Learning Curve for Robotic Colorectal Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. How the Learning Curve in Robotic Colorectal Surgery Is Assessed
3. Type of Statistical Analysis/Method
4. Variables Used for Analysis
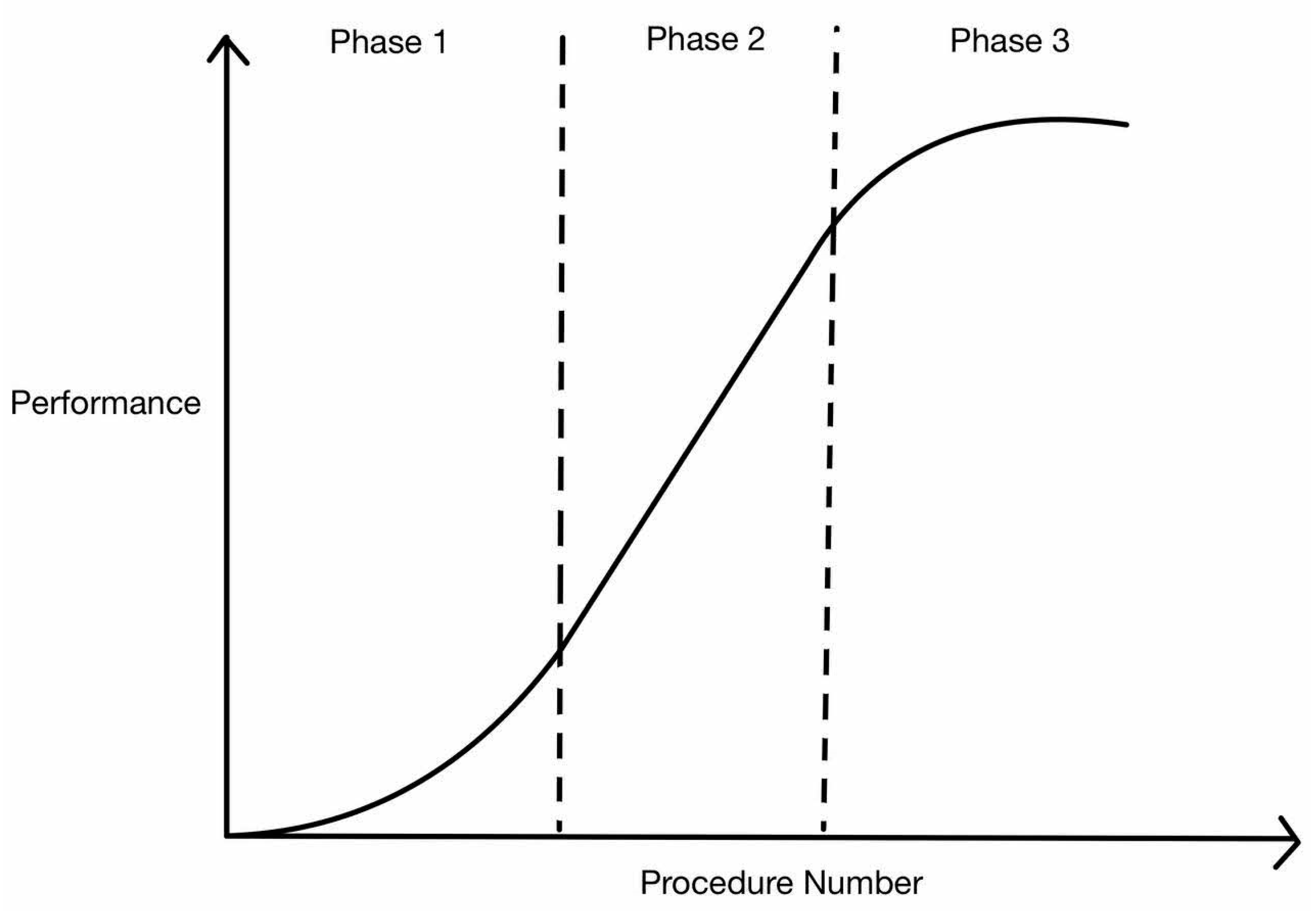
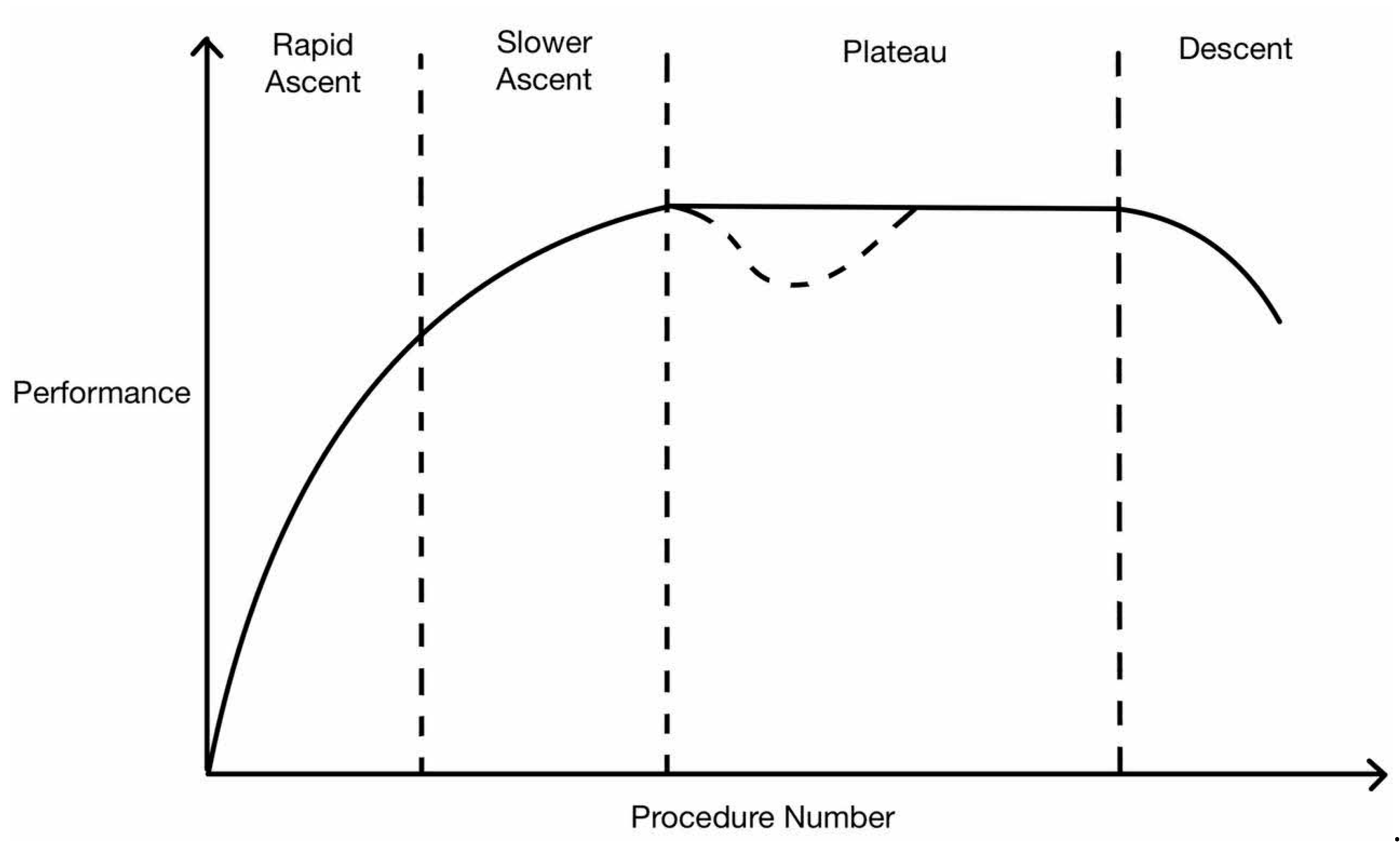
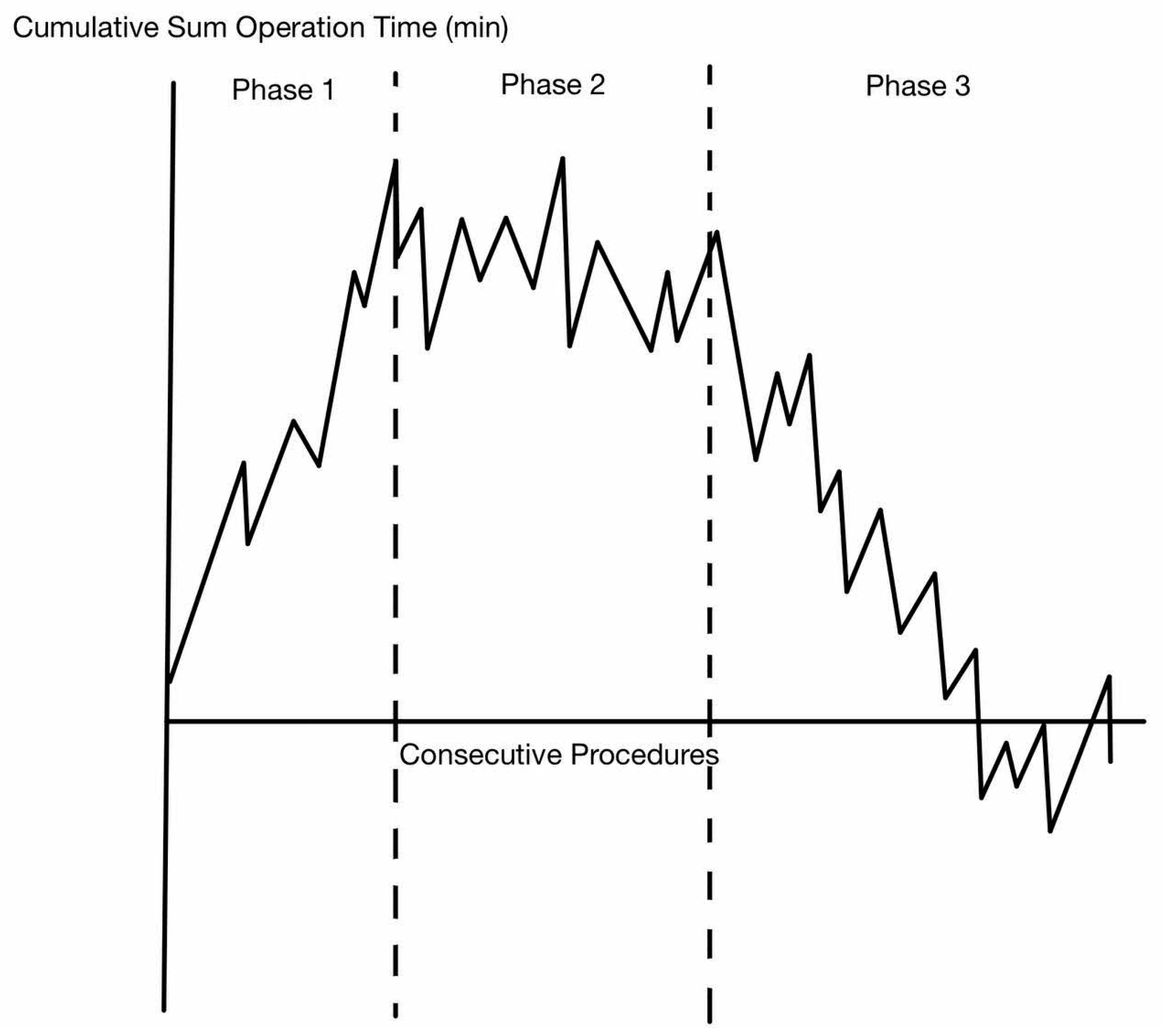
5. Interpretation of Learning Curve
6. RS Learning Curve in Terms of Operative Time
7. RS Learning Curve in Terms of Complication Rate or Patient Outcomes
8. Learning Curve of RS Compared to LS for Colorectal Operations
9. RS Learning Curve for Colon (CME)
10. Factors That Affect the Learning Curve
10.1. Prior Surgical Experience
10.2. Institutional Factor
10.3. Variety of Colorectal Operations/Case Mix
10.4. Case Complexity
10.5. Technology—daVinci Si vs. Xi vs. V
10.6. Surgical Simulation
10.7. Time Spent as First Assistant
10.8. Structured Training and Proctorship
11. How Information from Learning Curves Can Be/Have Been Used
12. Current Limitations in Learning Curve Assessment
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Study | Country | Analysis Type | Type of Surgery | Surgeons | Patients | Learning Curve Analysis | Other Variables Assessed | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IOC | POC | Pathological | Operative Time | Composite | ||||||||
| Gao et al. 2024 [30] | China | CUSUM |
TME with ISR (performed with Si) | 3 | 89 | No difference between phases | No difference between phases | Higher LN harvest in proficient phase. 14.8 vs 10.7 |
Phase 1: learning 1–47 Phase 2: proficiency > 47 | No difference in 3-year PFS, OS and LR | 47 cases required for proficiency | |
| Oshio et al. 2023 [15] | Japan | CUSUM and RA-CUSUM |
TME (performed with Si) | 1 | 100 |
Phase 1: 1–40 Phase 2: >40 |
