The Impact of Combined Chemotherapy and Intra-Tumoural Injection of Phosphorus-32 Microparticles on Vascularity in Locally Advanced Pancreatic Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
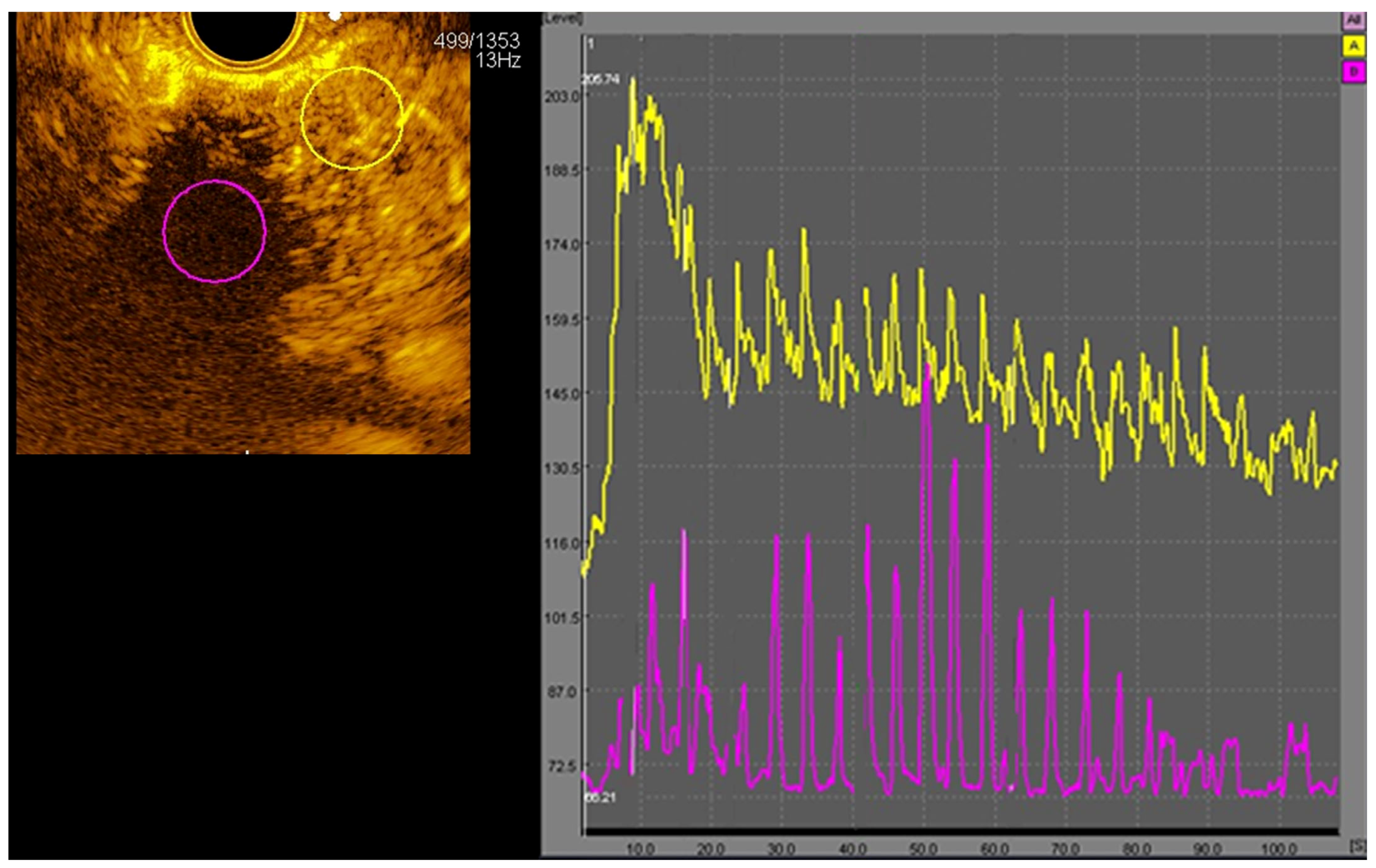
2.2. CE-EUS
2.3. 32P Implantation
2.4. Statistical Analysis
3. Results
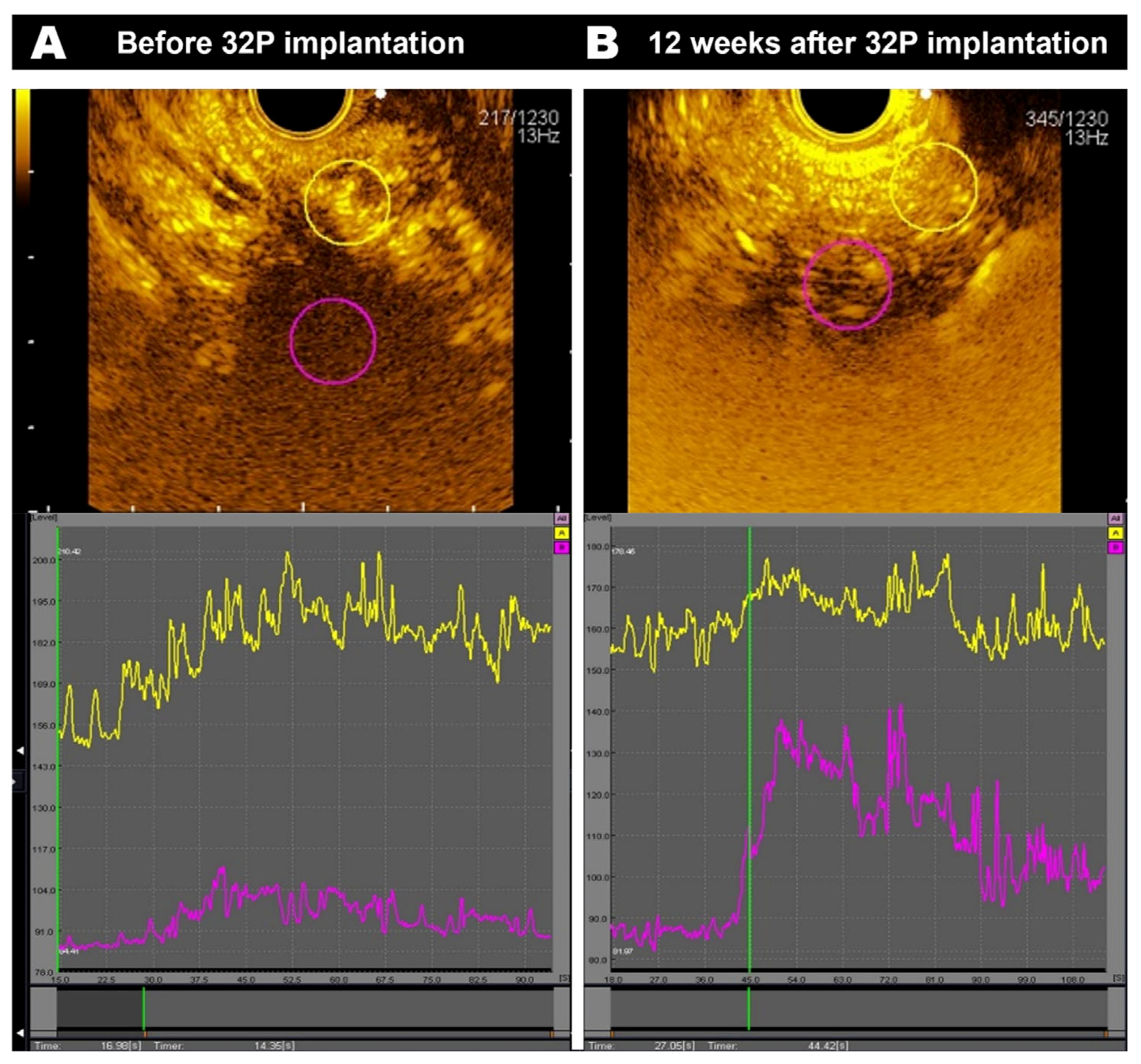
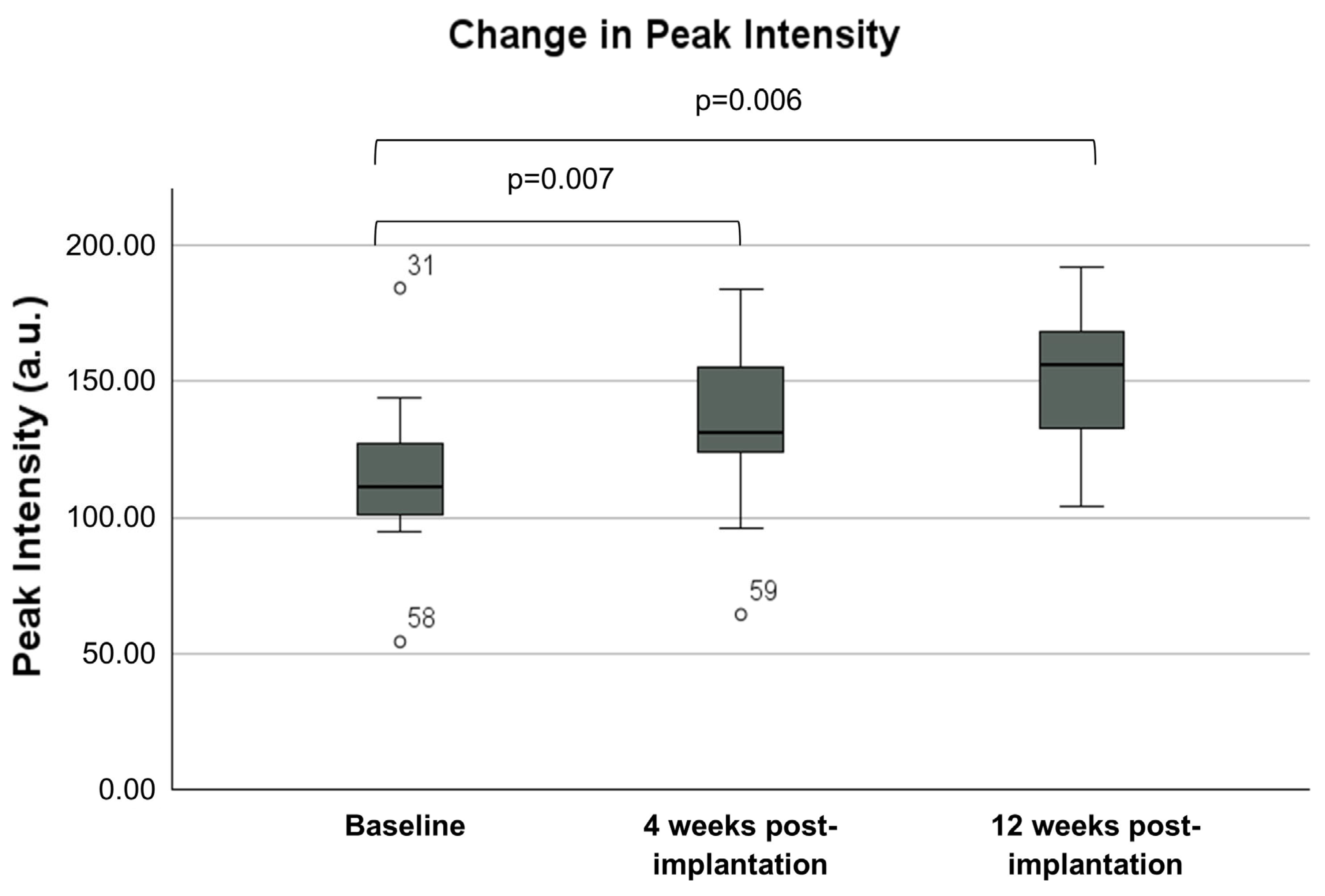
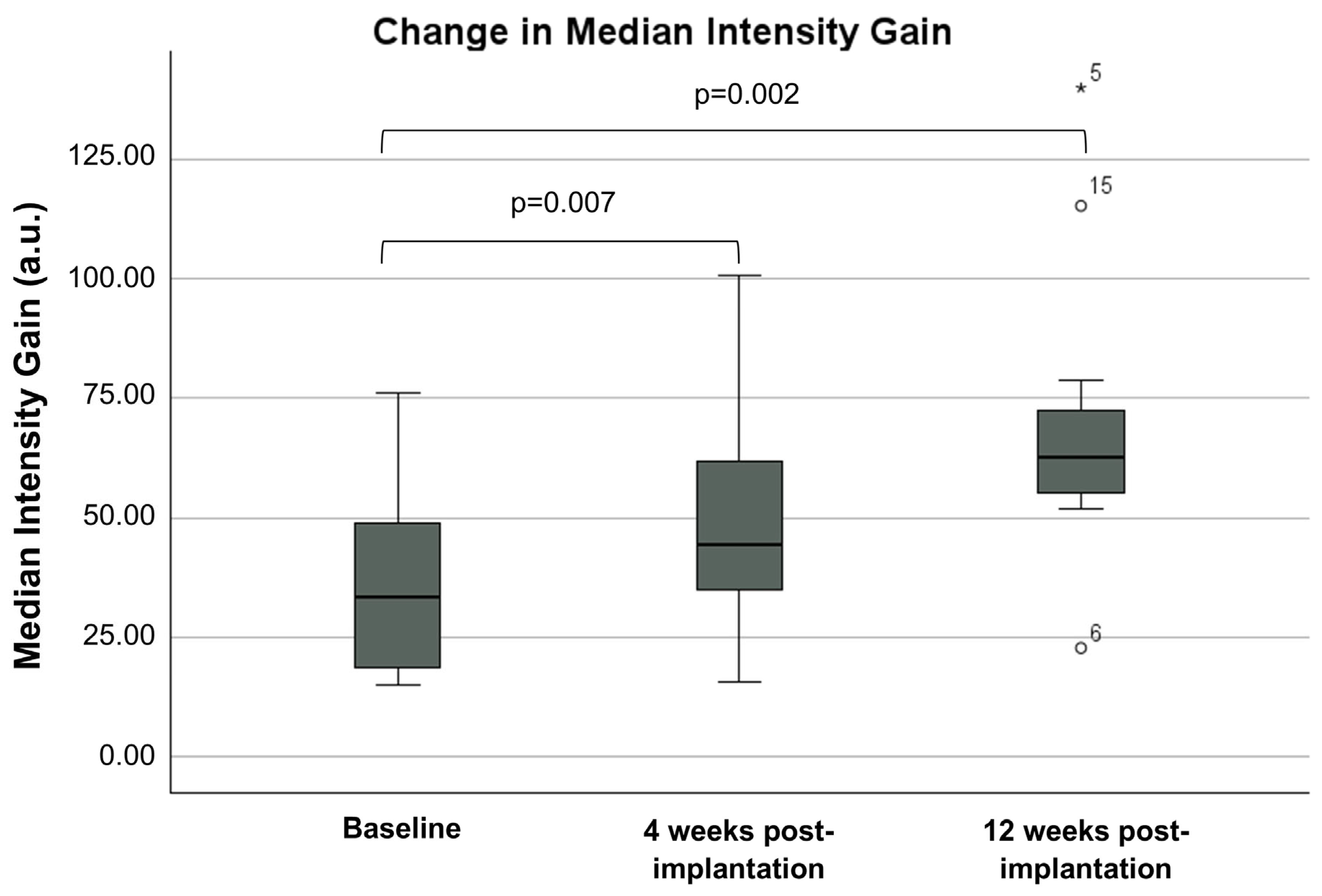
3.1. CE-EUS Results
3.2. Clinical Outcomes
3.3. Chemotherapy
3.4. GI Toxicity
3.5. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J.; et al. Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Sofuni, A.; Iijima, H.; Moriyasu, F.; Nakayama, D.; Shimizu, M.; Nakamura, K.; Itokawa, F.; Itoi, T. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J. Gastroenterol. 2005, 40, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Jurisica, I.; Do, T.; Hedley, D.W. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 2011, 71, 3110–3120. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; He, X.; Cheng, X.; Cao, G.; Chen, B.; Chen, S.; Xiong, M. A 4-gene-based hypoxia signature is associated with tumor immune microenvironment and predicts the prognosis of pancreatic cancer patients. World J. Surg. Oncol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Sofuni, A.; Itoi, T.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Moriyasu, F. Usefulness of contrast-enhanced ultrasonography in determining treatment efficacy and outcome after pancreatic cancer chemotherapy. World J. Gastroenterol. 2008, 14, 7183–7191. [Google Scholar] [CrossRef]
- Overgaard, J.; Horsman, M.R. Modification of Hypoxia-Induced Radioresistance in Tumors by the Use of Oxygen and Sensitizers. Semin Radiat. Oncol. 1996, 6, 10–21. [Google Scholar] [CrossRef]
