Simple Summary
There is no literature that compares the clinical outcomes between intraperitoneal chemotherapy without bevacizumab and triweekly intravenous chemotherapy plus bevacizumab. The aim of this study was to elucidate whether, in the frontline treatment of advanced ovarian, fallopian tube and primary peritoneal cancer, intraperitoneal cisplatin/paclitaxel chemotherapy without bevacizumab will confer an improved survival benefit when compared with the combination of triweekly intravenous carboplatin/paclitaxel chemotherapy and bevacizumab. We found that intraperitoneal chemotherapy without bevacizumab is associated with better survival when compared with intravenous chemotherapy with bevacizumab.
Abstract
Objectives: To compare the clinical outcomes of intravenous carboplatin/paclitaxel chemotherapy plus bevacizumab versus intraperitoneal cisplatin/paclitaxel chemotherapy without bevacizumab as the frontline treatment in women with advanced ovarian, fallopian tube and primary peritoneal cancer. Methods: Between November 2012 and January 2024, medical records of all consecutive women with stage II~IV cancer treated with either frontline adjuvant intraperitoneal cisplatin/paclitaxel without bevacizumab (IP group), intravenous carboplatin/paclitaxel without bevacizumab (IV group) or intravenous carboplatin/paclitaxel with bevacizumab (IVB group) at a tertiary referral center were reviewed. Results: A total of 143 women (IP group, n = 57; IVB group, n = 23; IV group, n = 63) were reviewed. The IP group had greater progression-free survival compared to the IVB group (49.1 months, 95% confidence interval [CI] = 27.8 months to infinity, versus 11.9 months, 95% CI = 11.2 to 16.2 months; adjusted hazard ratio [HR] = 0.45, 95% CI = 0.24 to 0.87, p = 0.017). Additionally, the IP group also had a higher overall survival compared to the IVB group (not reached, 95% CI = 55.6 months to infinity, versus 38.9 months, 95% CI = 21.9 months to infinity; adjusted HR = 0.34, 95% CI = 0.15 to 0.79, p = 0.012). Conclusions: Intraperitoneal cisplatin/paclitaxel chemotherapy without bevacizumab seems to offer a survival advantage when compared with intravenous carboplatin/paclitaxel with bevacizumab in the frontline treatment of women with advanced ovarian cancer.
1. Introduction
Platinum-based chemotherapy is the mainstay for the treatment of advanced ovarian, fallopian tube and peritoneal cancer following cytoreductive surgery. Nevertheless, the majority of women will develop recurrent cancer and eventually succumb to the disease. Various treatment approaches, such as different dosing schedules, routes of administration and integration of maintenance therapy, have been tested in prospective trials, in an attempt to improve survival. National guidelines including those of the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology and European Society of Gynecological Oncology (ESMO-ESGO) have recommended genetic testing (germline and/or somatic) that may inform future options for maintenance therapy in women with ovarian cancer, but there is no consensus on the standard testing strategy [1,2,3].
Several trials (including the Gynecology Oncologic Group (GOG) 172 trial) [4] have demonstrated a clinically significant survival advantage associated with intraperitoneal (IP) platinum-based combination chemotherapy compared to intravenous platinum-based combination chemotherapy in advanced disease [4,5,6]. On the other hand, the final results of both GOG 218 and ICON 7 studies demonstrated no survival benefit with the addition of bevacizumab to carboplatin/paclitaxel chemotherapy [7,8,9,10]. The overall survival (OS) benefit was recorded only in poor-prognosis women (i.e., stage IV disease, inoperable stage III disease or suboptimally debulked (>1 cm) stage III disease in the ICON 7 trial) [10]. Based on the conclusions drawn from the GOG 172, GOG 218 and ICON 7 studies, IP chemotherapy seems to be a favorable option over intravenous chemotherapy plus bevacizumab in the frontline treatment of women with advanced disease [4,7,8,9,10]. However, the GOG 252 study reported no progression-free survival (PFS) or OS benefit with the use of IP chemotherapy when compared with intravenous chemotherapy, both combined with bevacizumab [11]. Thus, the incorporation of IP chemotherapy in the treatment of advanced ovarian cancer becomes questionable in the era of bevacizumab [12].
