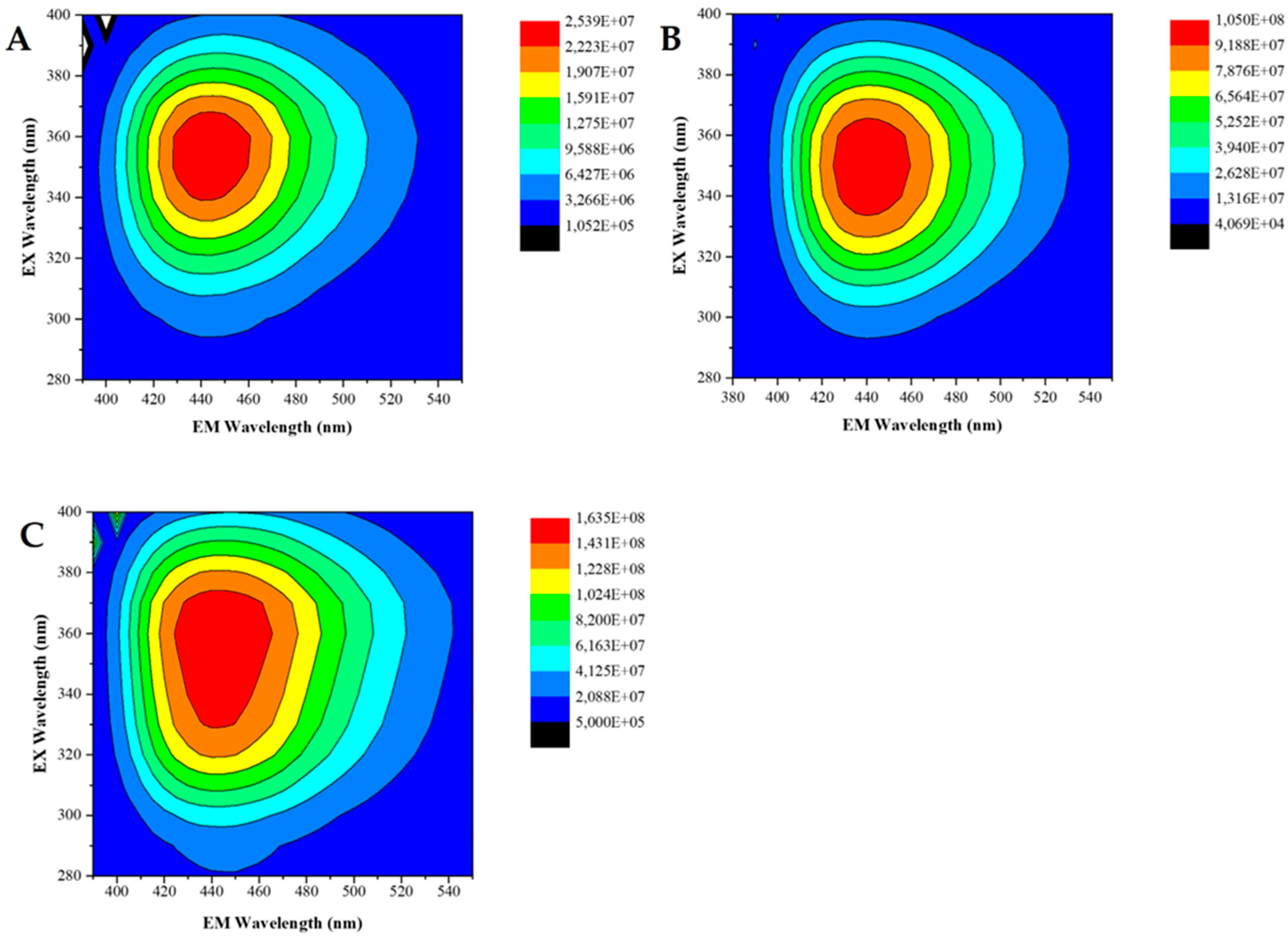
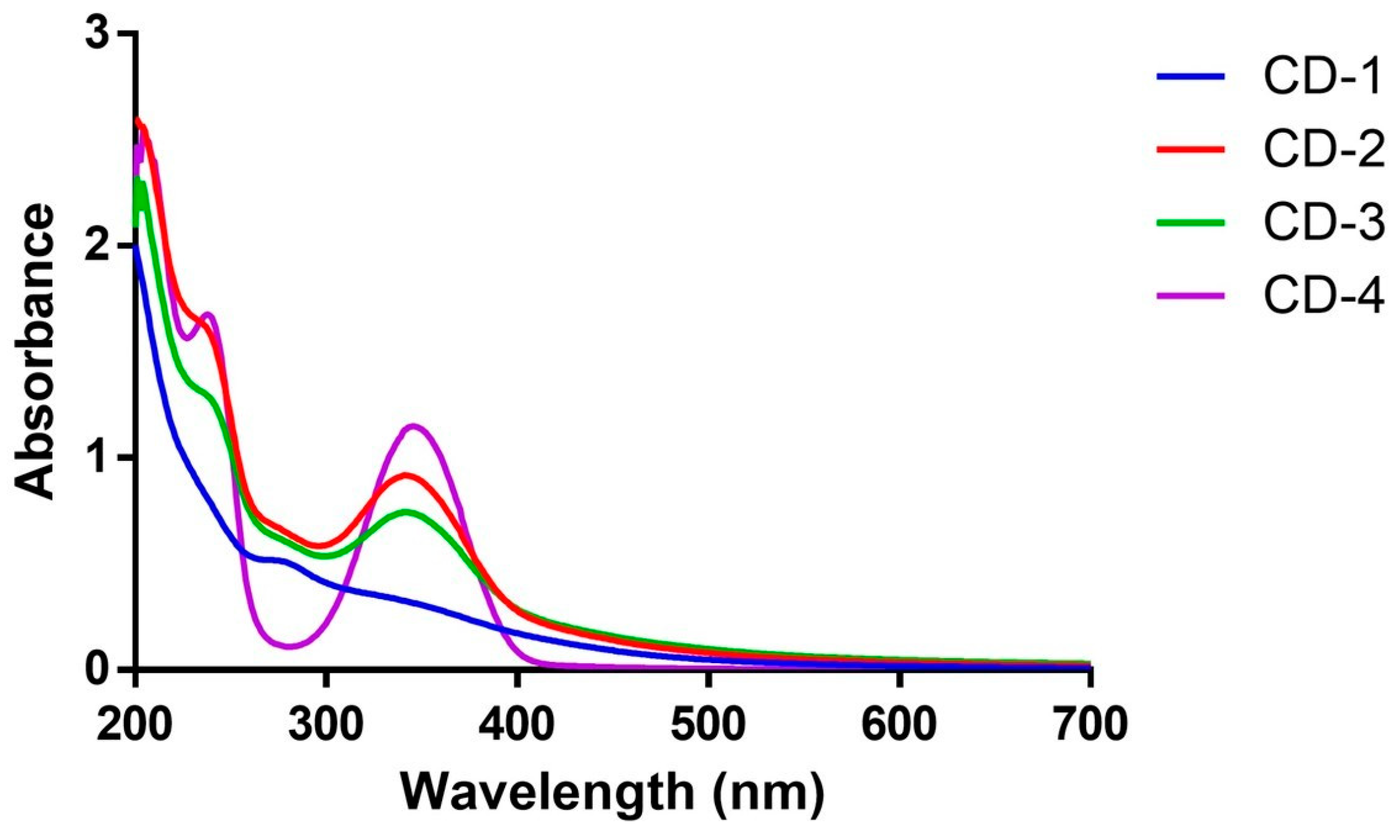
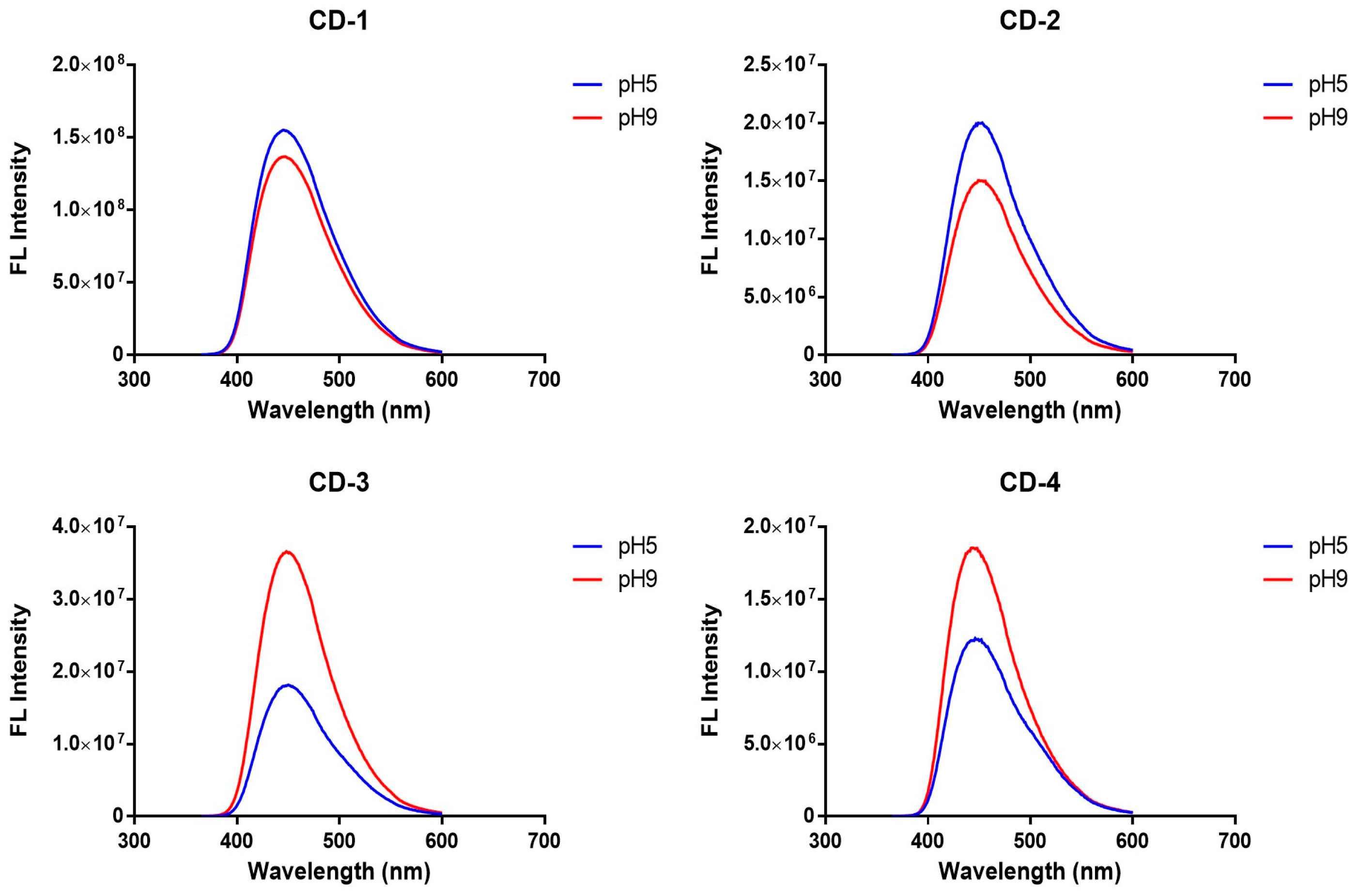
