Systemic and Local Strategies for Primary Prevention of Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
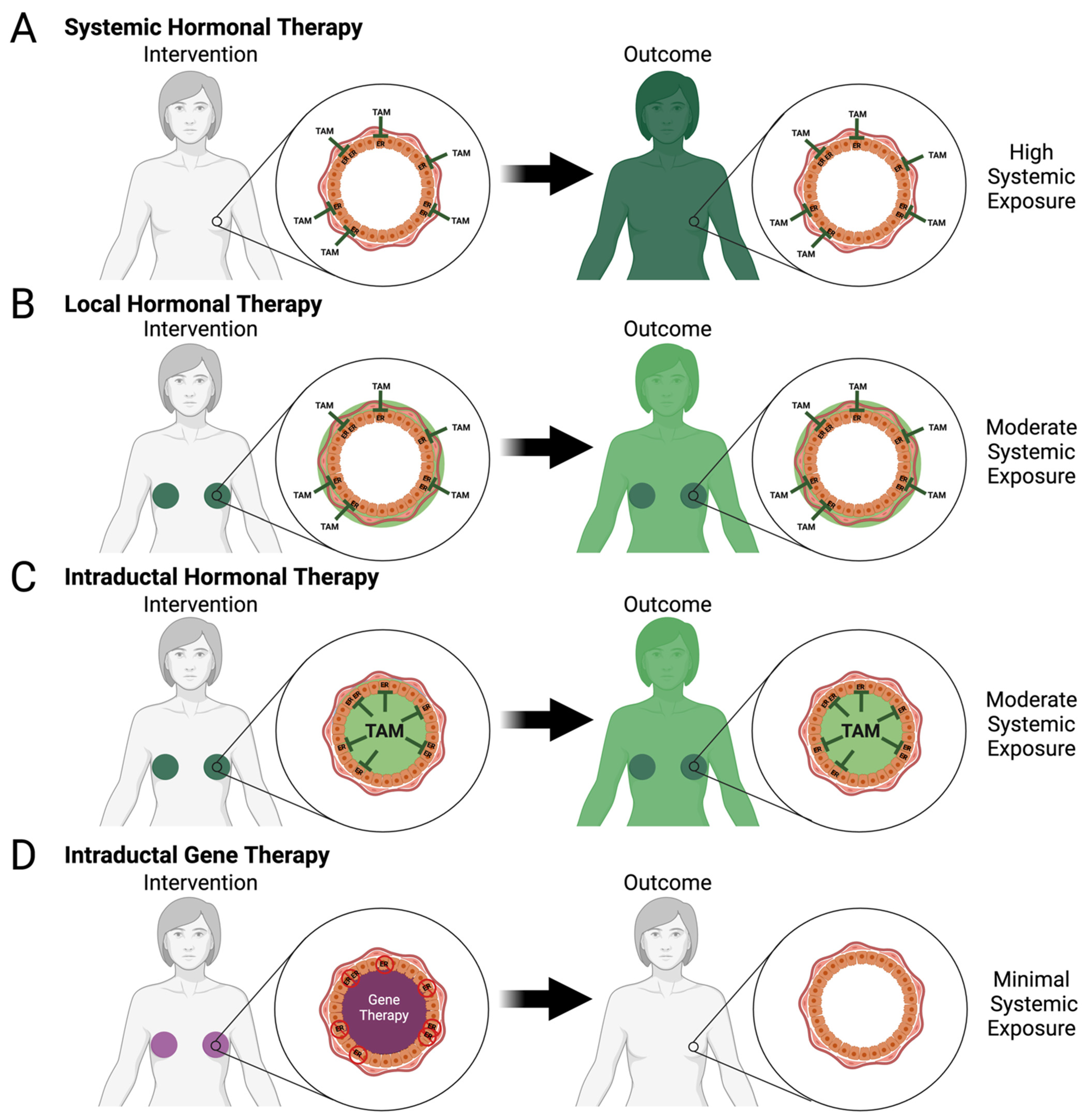
2. Evidence-Based Interventions for Primary Prevention
2.1. Surgical Intervention
2.2. Hormonal Modulation
2.3. Watchful Waiting
3. Investigational Approaches for Primary Prevention
| Approach | Intervention | Active Agent | Number of Participants | Results | Reference(s) |
|---|---|---|---|---|---|
| Hormonal therapy | Local | Endoxifen gel | 90 | 1.9% Reduction in mammographic density | NCT04616430, Completed [71] |
| 4-Hydroxytamoxifen transdermal gel | 194 | 52% Decrease in Ki-67 labeling index | NCT03063619, Active [72] | ||
| Fulvestrant | 3 | N/A | NCT02540330, Terminated | ||
| Systemic | Aromatase inhibitors (Anastrozole) | 3864 | N/A | NCT00078832, Completed | |
| Aromatase inhibitors (Letrozole) | 55 | N/A | NCT00579826 Completed | ||
| Chemoprevention | Retinoid (Fenretinide) | 20 | ≤50% Risk reduction | NCT01479192 [58,73] Terminated |
| Approach | Active Agent | Experimental Model 1 | Level of Evidence 2 | Results | Reference(s) |
|---|---|---|---|---|---|
| Prophylactic Vaccine | α-Lactalbumin | MMTV-HER2 (n = 6) | A | Increased latency (p-value = 0.0004) | [75] |
| MMTV-PyMT (n = 8) | D | Reduced tumor burden (p-value < 0.0006) | |||
| 4T1 isograft (n = 8) | D | Reduced tumor burden until day 13 post injection (p value = 0.0006) | |||
| HER2 | MMTV-HER2 (n = 10) | A | Increased latency (p-value < 0.01) | [76] | |
| HER2 | MMTV-HER2 (n = 5–8) | A | Increased latency (p-value < 0.02) | [77] | |
| Chemoprevention | Erlotinib | Brca1fl/fl;Trp53+/−; MMTV-Cre (n = 13) | A | Increased latency (p-value = 0.0001) | [78] |
| I-BET 762 | MMTV-PyMT (n = 13) | B | Increased latency (p-value < 0.05) | [79] | |
| CCDO-Me | Brca1fl/fl;Tpr53+/; MMTV-Cre (n = 15) | A | Increased latency (p-value < 0.05) | [80] | |
| RankL inhibitor | Brca1fl/fl;Trp53+/−; MMTV-Cre (n = 17) | A | Increased latency (p-value < 0.001) | [81] | |
| RankL monoclonal antibody | Brca1fl/fl; MMTV-Cre (n = 9) | A | Increased latency (p-value < 0.001) | [82] | |
| Cox-2 inhibitor | MMTV-Erbb2 (n = 24) | A | Reduced tumor incidence (p-value = 0.003) | [83] | |
| Curcumin | 4T1 isograft (n = 9) | C | Reduced tumor burden (p-value < 0.05) | [84] | |
| Bisphosphonates (zolendronic acid and risdronate) | MDA-MB-231 xenograft (n = 12) | D | Reduced tumor burden (p-value < 0.05) | [85] | |
| Rexinoids (Bexarotene) | MMTV-Erbb2 (n = 20) | A | Increased latency (p-value < 0.0001) | [86] | |
| MMTV-Erbb2 (n = 19) | A | Increased latency (p-value < 0.001) | [87] | ||
| JAK3 and EGFR inhibitor (WHI-P131 | DMBA-induced Balb/c mice (n = 20) | B | Increased latency (p-value = 0.0014) | [88] | |
| Cytotoxic | Paclitaxel | DMBA-induced Balb/c mice (n = 20) | B | Increased latency (p-value = 0.0041) | [88] |
| Intervention | Active Agent | Experimental Model 1 | Level of Evidence 2 | Results | Reference(s) |