To the best of our knowledge, there is no literature that compares the clinical outcomes between IP cisplatin/paclitaxel chemotherapy without bevacizumab and intravenous carboplatin/paclitaxel chemotherapy plus bevacizumab. Thus, we were interested in defining the role of IP chemotherapy without bevacizumab, and we aimed to elucidate whether there was a survival difference between the IP cisplatin/paclitaxel chemotherapy without bevacizumab and the combination of intravenous carboplatin/paclitaxel chemotherapy and bevacizumab in the frontline treatment of advanced ovarian, fallopian tube and primary peritoneal cancer.
2. Materials and Methods
From November 2012 to January 2024, the medical records of all consecutive women aged 20 and above with International Federation of Gynecology and Obstetrics (FIGO) stage II–IV advanced ovarian, fallopian tube or primary peritoneal cancer who received debulking surgery, followed by either intravenous chemotherapy with bevacizumab (i.e., the IVB group), intravenous chemotherapy without bevacizumab (i.e., the IV group) or IP chemotherapy (i.e., the IP group) in a tertiary referral center were reviewed. Women who received neoadjuvant chemotherapy followed by interval debulking surgery were also eligible for participation. IP chemotherapy was defined as having one or more cycles of an IP regimen administered. Optimal debulking surgery was defined as having residual tumor with a maximum diameter less than 1 cm. The choice of chemotherapy was solely based on the physician’s preference. IP chemotherapy was performed by four of six physicians, and it was mainly delivered by two of the physicians (i.e., IP-preferring physicians), who are familiar with IP chemotherapy port implantation, irrespective of the amount of residual disease after primary surgery, as long as the women consented to the treatment plan. Bevacizumab was added to the intravenous chemotherapy by the rest of the physicians, if affordable, since its cost was not covered by the national health insurance. This study was approved by the Research Ethics Review Committee of the hospital.
The intravenous chemotherapy regimen was given as 175 mg/m2 paclitaxel and carboplatin at a dose calculated to produce an area under the curve (AUC) of 6 mg/mL per min on day 1. The dose of carboplatin was calculated with the formula of Calvert and colleagues [13]. Bevacizumab was given at a dose of 7.5 mg/kg intravenously on day 2 from cycle 2. The treatments were repeated every 3 weeks for six cycles. Women who did not show a complete response after six cycles of chemotherapy were potentially treated with an additional one or two cycles of chemotherapy. Bevacizumab was continued for 12 additional cycles or until disease progression, death, unacceptable toxic effects or patient voluntary withdrawal [8]. The IP regimen was given as 135 mg/m2 intravenous paclitaxel over a 3 h period on day 1, followed by 100 mg/m2 intraperitoneal cisplatin on day 2 and 60 mg/m2 intraperitoneal paclitaxel on day 8 [4,14]. For women with significantly impaired renal function (i.e., estimated glomerular filtration rate < 50 mL/min per 1.73 m2), carboplatin was used instead of cisplatin.
In women with measurable tumors, the treatment response was evaluated according to the World Health Organization criteria [15]. Otherwise, Rustin’s criteria were used in women with a cancer antigen-125 (CA-125) ≥ 40 IU/mL and without measurable tumors [16,17]. A complete response (CR) was defined as the disappearance of all evidence (including CA-125 and images) of the tumor for at least 4 weeks. A partial response (PR) was defined as a ≥50% reduction in the sum of the products of the perpendicular diameters of all measurable lesions when compared with baseline in two observations at least 4 weeks apart, or a ≥50% reduction in CA-125 for at least 4 weeks. Progressive disease (PD) was defined as a ≥25% increase in the sum of the products of the perpendicular diameters of all measurable lesions, the appearance of new lesions or a ≥25% increase in CA-125. Stable disease (SD) was defined as any condition not meeting any of the above criteria [15,16,17].
Disease recurrence was assessed according to the CA-125 criteria of disease progression [15,16], the appearance of abnormal radiological findings or histological proof from biopsy analyses, whichever occurred first.
OS was calculated as the time interval from the date of surgery or the start of neoadjuvant chemotherapy to the date of death from any cause or last follow-up. PFS was defined as the time interval from the date of surgery or the start of neoadjuvant chemotherapy to clinically defined recurrence, disease progression, death from any cause or last follow-up.
Stata version 11.0 (Stata Corp, College Station, TX, USA) was used for statistical analyses. Survival curves were generated using the Kaplan–Meier method, and differences in the survival curves were calculated with the log-rank test. A p-value less than 0.05 was considered statistically significant. A multivariable Cox proportional-hazards model was run using all statistically significant variables (p < 0.05) in the univariate analysis to identify independent predictors of PFS and OS. Correlation coefficients were calculated to reveal the interactions between variables, particularly between the type of chemotherapy and other covariates. Correlation coefficients > 0.50 were considered to be strong correlations. Proportional-hazards assumption testing using Schoenfeld residuals was also applied. If the p-value of the global test was <0.05, then the Cox model did not meet the proportional-hazards assumption.