Surgical failure defined as RM positivity, CD >1, conversion Phase 1: 1–48 Phase 2: 49–80 Phase 3: >80 | After 80 cases, quality of surgery improved | ||||
| Burghgraef 2023 [58] | Netherlands | RA-CUSUM and CUSUM |
TME (combination of Si and Xi) | 7 | 531 | All surgeons stayed within pre specified literature-based limits of an in-control state | All surgeons stayed within pre specified literature-based limits of an in-control state | All surgeons stayed within pre specified literature-based limits of an in-control state | 12–35 | 12–35 cases required to attain efficiency | ||
| Zaepfel 2023 [21] | France | CUSUM | All colorectal resection (performed with Xi) | 1 | 174 | 57 | Without mentoring 57 cases required | |||||
| Sugishita 2022 [25] | Japan | CUSUM |
TME (performed with S or Xi) | 1 | 149 |
Phase 1: 1–32 Phase 2: 33–86 Phase 3: >86 | 32 cases for consolidation and stabilization of technique and 86 cases required for mastery in TME | |||||
| Tang 2022 [17] | China | CUSUM and RA-CUSUM |
TME (performed with Si) | 1 | 389 |
Phase 1: 1–36 Phase 2: >36 |
Phase 1: 1–34 Phase 2: 35–151 Phase 3: >151 | No difference oncological outcome in the different learning phases |
Learning curve for operative time was 34 Learning curve for postoperative complication was 36 | |||
| Huang 2022 [39] | China | CUSUM |
Right hemicolectomy (performed with Si) | 1 | 76 |
Phase 1: 1–27 Phase 2: >27 | Learning curve for robotic right hemicolectomy was 27 cases based on operative time | |||||
| Wong 2022 [22] | Australia | CUSUM |
All colorectal resections (performed with Xi) | 1 | 117 |
Phase 1: 1–44 Phase 2: 45–88 Phase 3: >88 | 88 cases required for mastery | |||||
| Parascandola 2021 [59] | United States | SGA and CUSUM |
All colorectal resections (performed with Si/Si/Xi) | 1 | 502 |
Anterior and Low Anterior Plateau at: 55–65 Left hemicolectomy and sigmoidectomy Plateau at: 35–45 | Plateau performance achieved after 65 anterior/low anterior and 45 left and sigmoid colectomies | |||||