|---|---|---|---|---|---|
| Chemoprevention | Oral-free curcumin | MNU-induced Sprague Dawley rats (n = 12) | A | Reduced tumor incidence (HR = 3.95, p-value 0.007) | [89] |
| Intraductal free curcumin | Reduced tumor incidence (HR = 2.85, p-value 0.020) | ||||
| Nanocurc encapsulated curcumin | A | Reduced tumor incidence (HR = 2.88, p-value 0.028) | |||
| Cytotoxic | Paclitaxel | MNU-induced Sprague-Dawley rats (n = 15) | A | Reduced tumor burden (p-value < 0.05) | [90] |
| Pegylated Liposomal Doxorubicin | MMTV-Erbb2 (n = 12) | B | Reduced tumor incidence (HR = 6.40, p-value < 0.0001) | [91] | |
| MNU-induced Sprague Dawley rats (n = 15) | B | Reduced tumor incidence (p-value < 0.001) | [91] | ||
| MNU-induced Sprague Dawley rats (n = 5) | B | No change compared to control | [92] | ||
| 5-fluorouracil | MNU-induced Sprague Dawley rats (n = 5) | B | Reduced tumor incidence (HR = 3.30, p-value = 0.018) | ||
| Carboplatin | MNU-induced Sprague Dawley rats (n = 5) | B | Reduced tumor incidence (HR = 10.4, p-value < 0.0001) | ||
| Nanoparticle albumin-bound paclitaxel | MNU-induced Sprague Dawley rats (n = 5) | B | No change compared to control | ||
| Methotrexate | MNU-induced Sprague Dawley rats (n = 5) | B | No change compared to control | ||
| Nanoparticle albumin-bound paclitaxel | MNU-induced Sprague Dawley rats (n = 6) | B | Reduced tumor burden (p-value < 0.05) | [93] | |
| Cisplatin | Brca1fl/flTrp53L/L; WAPcre (n = 20) | A | Increased latency (p-value < 0.0001) | [94] | |
| Hormonal therapy | 4-hydroxytamoxifen (4-OHT) | MNU-induced Sprague Dawley rats (n = 20) | A | Reduce tumor incidence (p-value < 0.0001) | [91] |
| Fulvestrant | MIND MCF-7 xenograft (n = 3) | B | Reduced tumor burden (p-value < 0.001) | [95] | |
| MNU-induced Sprague Dawley rats (n = 10) | B | Increased latency (p < 0.0001), reduced tumor incidence (HR = 2.08) | |||
| Fulvestrant and silastic tubing | MCF-7 xenograft (n = 8) | C | Reduced tumor burden (p-value < 0.05) | [96] | |
| Suicidal gene vector | Adenovirus vector with thymidine kinase and gancyclovir | MNU-induced Wistar Furth rats (n = 30) | A | (paradoxical) Decreased latency and increased tumor incidence | [97] |
| Gene silencing | Liposomal Hox1A siRNA silencing | C3(1)-TAg (n = 8) | B | Reduced tumor Incidence | [98] |
| Radioimmunotherapy | Radio-conjugated trastuzumab | MIND SUM225 xenograft (n = 3, 4) | C | Dose-dependent reduced tumor burden | [99] |
| Targeted immunotoxin | Anti-transferrin receptor-antibody conjugated pseudomonas exotoxin | MIND MCF7 xenograft (n = 20) | C | Increased latency and reduced tumor burden (p-value < 0.001) | [100] |
| Chemical ablation | Ethanol | C3(1)-TAg (n = 13) | A | Increased latency (p-value < 0.0001), reduced incidence (HR = 4.76, p-value < 0.0001) | [101] |
4. Systemic Approaches for Primary Prevention in Preclinical Models and Clinical Trials