- Bai, X.; Zhi, X.; Zhang, Q.; Liang, F.; Chen, W.; Liang, C.; Hu, Q.; Sun, X.; Zhuang, Z.; Liang, T. Inhibition of protein phosphatase 2A sensitizes pancreatic cancer to chemotherapy by increasing drug perfusion via HIF-1alpha-VEGF mediated angiogenesis. Cancer Lett. 2014, 355, 281–287. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Angiogenesis in Pancreatic Cancer: Pre-Clinical and Clinical Studies. Cancers 2019, 11, 381. [Google Scholar] [CrossRef]
- Rofstad, E.K.; Galappathi, K.; Mathiesen, B.S. Tumor interstitial fluid pressure-a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia 2014, 16, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, A.L.; Mills, I.G. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br. J. Cancer 2021, 125, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, K. Normalization of the tumor microenvironment by harnessing vascular and immune modulation to achieve enhanced cancer therapy. Exp. Mol. Med. 2023, 55, 2308–2319. [Google Scholar] [CrossRef]
- Kindler, H.L.; Niedzwiecki, D.; Hollis, D.; Sutherland, S.; Schrag, D.; Hurwitz, H.; Innocenti, F.; Mulcahy, M.F.; O’Reilly, E.; Wozniak, T.F.; et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 2010, 28, 3617–3622. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Wang, X.; Wang, Y.; Cha, N. Effects of chemoradiotherapy and chemotherapy on survival of patients with locally advanced pancreatic cancer: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12260. [Google Scholar] [CrossRef]