3. Results
A total of 143 consecutive women who underwent debulking surgery and adjuvant intravenous/intraperitoneal chemotherapy with/without bevacizumab (IVB group, n = 23; IV group, n = 63; IP group, n = 57) were reviewed (Table 1). There were no significant differences in the baseline characteristics between the three groups of women except the baseline performance status (Table 1). Despite two physicians being preferred to perform IP chemotherapy compared to the other four physicians (correlation coefficient = 0.538, Table 1), there was no difference in the residual disease status between the IP-preferring physician group and the other physician group (i.e., the IP-preferring physician group vs. the other physician group: no residual—22 vs. 12, optimal—29 vs. 10, suboptimal—41 vs. 29, correlation coefficient = −0.075). Seven women underwent neoadjuvant chemotherapy. One woman had R0, two women had an <1 cm residual tumor and four women had an >1 cm residual tumor after interval debulking surgery.

Table 1.
Baseline characteristics of women with advanced ovarian, fallopian tube or primary peritoneal cancer (n = 143).
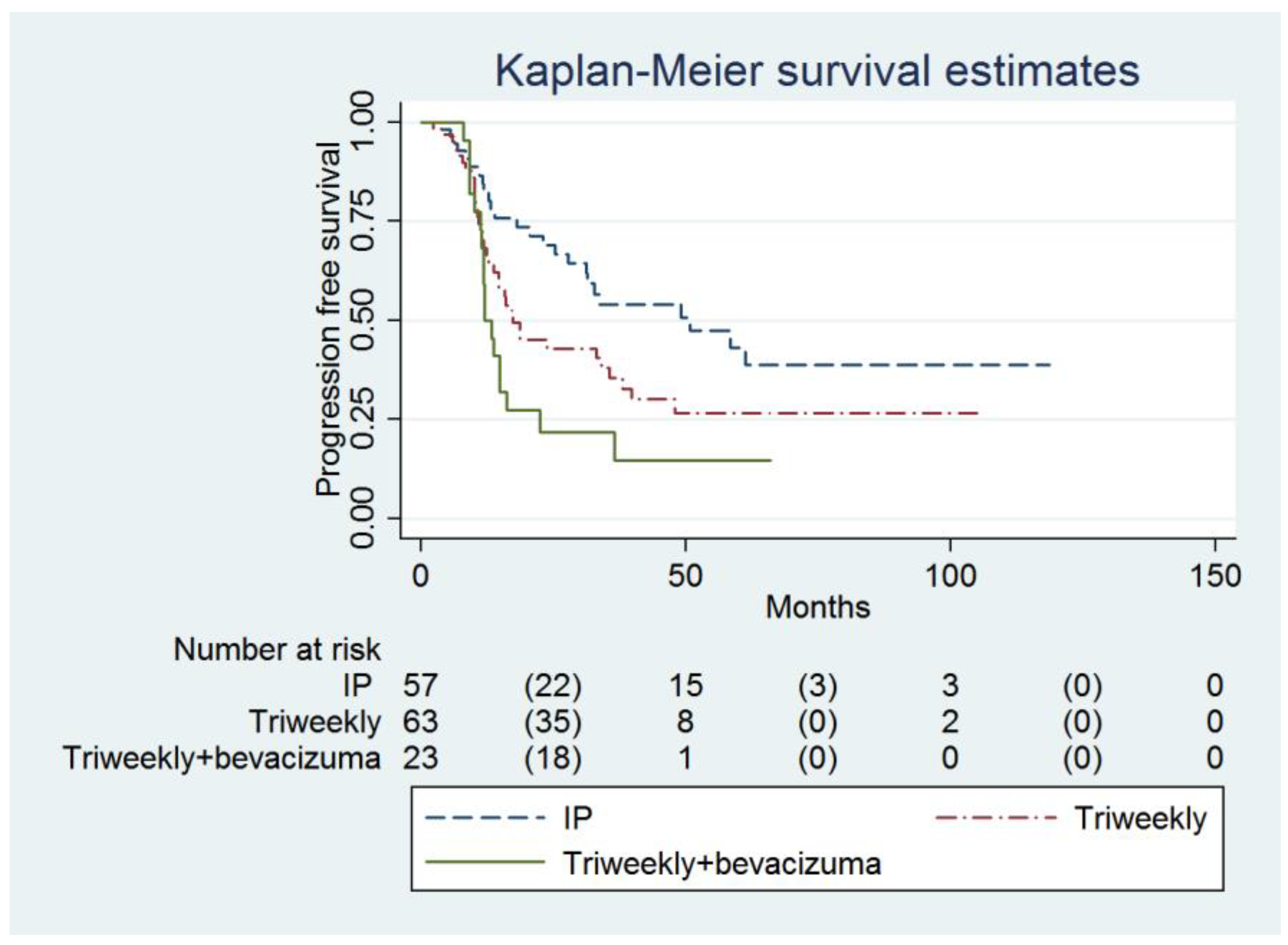
Multivariable analysis showed that the IP group had a higher PFS compared to the IVB group (49.1 months, 95% confidence interval [CI] = 27.8 months to infinity, versus 11.9 months, 95% CI = 11.2 to 16.2 months; adjusted hazard ratio [HR] = 0.45, 95% CI = 0.24 to 0.87, p = 0.017, Table 2, Figure 1). The baseline CA-125 value (adjusted HR = 1.00007, 95% CI = 1.00001 to 1.00014, p = 0.031) and FIGO Stage were determined to be the independent predictors of PFS (Table 2).

Table 2.
Cox proportional-hazards model to predict progression-free survival (n = 143).
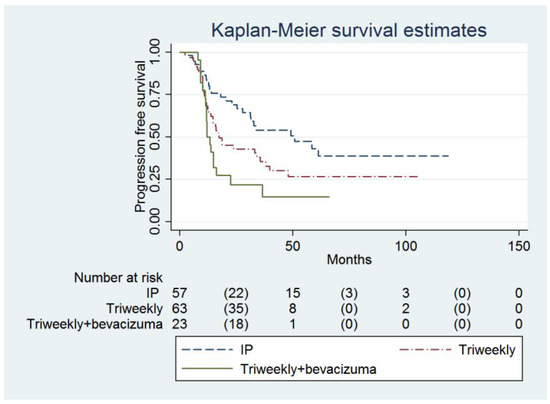
Figure 1.
Probabilities of progression-free survival in all enrolled women (n = 143).
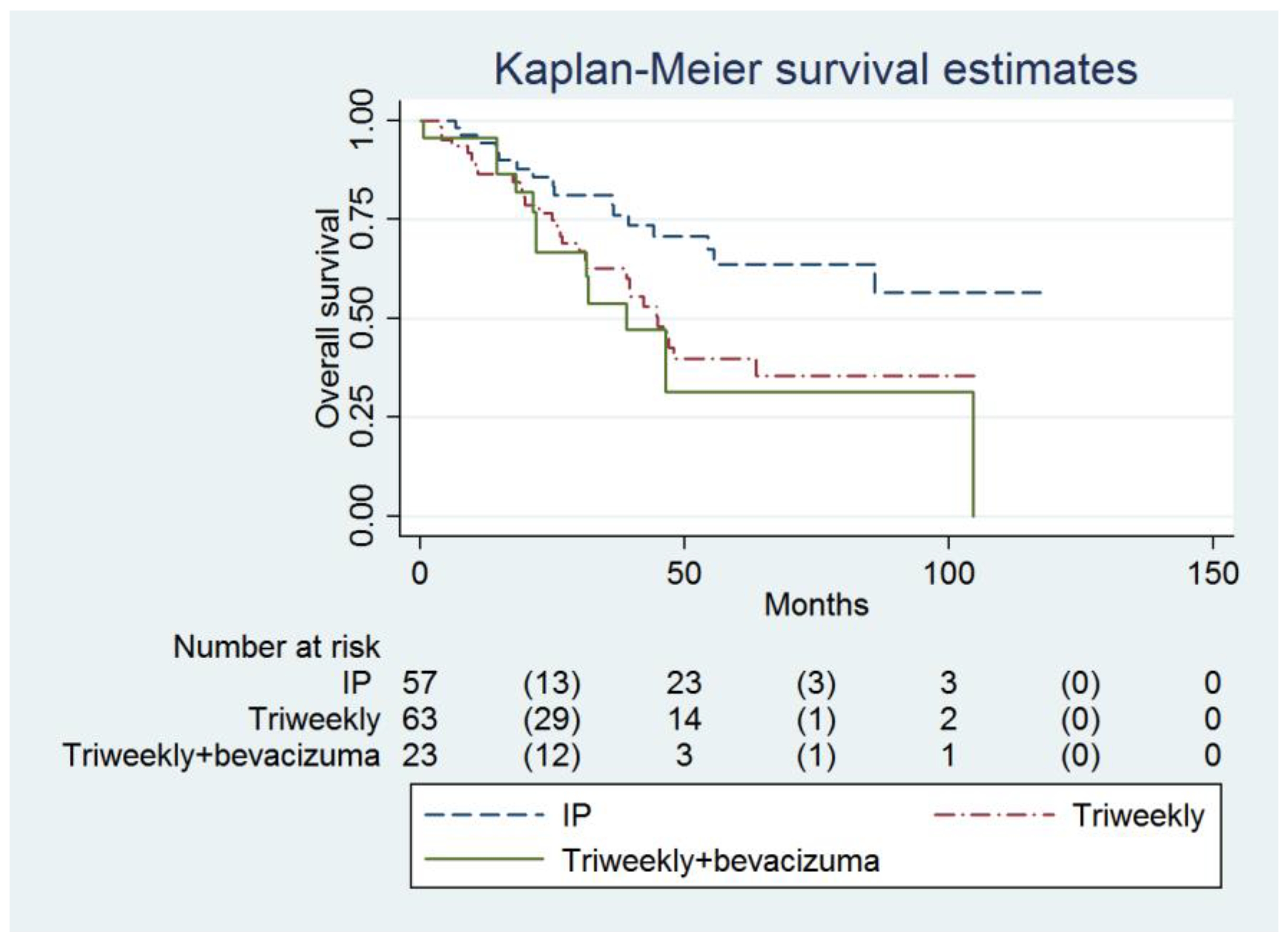
The IP group also had a higher OS compared to the IVB group (not reached, 95% CI = 55.6 months to infinity, versus 38.9 months, 95% CI = 21.9 months to infinity; adjusted HR = 0.34, 95% CI = 0.15 to 0.79, p = 0.012, Table 