4.1. Hormonal Therapy with Aromatase Inhibitors
4.2. Chemoprevention
4.3. Prophylactic Vaccines
5. Local Approaches for Primary Prevention in Preclinical Model and Clinical Trials
5.1. Gene Therapy
5.2. Local Hormone Therapy
5.3. Intraductal Chemotherapy and Targeted Treatments
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Michaels, E.; Worthington, R.O.; Rusiecki, J. Breast Cancer: Risk Assessment, Screening, and Primary Prevention. Med. Clin. North Am. 2023, 107, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Engmann, N.J.; Golmakani, M.K.; Miglioretti, D.L.; Sprague, B.L.; Kerlikowske, K. Breast Cancer Surveillance C: Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017, 3, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2016, 2, 1295–1302. [Google Scholar] [CrossRef]
- National Collaborating Centre for Cancer (UK). Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer; National Collaborating Centre for Cancer: Cardiff, UK, 2013. [Google Scholar]
- Rosenthal, E.T.; Evans, B.; Kidd, J.; Brown, K.; Gorringe, H.; van Orman, M.; Manley, S. Increased Identification of Candidates for High-Risk Breast Cancer Screening Through Expanded Genetic Testing. J. Am. Coll. Radiol. 2017, 14, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.; Hughes, E.; Wagner, S.; Tshiaba, P.; Rosenthal, E.; Roa, B.B.; Kurian, A.W.; Domchek, S.M.; Garber, J.; Lancaster, J.; et al. Association of a Polygenic Risk Score With Breast Cancer Among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw. Open 2020, 3, e208501. [Google Scholar] [CrossRef]
- Hughes, E.; Tshiaba, P.; Gallagher, S.; Wagner, S.; Judkins, T.; Roa, B.; Rosenthal, E.; Domchek, S.; Garber, J.; Lancaster, J.; et al. Development and Validation of a Clinical Polygenic Risk Score to Predict Breast Cancer Risk. JCO Precis. Oncol. 2020, 4, 585–592. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef]
- Breast Cancer Association Consortium; Dorling, L.; Carvalho, S.; Allen, J.; Gonzalez-Neira, A.; Luccarini, C.; Wahlstrom, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Howell, A. Can the breast screening appointment be used to provide risk assessment and prevention advice? Breast Cancer Res. 2015, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Gail, M.H.; Anderson, W.F.; Garcia-Closas, M.; Sherman, M.E. Absolute risk models for subtypes of breast cancer. J. Natl. Cancer Inst. 2007, 99, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.W.; Hughes, E.; Simmons, T.; Bernhisel, R.; Probst, B.; Meek, S.; Caswell-Jin, J.L.; John, E.M.; Lanchbury, J.S.; Slavin, T.P.; et al. Performance of the IBIS/Tyrer-Cuzick model of breast cancer risk by race and ethnicity in the Women’s Health Initiative. Cancer 2021, 127, 3742–3750. [Google Scholar] [CrossRef] [PubMed]