- Bhutani, M.S.; Cazacu, I.M.; Luzuriaga Chavez, A.A.; Singh, B.S.; Wong, F.C.L.; Erwin, W.D.; Tamm, E.P.; Mathew, G.G.; Le, D.B.; Koay, E.J.; et al. Novel EUS-guided brachytherapy treatment of pancreatic cancer with phosphorus-32 microparticles: First United States experience. VideoGIE 2019, 4, 223–225. [Google Scholar] [CrossRef]
- Ross, P.J.; Wasan, H.S.; Croagh, D.; Nikfarjam, M.; Nguyen, N.; Aghmesheh, M.; Nagrial, A.M.; Bartholomeusz, D.; Hendlisz, A.; Ajithkumar, T.; et al. Results of a single-arm pilot study of (32)P microparticles in unresectable locally advanced pancreatic adenocarcinoma with gemcitabine/nab-paclitaxel or FOLFIRINOX chemotherapy. ESMO Open 2022, 7, 100356. [Google Scholar] [CrossRef]
- Nagase, M.; Furuse, J.; Ishii, H.; Yoshino, M. Evaluation of contrast enhancement patterns in pancreatic tumors by coded harmonic sonographic imaging with a microbubble contrast agent. J. Ultrasound Med. 2003, 22, 789–795. [Google Scholar] [CrossRef]
- Emanuel, A.L.; Meijer, R.I.; van Poelgeest, E.; Spoor, P.; Serne, E.H.; Eringa, E.C. Contrast-enhanced ultrasound for quantification of tissue perfusion in humans. Microcirculation 2020, 27, e12588. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Dahel, Y.; Chanez, B.; Zemmour, C.; Piana, G.; Mitry, E.; Giovannini, M. Assessment of biological effect of nab-paclitaxel combined with gemcitabine, using contrast enhanced ultrasonography and elastography, in advanced pancreatic ductal carcinoma: A single-center pilot study. Endosc. Ultrasound 2023, 12, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Griffin, R.J.; Hui, S.; Levitt, S.H.; Song, C.W. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 2012, 177, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Clement-Colmou, K.; Potiron, V.; Pietri, M.; Guillonneau, M.; Jouglar, E.; Chiavassa, S.; Delpon, G.; Paris, F.; Supiot, S. Influence of Radiotherapy Fractionation Schedule on the Tumor Vascular Microenvironment in Prostate and Lung Cancer Models. Cancers 2020, 12, 121. [Google Scholar] [CrossRef]