| Nasseri 2020 [20] | United States | CUSUM |
All colorectal resections (performed with Xi) | 1 | 111 |
Phase 1: 1–13 Phase 2: 14–83 Phase 3: >83 | Learning phase is 13 cases and mastery achieved after 83 cases | |||||
| Kawai 2018 [60] | Japan | CUSUM |
Rectum and sigmoid resections with/without LLND (performed with Si) | 1 | 131 |
Phase 1: 1–19 Phase 2: 20–78 Phase 3: >79 | First phase of learning curve is 19 cases | |||||
| Shaw 2018 [27] | United States | SGA and moving average |
All colorectal resections and rectopexy (system not specified) | 2 | 62 |
1–15 cases: 27% >15 cases: 6.3% |
1–15 cases: 426min >15 cases: 373 min | Overall complications reduced after 15 cases | ||||
| Guend 2016 [12] | United States | CUSUM |
All colorectal resections (performed with S/Si) | 5 | 418 | Surgeon 1 Phase 1: 1–74 Phase 2: 75–137 Phase 3: >137 Surgeons 2–4 Phase 1: 23–30 | To establish RAS colorectal surgery program require 75 cases, once established, learning curve shorter for subsequent surgeons (25–30) | |||||
| De’Angelis 2016 [36] | France | CUSUM | Right hemicolectomy (system not specified) | 1 | 30 | 16 cases for inflection point | LC 16 cases for RAS vs 25 for Lap, RAS had a faster learning curve | |||||
| Foo 2015 [61] | Hong Kong | CUSUM |
TME (performed with S) | 1 | 39 |
Phase 1: 1–8 Phase 2: 9–25 Phase 3: 26–39 | Learning curve for novice rectal surgeon is 25 cases | |||||
| Yamaguchi 2014 [14] | Japan | CUSUM |
TME (system not specified) | 1 | 80 |
Phase 1: 1–25 Phase 2: 26–50 Phase 3: 51–80 | First 25 cases formed the learning phase | |||||
| Kim 2014 [24] | Korea | Moving average and RA-CUSUM |
TME (system not specified) | 1 | 167 |
Hybrid variable of Op time, conversion, periop morbidity and circumferential margin 1st plateau 33 cases 2nd plateau 72 cases | Learning cruve greatest effect on the first 32 cases. | |||||