3.1. Characterization of CDs
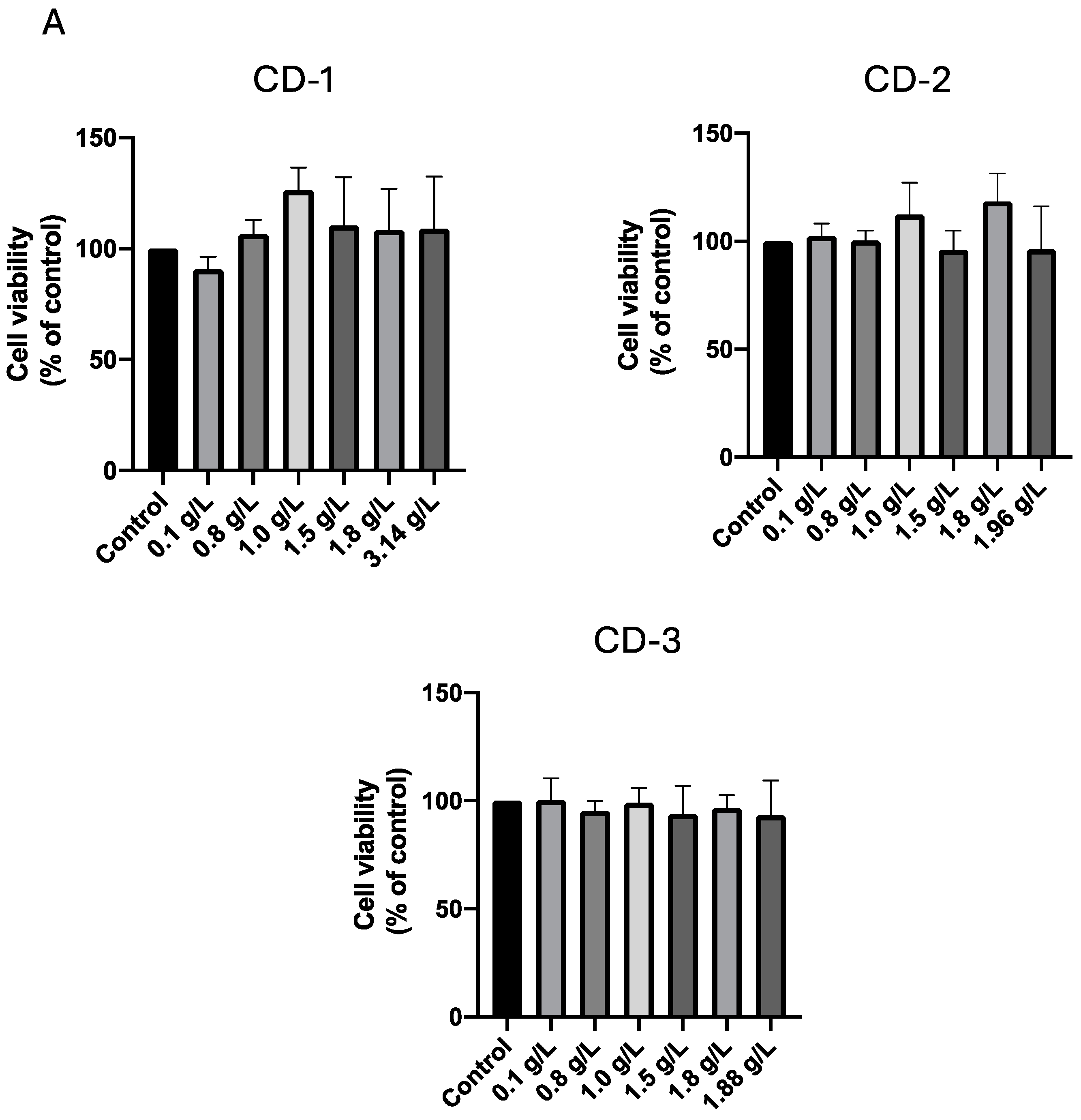
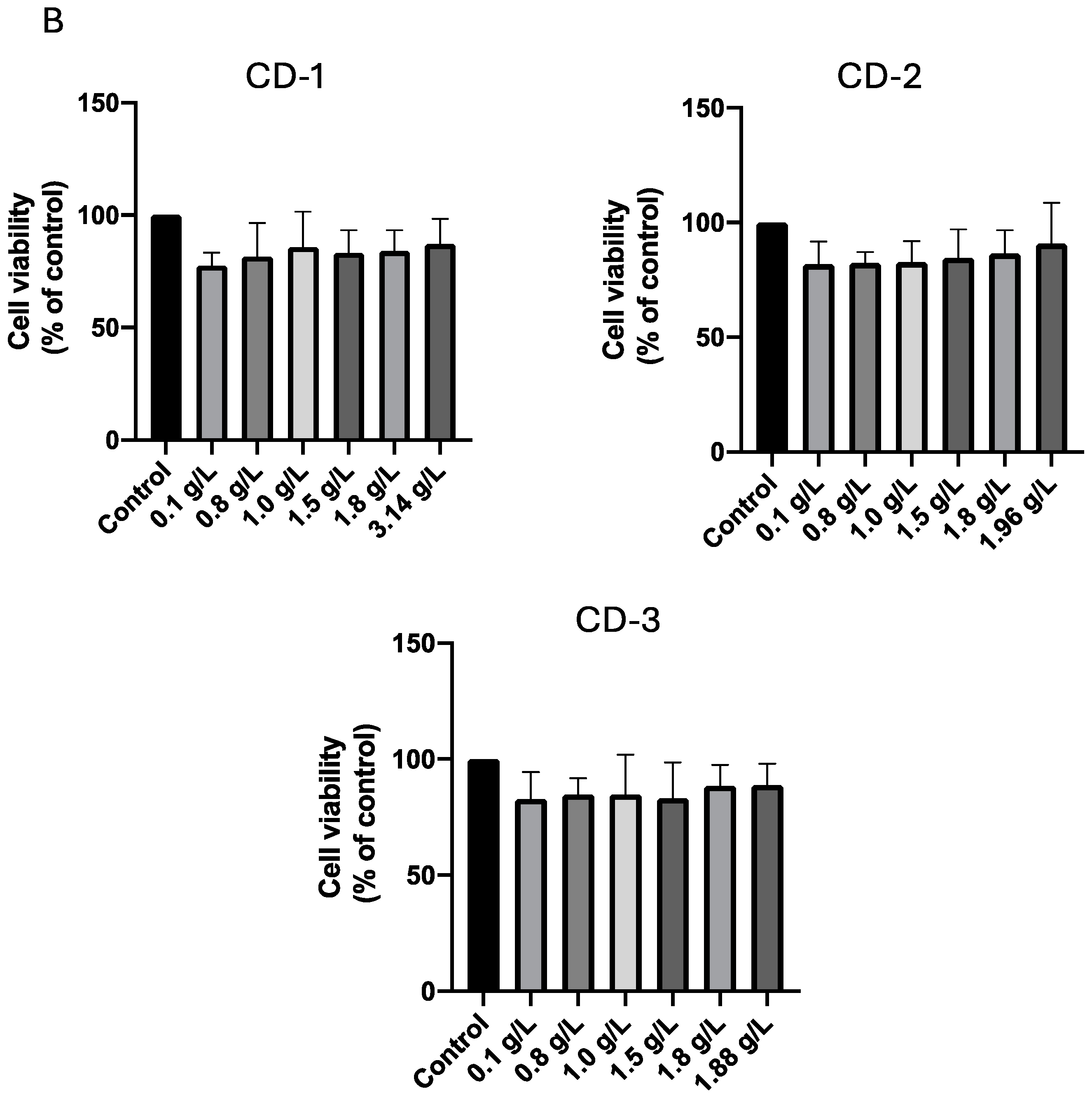
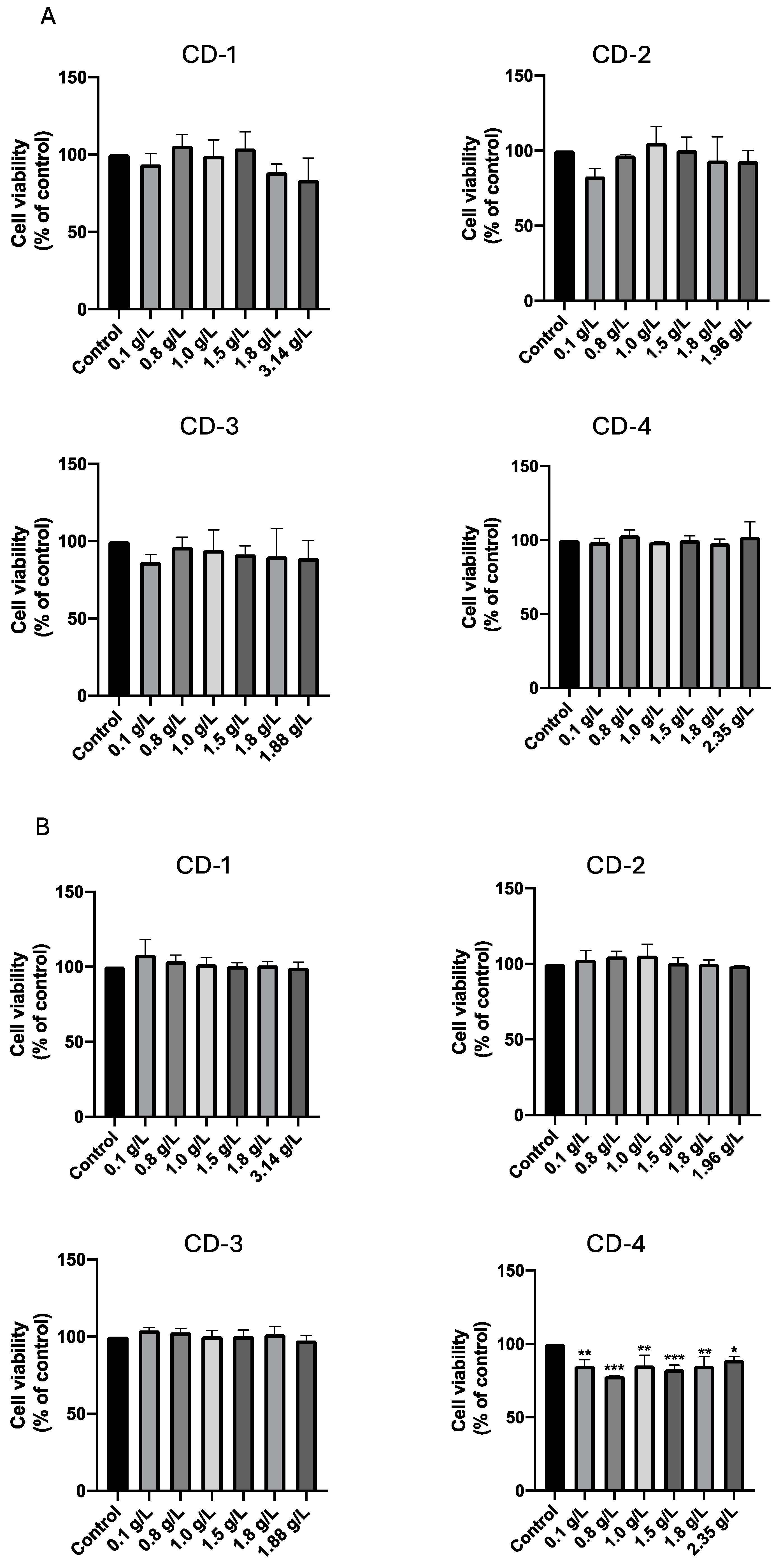