- Potiron, V.A.; Abderrahmani, R.; Clement-Colmou, K.; Marionneau-Lambot, S.; Oullier, T.; Paris, F.; Supiot, S. Improved functionality of the vasculature during conventionally fractionated radiation therapy of prostate cancer. PLoS ONE 2013, 8, e84076. [Google Scholar] [CrossRef]
- Kane, J.L.; Krueger, S.A.; Hanna, A.; Raffel, T.R.; Wilson, G.D.; Madlambayan, G.J.; Marples, B. Effect of Irradiation on Tumor Microenvironment and Bone Marrow Cell Migration in a Preclinical Tumor Model. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 170–178. [Google Scholar] [CrossRef]
- Potiron, V.; Clement-Colmou, K.; Jouglar, E.; Pietri, M.; Chiavassa, S.; Delpon, G.; Paris, F.; Supiot, S. Tumor vasculature remodeling by radiation therapy increases doxorubicin distribution and efficacy. Cancer Lett. 2019, 457, 1–9. [Google Scholar] [CrossRef]
- Ng, S.P.; Koay, E.J. Current and emerging radiotherapy strategies for pancreatic adenocarcinoma: Stereotactic, intensity modulated and particle radiotherapy. Ann. Pancreat. Cancer 2018, 1, 22. [Google Scholar] [CrossRef]
- Teriaca, M.A.; Loi, M.; Suker, M.; Eskens, F.; van Eijck, C.H.J.; Nuyttens, J.J. A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): Long-term outcome. Radiother. Oncol. 2021, 155, 232–236. [Google Scholar] [CrossRef]
- Ng, I.W.; Soon, Y.Y.; Chen, D.; Tey, J.C.S. Chemoradiotherapy versus chemotherapy for locally advanced unresectable pancreatic cancer: A systematic review and meta-analysis. Asia Pac. J. Clin. Oncol. 