| Park 2014 [23] | South Korea | CUSUM and RA-CUSUM |
TME (system not specified) | 1 | 130 |
Phase 1: 1–44 Phase 2: 45–78 Phase 3: >78 |
Surgical failure: R1 resection, conversion, LN < 12, local recurrence Minimized RA-CUSUM achieved at 75th case | Technical competence after 44 cases and good perioperative outcomes after 75 cases | ||||
| Byrn 2014 [62] | United States | SGA |
TME (system not specified) | 1 | 85 |
1–43 cases: 267 min 44–85 cases: 224 min | Operative time improved with number of cases performed | |||||
| Sng 2013 [51] | South Korea | CUSUM |
TME (performed with S) | 1 | 197 | Without |
Phase 1: 1–35 Phase 2: 36–129 Phase 3: 130–197 | First phase represents initial learning phase. Second phase involved more complex cases and the third phase represents the concluding phase of the learning curve | ||||
| Kim 2012 [63] | South Korea | SGA | TME | 1 | 62 | No difference in post operative complications between learning periods | No difference in LN count and distal margin between learning periods | Total operative and console time decreased after 20 cases | Experienced open surgeon with limited laparoscopic experience may begin RAS TME safely without a long learning period | |||
| Jimenez-Rodriguez 2012 [29] | Spain | CUSUM |
TME (system not specified) | 3 | 43 |
Phase 1: 1–11 Phase 2: 12–23 Phase 3: 24–43 | Estimated learning curve for RAS TME is 21–23 cases | |||||
| Bokhari 2010 [28] | United States | CUSUM |
APR, rectopexy, AR, LAR (system not specified) | 1 | 50 |
Phase 1: 1–15 Phase 2: 16–25 Phase 3: >25 | Learning phase achieved after 15–25 cases | |||||
References
- World Health Organization. Life Expectancy: Situation. Global Health Observatory (GHO) Data. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 27 July 2024).