3, Figure 2). Age (adjusted HR = 1.03, 95% CI = 1.00 to 1.06, p = 0.022), FIGO Stage and the number of chemotherapy cycles (adjusted HR = 0.70, 95% CI = 0.58 to 0.84, p <0.001) were independent predictors of OS (Table 3).

Table 3.
Cox proportional-hazards model to predict overall survival (n = 143).
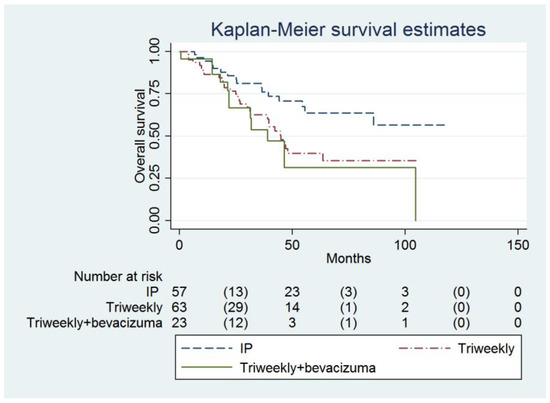
Figure 2.
Probabilities of overall survival in all enrolled women (n = 143).
A CA-125 value of ≥1176 U/mL was determined to be the optimal cut-off value for predicting disease recurrence/progression, with an AUC of 0.620 (95% CI = 0.527 to 0.712; sensitivity = 49.35%, specificity = 68.75%, Supplementary Figure S1). An age ≥ 55 years old was the optimal cut-off value for predicting death, with an AUC of 0.601 (95% CI = 0.507 to 0.695; sensitivity = 79.66%, specificity = 42.86%, Supplementary Figure S2). A number of chemotherapy cycles ≤ 5 was the optimal cut-off value for predicting death, with an AUC of 0.478 (95% CI = 0.389 to 0.567; sensitivity = 89.29%, specificity = 22.03%, Supplementary Figure S3).