2018, 14, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Walma, M.S.; Brada, L.J.; Patuleia, S.I.S.; Blomjous, J.G.; Bollen, T.L.; Bosscha, K.; Bruijnen, R.C.; Busch, O.R.; Creemers, G.J.; Daams, F.; et al. Treatment strategies and clinical outcomes in consecutive patients with locally advanced pancreatic cancer: A multicenter prospective cohort. Eur. J. Surg. Oncol. 2021, 47, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Jain, S.; Goldstein, M.; Miksad, R.; Pleskow, D.; Sawhney, M.; Brennan, D.; Callery, M.; Vollmer, C. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Terashima, K.; Nanno, Y.; Park, S.; Suga, M.; Takahashi, D.; Matsuo, Y.; Sulaiman, N.S.; Tokumaru, S.; Okimoto, T.; et al. Factors associated with long-term survival in gemcitabine-concurrent proton radiotherapy for non-metastatic locally advanced pancreatic cancer: A single-center retrospective study. Radiat. Oncol. 2022, 17, 32. [Google Scholar] [CrossRef]
- Malik, N.K.; May, K.S.; Chandrasekhar, R.; Ma, W.W.; Flaherty, L.; Iyer, R.; Gibbs, J.; Kuvshinoff, B.; Wilding, G.; Warren, G.; et al. Treatment of locally advanced unresectable pancreatic cancer: A 10-year experience. J. Gastrointest. Oncol. 2012, 3, 326–334. [Google Scholar]
- Chen, G.; Yu, Z.; Zhang, Y.; Liu, S.; Chen, C.; Zhang, S. Radiation-induced gastric injury during radiotherapy: Molecular mechanisms and clinical treatment. J. Radiat. Res. 2023, 64, 870–879. [Google Scholar] [CrossRef]
- Pollom, E.L.; Alagappan, M.; Chan, C.; Shultz, D.; Kunz, P.L.; Koong, A.; Chang, D.T. Outcomes and toxicity of SBRT for patients with unresectable pancreatic adenocarcinoma. J. Clin. Oncol. 2014, 32, 3. [Google Scholar] [CrossRef]
- Jung, J.; Yoon, S.M.; Park, J.H.; Seo, D.W.; Lee, S.S.; Kim, M.H.; Lee, S.K.; Park, D.H.; Song, T.J.; Ryoo, B.Y.; et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS ONE 2019, 14, e0214970. [Google Scholar] [CrossRef]