- Pascual, M.; Salvans, S.; Pera, M. Laparoscopic colorectal surgery: Current status and implementation of the latest technological innovations. World J. Gastroenterol. 2016, 22, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Wang, J.-X.; Chang, D.-W. A meta-analysis of robotic versus laparoscopic colectomy. J. Surg. Res. 2015, 195, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, Z.; Bie, M.; Peng, X.; Chen, C. Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: A meta-analysis. Surg. Endosc. 2016, 30, 5601–5614. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.A.; Merola S Fau-Wasielewski, A.; Wasielewski, A.; Fau-Ballantyne, G.H.; Ballantyne, G.H. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis. Colon Rectum 2002, 45, 1689–1696. [Google Scholar] [CrossRef]
- Khan, N.; Abboudi, H.; Khan, M.S.; Dasgupta, P.; Ahmed, K. Measuring the surgical ‘learning curve’: Methods, variables and competency. BJU Int. 2014, 113, 504–508. [Google Scholar] [CrossRef]
- Pernar, L.I.M.; Robertson, F.C.; Tavakkoli, A.; Sheu, E.G.; Brooks, D.C.; Smink, D.S. An appraisal of the learning curve in robotic general surgery. Surg. Endosc. 2017, 31, 4583–4596. [Google Scholar] [CrossRef] [PubMed]
- Hopper, A.N.; Jamison, M.H.; Lewis, W.G. Learning curves in surgical practice. Postgrad. Med. J. 2007, 83, 777–779. [Google Scholar] [CrossRef]
- Wong, S.W.; Crowe, P. Factors affecting the learning curve in robotic colorectal surgery. J. Robot. Surg. 2022, 16, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, C.R.; Grant Am Fau-Wallace, S.A.; Wallace Sa Fau-Garthwaite, P.H.; Garthwaite Ph Fau-Monk, A.F.; Monk Af Fau-Russell, I.T.; Russell, I.T. Statistical assessment of the learning curves of health technologies. Health Technol. Assess. 2001, 5, 12. [Google Scholar] [CrossRef]
- Dinçler, S.; Koller, M.T.; Steurer, J.; Bachmann, L.M.; Christen, D.; Buchmann, P. Multidimensional Analysis of Learning Curves in Laparoscopic Sigmoid Resection. Dis. Colon Rectum 2003, 46, 1371–1378. [Google Scholar] [CrossRef]
- Guend, H.; Widmar, M.; Patel, S.; Nash, G.M.; Paty, P.B.; Guillem, J.G.; Temple, L.K.; Garcia-Aguilar, J.; Weiser, M.R. Developing a robotic colorectal cancer surgery program: Understanding institutional and individual learning curves. Surg. Endosc. 2017, 31, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Wohl, H. The cusum plot: Its utility in the analysis of clinical data. N. Engl. J. Med. 1977, 296, 1044–1045. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kinugasa, Y.; Shiomi, A.; Sato, S.; Yamakawa, Y.; Kagawa, H.; Tomioka, H.; Mori, K. Learning curve for robotic-assisted surgery for rectal cancer: Use of the cumulative sum method. Surg. Endosc. 2015, 29, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Oshio, H.; Konta, T.; Oshima, Y.; Yunome, G.; Okazaki, S.; Kawamura, I.; Ashitomi, Y.; Kawai, M.; Musha, H.; Motoi, F. Learning curve of robotic rectal surgery using risk-adjusted cumulative summation: A 5-year institutional experience. Langenbeck’s Arch. Surg. 2023, 408, 89. [Google Scholar] [CrossRef]
- Soomro, N.A.; Hashimoto, D.A.; Porteous, A.J.; Ridley, C.J.A.; Marsh, W.J.; Ditto, R.; Roy, S. Systematic review of learning curves in robot-assisted surgery. BJS Open 2020, 4, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, T.; Gao, G.; Shi, J.; Li, T. Learning Curve of Robotic-Assisted Total Mesorectal Excision for Rectal Cancer. Front. Oncol. 2022, 12, 931426. [Google Scholar] [CrossRef]
- Green, C.A.; Levy, J.S.; Martino, M.A.; Porterfield, J.R., Jr. The current state of surgeon credentialing in the robotic era. Ann. Laparosc. Endosc. Surg. 2020, 5, 17. [Google Scholar] [CrossRef]
- Tou, S.; Au, S.; Clancy, C.; Clarke, S.; Collins, D.; Dixon, F.; Dreher, E.; Fleming, C.; Gallagher, A.G.; Gomez-Ruiz, M.; et al. European Society of Coloproctology guideline on training in robotic colorectal surgery (2024). Color. Dis. 2024, 26, 776–801. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, Y.; Stettler, I.; Shen, W.; Zhu, R.; Alizadeh, A.; Lee, A.; Cohen, J.; Barnajian, M. Learning curve in robotic colorectal surgery. J. Robot. Surg. 2021, 15, 489–495. [Google Scholar] [CrossRef]
- Zaepfel, S.; Marcovei, R.; Fernandez-de-Sevilla, E.; Sourrouille, I.; Honore, C.; Gelli, M.; Faron, M.; Benhaim, L. Robotic-assisted surgery for mid and low rectal cancer: A long but safe learning curve. J. Robot. Surg. 2023, 17, 2099–2108. [Google Scholar] [CrossRef]