3.2. Cytotoxic Effect of CDs on A549 and UM-UC-5 Cell Lines
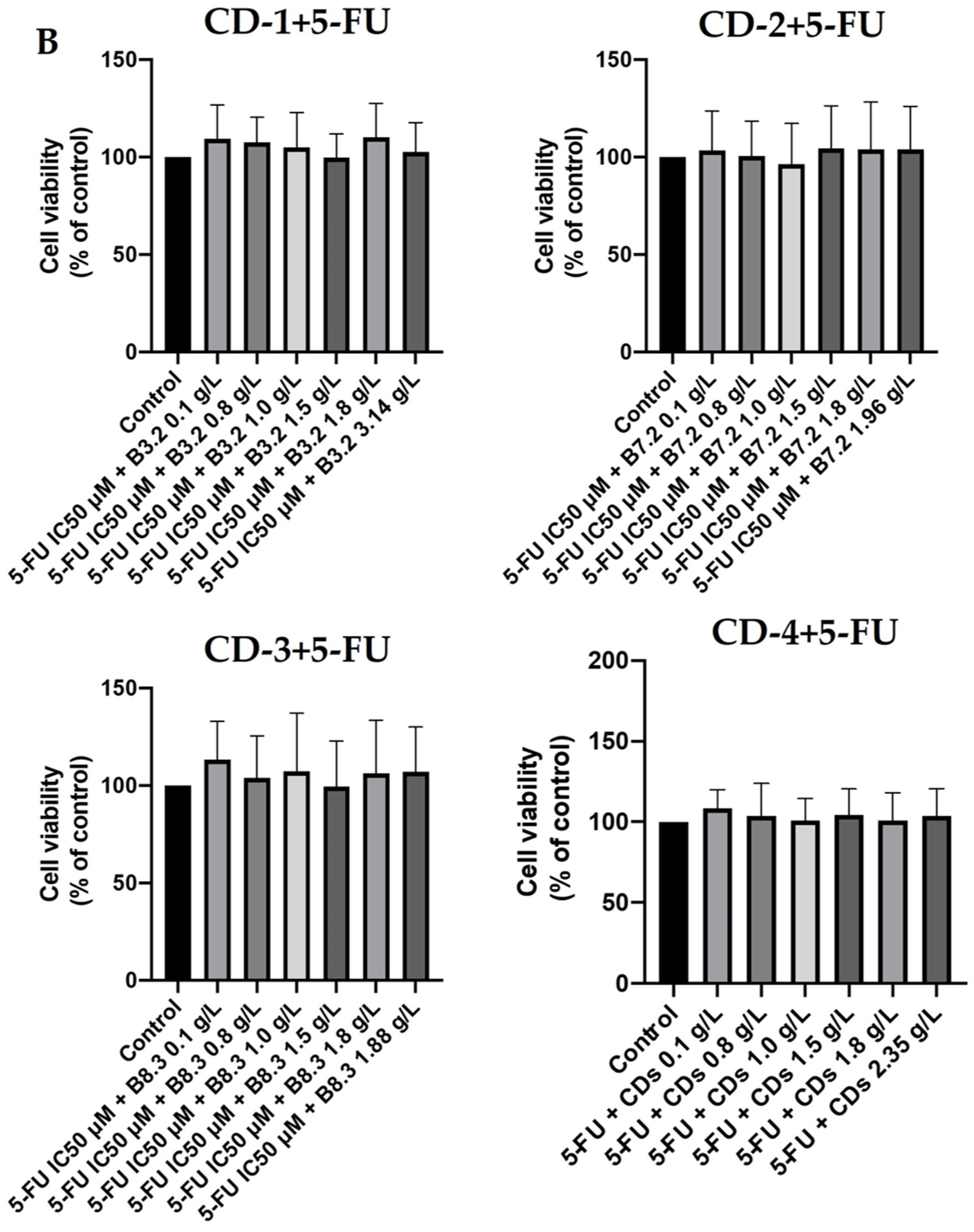
3.3. Combination Index Evaluation in the Combination of Carbon Dots CD-1–CD-4 with 5-FU in A549 and UM-UC-5 Cancer Cell Lines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Health Professional|Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/ (accessed on 24 October 2023).