4. Discussion
In our study, the use of IP without bevacizumab was associated with a better PFS and OS when compared with IVB. The adjusted HR of the IP was 0.45 in PFS compared to IVB (Table 2). The above result means that the risk of progression/recurrence reduced by 55% when using IP without bevacizumab as the frontline treatment compared to IVB. Additionally, compared to IVB, the adjusted HR of the IP was 0.34 in OS (Table 3). The above finding means that the risk of death reduced by 66% when using IP without bevacizumab as the frontline treatment compared to IVB. Similarly, a phase II trial also found that women with newly diagnosed ovarian cancer who received intraperitoneal chemotherapy without bevacizumab seemed to have a superior OS compared to those who received intraperitoneal chemotherapy plus bevacizumab (median OS: 79.7 versus 68 months) [18]. In addition, bevacizumab was reported to have no survival benefit in the GOG 218 and ICON 7 studies [9,10]. In the most recent Japanese phase II/III iPOCC trial, intraperitoneal carboplatin in combination with dose-dense intravenous paclitaxel without bevacizumab improved PFS versus an intravenous regimen in women with advanced ovarian cancer, regardless of the residual tumor size after initial surgery [19]. Taken together with our study, IP without bevacizumab seems to have a superior survival advantage as a frontline adjuvant treatment for advanced ovarian cancer compared to an intravenous regimen plus bevacizumab.
IP treatment provides a pharmacological advantage by directly exposing the tumor to a greater concentration of chemotherapeutic drugs [20], whereas bevacizumab causes a reduction in tumor vascularization and angiogenesis [7,8]. The reason why our IP group had a better survival benefit is unknown (Table 2 and Table 3). However, antiangiogenic therapy could prune tumor vessels excessively, rather than normalizing them, and thus decrease the delivery of chemotherapeutic agents [21]. Tumor angiogenesis can rapidly rebound when discontinuing vascular endothelial growth factor inhibition [22,23]. The above phenomenon might be a feasible explanation to justify our finding of a superior survival benefit in the IP group when compared with the IVB group.
An increased number of IP or intravenous chemotherapy cycles was a predictor of better OS (adjusted HR = 0.70, p < 0.001, Table 3). From our ROC analysis, we found that a number of IP or intravenous chemotherapy cycles ≤ 5 was the optimum cut-off value to predict death (Supplementary Figure S3). That is, an increase in the number of chemotherapy cycles seems to have a positive effect on the OS, and at least five cycles of chemotherapy was suggested. Similarly, both Yen et al. and Ting et al. reported that at least five cycles were needed to effectively prolong survival in women treated with IP [14,24].
In our study, advanced age was a predictor of poor OS (adjusted HR = 1.03, p = 0.026, Table 3), and the age cut-off was ≥55 years when using ROC analysis to predict death (Supplementary Figure S2). Kim et al. concluded in his study that an age cut-off of 66 years may be the prognostic indicator and the optimal starting point for a comprehensive geriatric assessment of ovarian cancer patients, especially in the serous histological subtype [25]. An older age at diagnosis was reported to have an adverse effect on the survival outcome in ovarian cancer patients, partly due to the greater likelihood of elderly patients having an advanced stage and higher-grade disease, but tumor biology differences in various age groups are also a potential explanation [26].
A higher baseline CA-125 level was associated with a poor PFS (adjusted HR = 1.00007, p = 0.031, cut-off value ≥ 1176 U/mL to predict recurrence/progression, Table 2). In the past, attempts have been made to determine an accurate CA-125 cut-off value to predict the optimal primary surgical cytoreduction, since the extent of surgery is predictive of cancer-related survival. It was proposed that higher preoperative serum CA125 values are directly related to a larger tumor burden. Baseline CA-125 has been used as a predictor of optimal cytoreduction [27,28,29], and 313.6 [27] and 500 U/mL [28] were identified to be optimal cut-off values for predicting the optimal cytoreduction. Nonetheless, Chi et al. reported that there was no identified threshold CA125 to predict the optimal cytoreduction [29]. In our study, a serum CA-125 value ≥ 1176 U/mL was the optimal cut-off level for predicting disease recurrence/progression. Similarly, a Gynecologic Oncology Group study also found that shorter PFS was observed with increasing CA-125 [30]. However, the role of CA-125 in predicting disease recurrence/progression seems to be limited due to its low AUC (AUC = 0.620 in our study). In any case, identifying a universal threshold serum CA-125 value to predict optimal cytoreduction or disease recurrence may not be possible because physicians vary in their surgical aggressiveness [28].