- Wong, S.W.; Ang, Z.H.; Crowe, P. The learning curve to attain surgical competency in robotic colorectal surgery. ANZ J. Surg. 2022, 92, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, C.W.; Cho, M.S.; Baik, S.H.; Kim, D.W.; Min, B.S.; Lee, K.Y.; Kim, N.K. Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg. Endosc. 2014, 28, 2821–2831. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, G.-S.; Park, J.S.; Park, S.Y. multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: Lessons from a single surgeon’s experience. Dis. Colon Rectum 2014, 57, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, T.; Tsukamoto, S.; Imaizumi, J.; Takamizawa, Y.; Inoue, M.; Moritani, K.; Kinugasa, Y.; Kanemitsu, Y. Evaluation of the learning curve for robot-assisted rectal surgery using the cumulative sum method. Surg. Endosc. 2022, 36, 5947–5955. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rodríguez, R.M.; Rubio-Dorado-Manzanares, M.; Díaz-Pavón, J.M.; Reyes-Díaz, M.L.; Vazquez-Monchul, J.M.; Garcia-Cabrera, A.M.; Padillo, J.; De la Portilla, F. Learning curve in robotic rectal cancer surgery: Current state of affairs. Int. J. Color. Dis. 2016, 31, 1807–1815. [Google Scholar] [CrossRef]
- Shaw, D.D.; Wright, M.; Taylor, L.; Bertelson, N.L.; Shashidharan, M.; Menon, P.; Menon, V.; Wood, S.; Ternent, C.A. Robotic Colorectal Surgery Learning Curve and Case Complexity. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 1163–1168. [Google Scholar] [CrossRef]
- Bokhari, M.B.; Patel, C.B.; Ramos-Valadez, D.I.; Ragupathi, M.; Haas, E.M. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg. Endosc. 2010, 25, 855–860. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez, R.M.; Díaz-Pavón, J.M.; de la Portilla de Juan, F.; Prendes-Sillero, E.; Dussort, H.C.; Padillo, J. Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int. J. Color. Dis. 2013, 28, 815–821. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, H.; Ye, J.; Ruan, H.; Jiang, W.; Chi, P.; Huang, Y.; Huang, S. Robotic intersphincteric resection for low rectal cancer: A cumulative sum analysis for the learning curve. Surg. Today 2024. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, H.J.; Huh, J.W.; Kim, Y.J.; Kim, H.R. Learning curve of laparoscopic low anterior resection in terms of local recurrence. J. Surg. Oncol. 2014, 110, 989–996. [Google Scholar] [CrossRef]
- Kayano, H.; Okuda, J.; Tanaka, K.; Kondo, K.; Tanigawa, N. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg. Endosc. 2011, 25, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y.; Chen, C.-C. Transanal total mesorectal excision for rectal cancer: It’s come a long way and here to stay. Ann. Coloproctol. 2022, 38, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Burghgraef, T.A.; Sikkenk, D.J.; Verheijen, P.M.; El Moumni, M.; Hompes, R.; Consten, E.C.J. The learning curve of laparoscopic, robot-assisted and transanal total mesorectal excisions: A systematic review. Surg. Endosc. 2022, 36, 6337–6360. [Google Scholar] [CrossRef] [PubMed]
- Melich, G.; Hong, Y.K.; Kim, J.; Hur, H.; Baik, S.H.; Kim, N.K.; Sender Liberman, A.; Min, B.S. Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: Analysis of novice MIS surgeon learning curves. Surg. Endosc. 2014, 29, 558–568. [Google Scholar] [CrossRef] [PubMed]
- de’Angelis, N.; Lizzi, V.; Azoulay, D.; Brunetti, F. Robotic Versus Laparoscopic Right Colectomy for Colon Cancer: Analysis of the Initial Simultaneous Learning Curve of a Surgical Fellow. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 882–892. [Google Scholar] [CrossRef]
- Odermatt, M.; Ahmed, J.; Panteleimonitis, S.; Khan, J.; Parvaiz, A. Prior experience in laparoscopic rectal surgery can minimise the learning curve for robotic rectal resections: A cumulative sum analysis. Surg. Endosc. 2017, 31, 4067–4076. [Google Scholar] [CrossRef]
- Kim, I.K.; Kang, J.; Fau-Park, Y.A.; Park Ya Fau-Kim, N.K.; Kim Nk Fau-Sohn, S.-K.; Sohn Sk Fau-Lee, K.Y.; Lee, K.Y. Is prior laparoscopy experience required for adaptation to robotic rectal surgery?: Feasibility of one-step transition from open to robotic surgery. Int. J. Color. Dis. 2014, 29, 693–699. [Google Scholar] [CrossRef]