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Puglisi, C.; Campolo, M.; Esposito, E.; Conoci, S. Carbon Dots as Promising Tools for Cancer Diagnosis and Therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef] [PubMed]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021, 16, 6593–6644. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology Approaches for Global Infectious Diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Yu, H.; Tang, X.; Xing, C.; Nie, G.; Akafzade, H.; Wang, S.; Chen, W. Exploration of Room-Temperature Phosphorescence and New Mechanism on Carbon Dots in a Polyacrylamide Platform and Their Applications for Anti-Counterfeiting and Information Encryption. Adv. Opt. Mater. 2022, 10, 2200678. [Google Scholar] [CrossRef]
- Fang, M.; Wang, B.; Qu, X.; Li, S.; Huang, J.; Li, J.; Lu, S.; Zhou, N. State-of-the-Art of Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Chin. Chem. Lett. 2024, 35, 108423. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yin, M.; Zhao, L.; Meng, F.; Luo, L. Recent Progress on Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Cancer Biol. Med. 2017, 14, 228. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in Drug Delivery: Past, Present and Future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as Drug Delivery Systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Adrita, S.; Tasnim, K.; Ryu, J.; Sharker, S. Nanotheranostic Carbon Dots as an Emerging Platform for Cancer Therapy. J. Nanotheranostics 2020, 1, 58–77. [Google Scholar] [CrossRef]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-Based Nanomaterials: Synthesis and Prospective Applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced Carbon Nanomaterials for State-of-the-Art Flexible Supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Su, W.; Wu, H.; Xu, H.; Zhang, Y.; Li, Y.; Li, X.; Fan, L. Carbon Dots: A Booming Material for Biomedical Applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Mikhraliieva, A.; Zaitsev, V.; Xing, Y.; Coelho-Júnior, H.; Sommer, R.L. Excitation-Independent Blue-Emitting Carbon Dots from Mesoporous Aminosilica Nanoreactor for Bioanalytical Application. ACS Appl. Nano Mater. 2020, 3, 3652–3664. [Google Scholar] [CrossRef]
- Innocenzi, P.; Stagi, L. Carbon-Based Antiviral Nanomaterials: Graphene, C-Dots, and Fullerenes. A Perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef]
- Pandiyan, S.; Arumugam, L.; Srirengan, S.P.; Pitchan, R.; Sevugan, P.; Kannan, K.; Pitchan, G.; Hegde, T.A.; Gandhirajan, V. Biocompatible Carbon Quantum Dots Derived from Sugarcane Industrial Wastes for Effective Nonlinear Optical Behavior and Antimicrobial Activity Applications. ACS Omega 2020, 5, 30363–30372. [Google Scholar] [CrossRef]
- Wang, B.; Song, H.; Qu, X.; Chang, J.; Yang, B.; Lu, S. Carbon Dots as a New Class of Nanomedicines: Opportunities and Challenges. Coord. Chem. Rev. 2021, 442, 214010. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Ali, H.; Ghosh, S.; Jana, N.R. Fluorescent Carbon Dots as Intracellular Imaging Probes. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1617. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.-T. Carbon Dots: Advances in Nanocarbon Applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Silva, S.; Duarte, D.; Crista, D.M.A.; Pinto da Silva, L.; Esteves da ValeVale, J.C.G. Normal Breast Epithelial MCF-10A Cells to Evaluate the Safety of Carbon Dots. RSC Med. Chem. 2021, 12, 245–253. [Google Scholar] [CrossRef]
- Crista, D.M.A.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of Different Bottom-up Routes for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Sendão, R.M.S.; Crista, D.M.A.; Afonso, A.C.P.; Martinez de Yuso, M.D.V.; Algarra, M.; Esteves da Silva, J.C.G.; Pinto da Silva, L.P. Insight into the Hybrid Luminescence Showed by Carbon Dots and Molecular Fluorophores in Solution. Phys. Chem. Chem. Phys. 2019, 21, 20919–20926. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C Mater. 2014, 2, 6921. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.-P. Carbon-Based Quantum Dots for Fluorescence Imaging of Cells and Tissues. RSC Adv. 2014, 4, 10791. [Google Scholar] [CrossRef]