- Goh, A.S.; Chung, A.Y.; Lo, R.H.; Lau, T.N.; Yu, S.W.; Chng, M.; Satchithanantham, S.; Loong, S.L.; Ng, D.C.; Lim, B.C.; et al. A novel approach to brachytherapy in hepatocellular carcinoma using a phosphorous32 (32P) brachytherapy delivery device--a first-in-man study. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 786–792. [Google Scholar] [CrossRef]


| Female Sex—No. (%) | 11 (55%) |
|---|---|
| Median age (IQR)—years | 65 (58–71) |
| ECOG performance-status score—no. (%) ¥ | |
| 0 | 9 (45%) |
| 1 | 11 (55%) |
| Median Charlson Comorbidity Index Score (IQR) | 5 (3–6) |
| Primary tumour stage—no. (%) | |
| T1 | 0 (0%) |
| T2 | 5 (25%) |
| T3 | 1 (5%) |
| T4 | 14 (70%) |
| Nodal status—no. (%) | |
| N0 | 17 (85%) |
| N1 | 3 (15%) |
| Pancreatic tumour location—no. (%) | |
| Head | 7 (35%) |
| Neck | 4 (20%) |
| Body | 4 (20%) |
| Uncinate | 5 (25%) |
| Target lesion longest diameter—median (IQR), mm | 32 (27.5–38.75) |
| CA19.9—median (IQR); U/mL | 121 (51–243) |
| EORTC QLQ-C30 Scale | Median Score (/100) Pre-Implantation | Median Score (/100) 12 Weeks Post-Implantation | p-Value ¥ |
|---|---|---|---|
| Global health status/QoL | 50.0 | 58.3 | 0.036 * |
| Physical functioning | 73.3 | 73.3 | 0.582 |
| Role functioning | 66.7 | 66.7 | 0.440 |
| Emotional functioning | 75.0 | 70.8 | 0.262 |
| Cognitive functioning | 66.7 | 83.3 | 0.031 * |
| Social functioning | 58.3 | 66.7 | 0.345 |
| Fatigue | 44.4 | 38.9 | 0.138 |
| Nausea and vomiting | 16.7 | 0 | 0.439 |
| Pain | 33.3 | 16.7 | 0.152 |
| Dyspnoea | 33.3 | 0.0 | 0.492 |
| Insomnia | 33.3 | 33.3 | 0.739 |
| Appetite loss | 33.3 | 0.0 | 0.027 * |