We acknowledge that the clinical evidence of this study is limited due to its retrospective nature, limited sample size, nonrandomized nature and the choice of chemotherapy based on the physician’s preference. In addition, bevacizumab 7.5 mg/kg was used in this study, instead of 15 mg/kg. Furthermore, the 95%CI for PFS of the IP group was wide (“27.8 months to infinity”) (Table 1), which indicates uncertainty in the estimate. Further follow-up with a larger sample size of women receiving IP treatment might improve the estimate’s precision. Despite the iPOCC trial revealing that intraperitoneal therapy improved PFS regardless of the residual tumor size after initial surgery [19], the finding that our patients underwent intraperitoneal chemotherapy irrespective of the amount of residual disease after primary surgery might stand as one of the limitations of this study.
Furthermore, in this study, some baseline characteristics such as the extent of cytoreduction and performance status were not well-balanced between groups. In addition, some patients received neoadjuvant chemotherapy, and bevacizumab was added to the intravenous chemotherapy only if affordable. However, we used multivariable analysis in an attempt to minimize the bias.
5. Conclusions
In conclusion, intraperitoneal chemotherapy with cisplatin/paclitaxel seems to be associated with a better survival benefit compared to intravenous carboplatin/paclitaxel chemotherapy with bevacizumab in the frontline treatment of women with advanced ovarian, fallopian tube or primary peritoneal cancer.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers16193382/s1, Figure S1: The receiver operating characteristic curve for the serum CA-125 value as a predictor of disease recurrence; Figure S2: The receiver operating characteristic curve for age as a predictor of death; Figure S3: The receiver operating characteristic curve for the number of chemotherapy cycles as a predictor of death.
Author Contributions
Conceptualization, S.-M.H.; methodology, S.-M.H.; validation, W.-H.T. and S.-M.H.; formal analysis, W.-H.T. and S.-M.H.; investigation, W.-H.T. and H.-H.C.; resources, W.-H.T., H.-H.C., M.-C.W., H.-D.S. and S.-M.H.; data curation, W.-H.T. and S.-M.H.; writing—original draft preparation, W.-H.T.; writing—review and editing, H.-H.C., M.-C.W., H.-D.S. and S.-M.H.; visualization, W.-H.T., H.-H.C., M.-C.W., H.-D.S. and S.-M.H.; supervision, H.-H.C., M.-C.W., H.-D.S. and S.-M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of FAR Eastern Memorial Hospital (protocol code 113131-E and date of approval 25th June 2024).
Informed Consent Statement
Patient consent was waived due to retrospective nature of this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical issues.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Comprehensive Cancer Network Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 3.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 23 July 2024).
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Stegel, V.; Blatnik, A.; Škof, E.; Dragoš, V.Š.; Krajc, M.; Gregorič, B.; Škerl, P.; Strojnik, K.; Klančar, G.; Banjac, M.; et al. Real-World Data on Detection of Germline and Somatic Pathogenic/Likely Pathogenic Variants in BRCA1/2 and Other Susceptibility Genes in Ovarian Cancer Patients Using Next Generation Sequencing. Cancers 2022, 14, 1434. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gyne-cologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Liu, P.Y.; Hannigan, E.V.; O’Toole, R.; Williams, S.D.; Young, J.A.; Franklin, E.W.; Clarke-Pearson, D.L.; Malviya, V.K.; DuBeshter, B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996, 335, 1950–1955. [Google Scholar] [CrossRef]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef]
- Monk, B.J.; Chan, J.K. Is intraperitoneal chemotherapy still an acceptable option in primary adjuvant chemotherapy for ad-vanced ovarian cancer? Ann. Oncol. 2017, 28, viii40–viii45. [Google Scholar] [CrossRef] [PubMed]
- Calvert, A.H.; Newell, D.R.; Gumbrell, L.A.; O’Reilly, S.; Burnell, M.; Boxall, F.E.; Siddik, Z.H.; Judson, I.R.; Gore, M.E.; Wiltshaw, E. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J. Clin. Oncol. 1989, 7, 1748–1756. [Google Scholar] [CrossRef]