- Jing, H.; Bardakci, F.; Akgöl, S.; Kusat, K.; Adnan, M.; Alam, M.; Gupta, R.; Sahreen, S.; Chen, Y.; Gopinath, S.; et al. Green Carbon Dots: Synthesis, Characterization, Properties and Biomedical Applications. J. Funct. Biomater. 2023, 14, 27. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Chiriu, D.; Stagi, L.; Casula, M.F.; Thakkar, S.V.; Malfatti, L.; Suzuki, K.; Ricci, P.C.; Corpino, R. Carbon Dots in Water and Mesoporous Matrix: Chasing the Origin of Their Photoluminescence. J. Phys. Chem. C 2018, 122, 25638–25650. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence Mechanism of Carbon Dots: Triggering High-Color-Purity Red Fluorescence Emission through Edge Amino Protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence Phenomena of Carbon Dots Derived from Citric Acid and Urea—A Molecular Insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef]
- Simões, E.F.C.; Esteves da Silva, J.C.G.; Leitão, J.M.M. Peroxynitrite and Nitric Oxide Fluorescence Sensing by Ethylenediamine Doped Carbon Dots. Sens. Actuators B Chem. 2015, 220, 1043–1049. [Google Scholar] [CrossRef]
- Rao, L.; Tang, Y.; Lu, H.; Yu, S.; Ding, X.; Xu, K.; Li, Z.; Zhang, J. Highly Photoluminescent and Stable N-Doped Carbon Dots as Nanoprobes for Hg2+ Detection. Nanomaterials 2018, 8, 900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon Dots Doped with Heteroatoms for Fluorescent Bioimaging: A Review. Microchim. Acta 2017, 184, 343–368. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Lu, S.; Geng, T.; Xiao, G.; Zou, B.; Bi, H. Photoactivated Fluorescence Enhancement in F,N-Doped Carbon Dots with Piezochromic Behavior. Angew. Chem. 2020, 132, 10072–10077. [Google Scholar] [CrossRef]
- Crista, D.M.A.; El Mragui, A.; Algarra, M.; Esteves da Silva, J.C.G.; Luque, R.; Pinto da Silva, L. Turning Spent Coffee Grounds into Sustainable Precursors for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1209. [Google Scholar] [CrossRef]
- John, B.K.; Abraham, T.; Mathew, B. A Review on Characterization Techniques for Carbon Quantum Dots and Their Applications in Agrochemical Residue Detection. J. Fluoresc. 2022, 32, 449–471. [Google Scholar] [CrossRef]
- Long, W.-J.; Li, X.-Q.; Yu, Y.; He, C. Green Synthesis of Biomass-Derived Carbon Dots as an Efficient Corrosion Inhibitor. J. Mol. Liq. 2022, 360, 119522. [Google Scholar] [CrossRef]
- Li, C.-L.; Ou, C.-M.; Huang, C.-C.; Wu, W.-C.; Chen, Y.-P.; Lin, T.-E.; Ho, L.-C.; Wang, C.-W.; Shih, C.-C.; Zhou, H.-C.; et al. Carbon Dots Prepared from Ginger Exhibiting Efficient Inhibition of Human Hepatocellular Carcinoma Cells. J. Mater. Chem. B 2014, 2, 4564. [Google Scholar] [CrossRef]
- Xie, Y.; Filchakova, O.; Yang, Q.; Yesbolatov, Y.; Tursynkhan, D.; Kassymbek, A.; Bouhrara, M.; Wang, K.; Balanay, M.; Fan, H. Inhibition of Cancer Cell Proliferation by Carbon Dots Derived from Date Pits at Low-Dose. ChemistrySelect 2017, 2, 4079–4083. [Google Scholar] [CrossRef]
- Wan, J.; Xu, S.; Li, J.; Yu, M.; Zhang, K.; Wei, G.; Su, Z. Facile Synthesis of Multifunctional Pharmaceutical Carbon Dots for Targeted Bioimaging and Chemotherapy of Tumors. Nanoscale 2022, 14, 11359–11368. [Google Scholar] [CrossRef]
- Fernandes, S.; Algarra, M.; Gil, A.; Esteves da Silva, J.; Pinto da Silva, L. Development of a Facile and Green Synthesis Strategy for Brightly Fluorescent Carbon Dots from Various Waste Materials. ChemSusChem 2024, e202401702. [Google Scholar] [CrossRef]
- Katharotiya, K.; Shinde, G.; Katharotiya, D.; Shelke, S.; Patel, R.; Kulkarni, D.; Panzade, P. Development, Evaluation and Biodistribution of Stealth Liposomes of 5-Fluorouracil for Effective Treatment of Breast Cancer. J. Liposome Res. 2022, 32, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Mawalizadeh, F.; Mohammadzadeh, G.; Khedri, A.; Rashidi, M. Quercetin Potentiates the Chemosensitivity of MCF-7 Breast Cancer Cells to 5-Fluorouracil. Mol. Biol. Rep. 2021, 48, 7733–7742. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.F. Reference Materials for Fluorescence Measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- Sendão, R.M.S.; Algarra, M.; Ribeiro, E.; Pereira, M.; Gil, A.; Vale, N.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Carbon Dots–TiO2 Nanocomposites for the Enhanced Visible-Light Driven Photodegradation of Methylene Blue. Adv. Sustain. Syst. 2023. [Google Scholar] [CrossRef]
- Melhuish, W.H. Quantum Efficiencies of Fluorescence of Organic Substances: Effect of Solvent and Concentration of the Fluorescent Solute. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of Excitation Dependent Fluorescence in Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Embrechts, H.; Damm, C.; Cadranel, A.; Strauss, V.; Distaso, M.; Hinterberger, V.; Guldi, D.M.; Peukert, W. Shedding Light on the Effective Fluorophore Structure of High Fluorescence Quantum Yield Carbon Nanodots. RSC Adv. 2017, 7, 24771–24780. [Google Scholar] [CrossRef]