- Huang, P.; Li, S.; Li, P.; Jia, B. The Learning Curve of Da Vinci Robot-Assisted Hemicolectomy for Colon Cancer: A Retrospective Study of 76 Cases at a Single Center. Front. Surg. 2022, 9, 897103. [Google Scholar] [CrossRef]
- Shu, D.; Cai, Z.; Yin, X.; Zheng, M.; Li, J.; Yang, X.; Zhang, S.; Aikemu, B.; Qin, W.; Xu, X.; et al. Structured training curricula for robotic colorectal surgery in China: Does laparoscopic experience affect training effects? J. Gastrointest. Oncol. 2023, 14, 198–205. [Google Scholar] [CrossRef]
- Lebeau, T.; Rouprêt, M.; Ferhi, K.; Chartier-Kastler, E.; Bitker, M.O.; Richard, F.; Vaessen, C. The role of a well-trained team on the early learning curve of robot-assisted laparoscopic procedures: The example of radical prostatectomy. Int. J. Med. Robot. 2012, 8, 67–72. [Google Scholar] [CrossRef]
- Jamali, F.R.; Soweid, A.M.; Dimassi, H.; Bailey, C.; Leroy, J.; Marescaux, J. Evaluating the Degree of Difficulty of Laparoscopic Colorectal Surgery. Arch. Surg. 2008, 143, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Ozben, V.; Cengiz, T.B.; Fau-Atasoy, D.; Atasoy, D.; Fau-Bayraktar, O.; Bayraktar, O.; Fau-Aghayeva, A.; Aghayeva, A.; Fau-Erguner, I.; Erguner, I.; et al. Is da Vinci Xi Better than da Vinci Si in Robotic Rectal Cancer Surgery? Comparison of the 2 Generations of da Vinci Systems. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Di Franco, G.; Guadagni, S.; Rossi, L.; Palmeri, M.; Furbetta, N.; Gianardi, D.; Bianchini, M.; Caprili, G.; D’Isidoro, C.; et al. Robot-assisted total mesorectal excision for rectal cancer: Case-matched comparison of short-term surgical and functional outcomes between the da Vinci Xi and Si. Surg. Endosc. 2018, 32, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Culligan, P.; Gurshumov, E.; Lewis, C.; Priestley, J.; Komar, J.; Salamon, C. Predictive Validity of a Training Protocol Using a Robotic Surgery Simulator. Urogynecology 2014, 20, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.; Ang, Z.H.; Crowe, P. Improving ergonomics for the bedside assistant in robotic colorectal surgery. J. Surg. Case Rep. 2023, 2023, rjad007. [Google Scholar] [CrossRef]
- Cimen, H.I.; Atik, Y.T.; Gul, D.; Uysal, B.; Balbay, M.D. Serving as a bedside surgeon before performing robotic radical prostatectomy improves surgical outcomes. Int. Braz. J. Urol. 2019, 45, 1122–1128. [Google Scholar] [CrossRef]
- Favre, A.; Huberlant, S.; Carbonnel, M.; Goetgheluck, J.; Revaux, A.; Ayoubi, J.M. Pedagogic Approach in the Surgical Learning: The First Period of “Assistant Surgeon” May Improve the Learning Curve for Laparoscopic Robotic-Assisted Hysterectomy. Front. Surg. 2016, 3, 58. [Google Scholar] [CrossRef]
- Thomas, A.A.-O.; Altaf, K.A.-O.; Sochorova, D.; Gur, U.A.-O.; Parvaiz, A.; Ahmed, S. Effective implementation and adaptation of structured robotic colorectal programme in a busy tertiary unit. J. Robot. Surg. 2020, 15, 731–739. [Google Scholar] [CrossRef]
- Disbrow, D.E.; Pannell, S.M.; Shanker, B.A.; Albright, J.; Wu, J.; Bastawrous, A.; Soliman, M.; Ferraro, J.; Cleary, R.K. The Effect of Formal Robotic Residency Training on the Adoption of Minimally Invasive Surgery by Young Colorectal Surgeons. J. Surg. Educ. 2018, 75, 767–778. [Google Scholar] [CrossRef]
- Sng, K.K.; Hara, M.; Shin, J.-W.; Yoo, B.-E.; Yang, K.-S.; Kim, S.-H. The multiphasic learning curve for robot-assisted rectal surgery. Surg. Endosc. 2013, 27, 3297–3307. [Google Scholar] [CrossRef]
- Rice, M.K.; Hodges, J.C.; Bellon, J.; Borrebach, J.; Al Abbas, A.I.; Hamad, A.; Knab, L.M.; Moser, A.J.; Zureikat, A.H.; Zeh, H.J.; et al. Association of Mentorship and a Formal Robotic Proficiency Skills Curriculum with Subsequent Generations’ Learning Curve and Safety for Robotic Pancreaticoduodenectomy. JAMA Surg. 2020, 155, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, L.; Formisano, G.; Salaj, A.; Giuratrabocchetta, S.; Giuliani, G.; Salvischiani, L.; Bianchi, P.P. Robotic right colectomy with complete mesocolic excision: Senior versus junior surgeon, a case-matched retrospective analysis. Int. J. Med. Robot. 2022, 18, e2383. [Google Scholar] [CrossRef]