- Ting, W.H.; Hsiao, C.H.; Chen, H.H.; Wei, M.C.; Lin, H.H.; Hsiao, S.M. Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab. Int. J. Environ. Res. Public. Health 2020, 17, 3603. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; Nelstrop, A.E.; McClean, P.; Brady, M.F.; McGuire, W.P.; Hoskins, W.J.; Mitchell, H.; Lambert, H.E. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J. Clin. Oncol. 1996, 14, 1545–1551. [Google Scholar] [CrossRef]
- Vergote, I.; Rustin, G.J.; Eisenhauer, E.A.; Kristensen, G.B.; Pujade-Lauraine, E.; Parmar, M.K.; Friedlander, M.; Jakobsen, A.; Vermorken, J.B. Re: New guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J. Natl. Cancer Inst. 2000, 92, 1534–1535. [Google Scholar] [CrossRef]
- Krasner, C.N.; Castro, C.; Penson, R.T.; Roche, M.; Matulonis, U.A.; Morgan, M.A.; Drescher, C.; Armstrong, D.K.; Wolfe, J.K.; Lee, H.; et al. Final report on serial phase II trials of all-intraperitoneal chemotherapy with or without bevacizumab for women with newly diagnosed, optimally cytoreduced carcinoma of Müllerian origin. Gynecol. Oncol. 2019, 153, 223–229. [Google Scholar] [CrossRef]
- Nagao, S.; Fujiwara, K.; Yamamoto, K.; Tanabe, H.; Okamoto, A.; Takehara, K.; Saito, M.; Fujiwara, H.; Tan, D.S.P.; Yamaguchi, S.; et al. Intraperitoneal Carboplatin for Ovarian Cancer—A Phase 2/3 Trial. NEJM Evid. 2023, 2, EVIDoa2200225. [Google Scholar] [CrossRef]
- Derick, R.L.; Flessner, M.F. Pharmacokinetic Problems in Peritoneal Drug Administration: Tissue Penetration and Surface Exposure. J. Natl. Cancer Inst. 1997, 89, 480–487. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, J.; Righi, E.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef]
- Mancuso, M.R.; Davis, R.; Norberg, S.M.; O’Brien, S.; Sennino, B.; Nakahara, T.; Yao, V.J.; Inai, T.; Brooks, P.; Freimark, B.; et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Investig. 2006, 116, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Lambrechts, D.; Prenen, H.; Jain, R.K.; Carmeliet, P. Lessons from the adjuvant bevacizumab trial on colon cancer: What next? J. Clin. Oncol. 2011, 29, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.S.; Twu, N.F.; Lai, C.R.; Horng, H.C.; Chao, K.C.; Juang, C.M. Importance of delivered cycles and nomo-gram for intraperitoneal chemotherapy in ovarian cancer. Gynecol. Oncol. 2009, 114, 415–419. [Google Scholar] [CrossRef]
- Kim, J.; Chang, Y.; Kim, T.J.; Lee, J.W.; Kim, B.G.; Bae, D.S.; Choi, C.H. Optimal cutoff age for predicting prognosis associated with serous epithelial ovarian cancer: What is the best age cutoff? J. Gynecol. Oncol. 2019, 30, e11. [Google Scholar] [CrossRef]
- Chan, J.K.; Urban, R.; Cheung, M.K.; Osann, K.; Shin, J.Y.; Husain, A.; Teng, N.N.; Kapp, D.S.; Berek, J.S.; Leiserowitz, G.S. Ovarian cancer in younger vs older women: A population-based analysis. Br. J. Cancer 2006, 95, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Liao, S.B.; Li, L. Preoperative serum levels of HE4 and CA125 predict primary optimal cytoreduction in advanced epithelial ovarian cancer: A preliminary model study. J. Ovarian Res. 2020, 13, 17. [Google Scholar] [CrossRef]
- Obeidat, B.; Latimer, J.; Crawford, R. Can optimal primary cytoreduction be predicted in advanced stage epithelial ovarian cancer? Role of preoperative serum CA-125 level. Gynecol. Obstet. Investig. 2004, 57, 153–156. [Google Scholar] [CrossRef]
- Chi, D.S.; Zivanovic, O.; Palayekar, M.J.; Eisenhauer, E.L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Leitao, M.M.; Brown, C.L.; Barakat, R.R. A contemporary analysis of the ability of preoperative serum CA-125 to predict primary cytoreductive outcome in patients with advanced ovarian, tubal and peritoneal carcinoma. Gynecol. Oncol. 2009, 112, 6–10. [Google Scholar] [CrossRef]
- Zorn, K.K.; Tian, C.; McGuire, W.P.; Hoskins, W.J.; Markman, M.; Muggia, F.M.; Rose, P.G.; Ozols, R.F.; Spriggs, D.; Armstrong, D.K. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: A Gynecologic Oncology Group study. Cancer 2009, 115, 1028–1035. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).