- Christé, S.; Esteves da Silva, J.C.; Pinto da Silva, L. Evaluation of the Environmental Impact and Efficiency of N-Doping Strategies in the Synthesis of Carbon Dots. Materials 2020, 13, 504. [Google Scholar] [CrossRef]
- Sendão, R.M.S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Photocatalytic Removal of Pharmaceutical Water Pollutants by TiO2—Carbon Dots Nanocomposites: A Review. Chemosphere 2022, 301, 134731. [Google Scholar] [CrossRef]
- Esfandiari, N.; Bagheri, Z.; Ehtesabi, H.; Fatahi, Z.; Tavana, H.; Latifi, H. Effect of Carbonization Degree of Carbon Dots on Cytotoxicity and Photo-Induced Toxicity to Cells. Heliyon 2019, 5, e02940. [Google Scholar] [CrossRef]
- Kaurav, H.; Verma, D.; Bansal, A.; Kapoor, D.N.; Sheth, S. Progress in Drug Delivery and Diagnostic Applications of Carbon Dots: A Systematic Review. Front. Chem. 2023, 11, 1227843. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, J.; Gong, P.; Yang, Y.; Jiang, Y. Carbon Dots as Potential Antioxidants for the Scavenging of Multi-Reactive Oxygen and Nitrogen Species. Chem. Eng. J. 2023, 462, 142338. [Google Scholar] [CrossRef]









| Cell Line | Carbon Dot (g/L) | CD-1 | CD-2 | CD-3 | CD-4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| A549 | Fa | CI | Fa | CI | Fa | CI | Fa | CI | |
| 0.1 | 0.32571 | >10 | 0.35358 | 4.07950 | 0.29050 | >10 | 0.29327 | >10 | |
| 0.8 | 0.25045 | >10 | 0.25625 | >10 | 0.14106 | >10 | 0.27112 | >10 | |
| 1.0 | 0.21080 | >10 | 0.23775 | >10 | 0.17399 | >10 | 0.25960 | >10 | |
| 1.5 | 0.14973 | >10 | 0.11464 | >10 | 0.24125 | >10 | 0.23729 | >10 | |
| 1.8 | 0.17149 | >10 | 0.20749 | >10 | 0.21533 | >10 | 0.30515 | >10 | |
| 3.14/1.96/1.88/2.35 | 0.24841 | 2.5 × 107 | 0.30239 | >10 | 0.32533 | >10 | 0.35487 | 2.0 × 107 | |
| UM-UC-5 | 0.1 | 0.04691 | 1.38739 | 0.12282 | 0.24168 | 0.16829 | 0.24545 | 0.17125 | 1.42858 |
| 0.8 | 0.07871 | 9.49265 | 0.03349 | 0.52381 | 0.02238 | 1.25008 | 0.10344 | 6.23997 | |
| 1.0 | 0.12014 | >10 | 0.09244 | 0.31672 | 1.0 × 10−6 | >10 | 0.11796 | 8.72487 | |
| 1.5 | 1.0x10−6 | >10 | 0.12338 | 0.28694 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
| 1.8 | 0.05181 | >10 | 0.04826 | 0.48553 | 0.06963 | 1.42030 | 0.16593 | >10 | |
| 3.14/1.96/1.88/2.35 | 0.09961 | >10 | 0.11033 | 0.32097 | 0.13757 | 1.16018 | 0.16034 | >10 | |
| Cell Line | Carbon Dot (g/L) | CD-1 | CD-2 | CD-3 | CD-4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| A549 | Fa | CI | Fa | CI | Fa | CI | Fa | CI | |
| 0.1 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
| 0.8 | 0.00689 | 0.76924 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
| 1.0 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 0.00228 | 1.23695 | 1.0 × 10−6 | >10 | |
| 1.5 | 1.0 × 10−6 | >10 | 0.00368 | 0.98253 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
| 1.8 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 0.00365 | 0.99106 | 1.0 × 10−6 | >10 | |
| 3.14/1.96/1.88/2.35 | 1.0 × 10−6 | >10 | 0.00187 | 1.36813 | 1.0 × 10−6 | >10 | 0.00760 | >10 | |
| UM-UC-5 | 0.1 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 |
| 0.8 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
| 1.0 | 1.0 × 10−6 | >10 | 0.03624 | 0.45725 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
| 1.5 | 0.00255 | 1.73160 | 1.0 × 10−6 | >10 | 0.00554 | 1.17674 | 1.0 × 10−6 | >10 | |
| 1.8 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
| 3.14/1.96/1.88/2.35 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | 1.0 × 10−6 | >10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, C.M.; Ribeiro, E.; Fernandes, S.; Esteves da Silva, J.; Vale, N.; Pinto da Silva, L. Safety Evaluation of Carbon Dots in UM-UC-5 and A549 Cells for Biomedical Applications. Cancers 2024, 16, 3332. https://doi.org/10.3390/cancers16193332
Magalhães CM, Ribeiro E, Fernandes S, Esteves da Silva J, Vale N, Pinto da Silva L. Safety Evaluation of Carbon Dots in UM-UC-5 and A549 Cells for Biomedical Applications. Cancers. 2024; 16(19):3332. https://doi.org/10.3390/cancers16193332
Chicago/Turabian StyleMagalhães, Carla M., Eduarda Ribeiro, Sónia Fernandes, Joaquim Esteves da Silva, Nuno Vale, and Luís Pinto da Silva. 2024. "Safety Evaluation of Carbon Dots in UM-UC-5 and A549 Cells for Biomedical Applications" Cancers 16, no. 19: 3332. https://doi.org/10.3390/cancers16193332