- Soliman, M.K.; Tammany, A.J. Teaching and Training Surgeons in Robotic Colorectal Surgery. Clin. Colon Rectal Surg. 2021, 34, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.; Miskovic, D. Proctorship in Minimally Invasive Colorectal Surgery. Clin. Colon Rectal Surg. 2021, 34, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.; Marshall, H.; Croft, J.; Copeland, J.; Jayne, D.; Brown, J. Exploring and adjusting for potential learning effects in ROLARR: A randomised controlled trial comparing robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection. Trials 2018, 19, 339. [Google Scholar] [CrossRef]
- Lin, P.-L.; Zheng, F.; Shin, M.; Liu, X.; Oh, D.; D’Attilio, D. CUSUM learning curves: What they can and can’t tell us. Surg. Endosc. 2023, 37, 7991–7999. [Google Scholar] [CrossRef]
- Burghgraef, T.A.; Sikkenk, D.J.; Crolla, R.; Fahim, M.; Melenhorst, J.; Moumni, M.E.; Schelling, G.V.; Smits, A.B.; Stassen, L.P.S.; Verheijen, P.M.; et al. Assessing the learning curve of robot-assisted total mesorectal excision: A multicenter study considering procedural safety, pathological safety, and efficiency. Int J Colorectal Dis. 2023, 38, 9. [Google Scholar] [CrossRef]
- Parascandola, S.A.; Horsey, M.L.; Hota, S.; Paull, J.O.; Graham, A.; Pudalov, N.; Smith, S.; Amdur, R.; Obias, V. The robotic colorectal experience: An outcomes and learning curve analysis of 502 patients. Colorectal Dis. 2021, 23, 226–236. [Google Scholar] [CrossRef]
- Kawai, K.; Hata, K.; Tanaka, T.; Nishikawa, T.; Otani, K.; Murono, K.; Sasaki, K.; Kaneko, M.; Emoto, S.; Nozawa, H. Learning Curve of Robotic Rectal Surgery With Lateral Lymph Node Dissection: Cumulative Sum and Multiple Regression Analyses. J. Surg. Educ. 2018, 75, 1598–1605. [Google Scholar] [CrossRef]
- Foo, C.C.; Law, W.L. The Learning Curve of Robotic-Assisted Low Rectal Resection of a Novice Rectal Surgeon. World J Surg. 2016, 40, 456–462. [Google Scholar] [CrossRef]
- Byrn, J.C.; Hrabe, J.E.; Charlton, M.E. An initial experience with 85 consecutive robotic-assisted rectal dissections: Improved operating times and lower costs with experience. Surg Endosc. 2014, 28, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Lee, H.M.; Kim, N.K.; Min, B.S.; Lee, K.Y. The learning curve for robot-assisted total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutan Tech. 2012, 22, 400–405. [Google Scholar] [CrossRef]
| Type of Statistical Analysis | Advantages | Disadvantages |
|---|---|---|
| Regression analysis | Easy to perform | Oversimplified |
| Split group analysis | Able to compare large case numbers | Split between groups arbitrary with no rationale for cut-off point Difficult to pinpoint exact number required to overcome learning curve |
| Moving average analysis | Simple and easy analysis of consecutive cases Decreases random fluctuations that occur with serial data | Can only be used for operating time analysis Order of moving average is arbitrarily decided |
| Cumulative sum (CUSUM) analysis | Detects change in individual surgeon performance | Does not take into account heterogeneity of cases and different case complexities Influenced by total case number assessed |
| Risk-adjusted cumulative sum (RA-CUSUM) analysis | Ability to correct for case mix that may influence the risk of an event | Difficult to perform Requires large data sets, especially if negative variables/confounders are rare events |
| Indicator of Surgical Performance | Examples of Variables |
|---|---|
| Time | Total operative time Console time Time taken for each surgical phase |
| Intraoperative morbidity | Injury to bladder/urethra/ureter/vagina/intestine Bleeding requiring transfusion Conversion |
| Postoperative morbidity | Clavien Dindo grade 2 or higher Reoperation |
| Pathological outcome | Resection margin positivity Incomplete TME Lymph node yield |
| Functional outcome | International prostate symptom score International index of erectile function Quality of life |
| Composite | Combination of above variables |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, N.W.; Teo, N.Z.; Ngu, J.C.-Y. Learning Curve for Robotic Colorectal Surgery. Cancers 2024, 16, 3420. https://doi.org/10.3390/cancers16193420
Wong NW, Teo NZ, Ngu JC-Y. Learning Curve for Robotic Colorectal Surgery. Cancers. 2024; 16(19):3420. https://doi.org/10.3390/cancers16193420
Chicago/Turabian StyleWong, Neng Wei, Nan Zun Teo, and James Chi-Yong Ngu. 2024. "Learning Curve for Robotic Colorectal Surgery" Cancers 16, no. 19: 3420. https://doi.org/10.3390/cancers16193420