| Constipation | 16.7 | 0.0 | 0.039 * |
| Diarrhoea | 33.3 | 33.3 | 0.230 |
| Financial difficulties | 0.0 | 0.0 | 0.236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.H.W.; Zobel, J.; Bills, M.; Hsieh, W.; Crouch, B.; Joshi, R.; Thomson, J.-E.; Neo, E.; Kuan, L.L.; Safaeian, R.; et al. The Impact of Combined Chemotherapy and Intra-Tumoural Injection of Phosphorus-32 Microparticles on Vascularity in Locally Advanced Pancreatic Carcinoma. Cancers 2024, 16, 3412. https://doi.org/10.3390/cancers16193412
Lim AHW, Zobel J, Bills M, Hsieh W, Crouch B, Joshi R, Thomson J-E, Neo E, Kuan LL, Safaeian R, et al. The Impact of Combined Chemotherapy and Intra-Tumoural Injection of Phosphorus-32 Microparticles on Vascularity in Locally Advanced Pancreatic Carcinoma. Cancers. 2024; 16(19):3412. https://doi.org/10.3390/cancers16193412
Chicago/Turabian StyleLim, Amanda Huoy Wen, Joshua Zobel, Madison Bills, William Hsieh, Benjamin Crouch, Rohit Joshi, John-Edwin Thomson, EuLing Neo, Li Lian Kuan, Romina Safaeian, and et al. 2024. "The Impact of Combined Chemotherapy and Intra-Tumoural Injection of Phosphorus-32 Microparticles on Vascularity in Locally Advanced Pancreatic Carcinoma" Cancers 16, no. 19: 3412. https://doi.org/10.3390/cancers16193412
APA StyleLim, A. H. W., Zobel, J., Bills, M., Hsieh, W., Crouch, B., Joshi, R., Thomson, J.-E., Neo, E., Kuan, L. L., Safaeian, R., Tse, E., Rayner, C. K., Ruszkiewicz, A., Singhal, N., Bartholomeusz, D., & Nguyen, N. Q. (2024). The Impact of Combined Chemotherapy and Intra-Tumoural Injection of Phosphorus-32 Microparticles on Vascularity in Locally Advanced Pancreatic Carcinoma. Cancers, 16(19), 3412. https://doi.org/10.3390/cancers16193412