- Padamsee, T.J.; Wills, C.E.; Yee, L.D.; Paskett, E.D. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Gandhi, A.; Wisely, J.; Clancy, T.; Woodward, E.R.; Harvey, J.; Highton, L.; Murphy, J.; Barr, L.; Howell, S.J.; et al. Uptake of bilateral-risk-reducing-mastectomy: Prospective analysis of 7195 women at high-risk of breast cancer. Breast 2021, 60, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Jones, C.; Rahman, R.L. Endocrine prevention of breast cancer. Mol. Cell. Endocrinol. 2021, 530, 111284. [Google Scholar] [CrossRef]
- Skytte, A.B.; Gerdes, A.M.; Andersen, M.K.; Sunde, L.; Brondum-Nielsen, K.; Waldstrom, M.; Kolvraa, S.; Cruger, D. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin. Genet. 2010, 77, 342–349. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Mian, N.; Enmore, M.; Poll, A.; Llacuachaqui, M.; Nanda, S.; Sun, P.; Hughes, K.S.; Narod, S.A. Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res. Treat. 2012, 133, 735–740. [Google Scholar] [CrossRef]
- Ralph, A.F.; Ager, B.; Bell, M.L.; Collins, I.M.; Andrews, L.; Tucker, K.; Phillips, K.A.; Butow, P. Women’s preferences for selective estrogen reuptake modulators: An investigation using protection motivation theory. Patient Educ. Couns. 2014, 96, 106–112. [Google Scholar] [CrossRef]
- Gareth Evans, D.; McWilliams, L.; Astley, S.; Brentnall, A.R.; Cuzick, J.; Dobrashian, R.; Duffy, S.W.; Gorman, L.S.; Harkness, E.F.; Harrison, F.; et al. Quantifying the effects of risk-stratified breast cancer screening when delivered in real time as routine practice versus usual screening: The BC-Predict non-randomised controlled study (NCT04359420). Br. J. Cancer 2023, 128, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.S.; Evans, D.G.; Wiseman, J.; Fox, J.; Greenhalgh, R.; Affen, J.; Juraskova, I.; Stavrinos, P.; Dawe, S.; Cuzick, J.; et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br. J. Cancer 2014, 110, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Gandhi, A.; Howell, S.; Wilson, M.; Maxwell, A.; Astley, S.; Harvie, M.; Pegington, M.; Barr, L.; Baildam, A.; et al. Long-Term Evaluation of Women Referred to a Breast Cancer Family History Clinic (Manchester UK 1987–2020). Cancers 2020, 12, 3697. [Google Scholar] [CrossRef] [PubMed]
- Tot, T.; Gere, M.; Hofmeyer, S.; Bauer, A.; Pellas, U. The clinical value of detecting microcalcifications on a mammogram. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 72, pp. 165–174. [Google Scholar]
- Tan, M.P.; Tot, T. The sick lobe hypothesis, field cancerisation and the new era of precision breast surgery. Gland. Surg. 2018, 7, 611–618. [Google Scholar] [CrossRef]
- Love, S.M.; Barsky, S.H. Anatomy of the nipple and breast ducts revisited. Cancer 2004, 101, 1947–1957. [Google Scholar] [CrossRef]
- King, B.L.; Love, S.M. The intraductal approach to the breast: Raison d’etre. Breast Cancer Res. 2006, 8, 206. [Google Scholar] [CrossRef]
- Cooper, A.P. On the Anatomy of the Breast; Longman: London, UK, 1840; Volume 1. [Google Scholar]
- Newman, L.A.; Kuerer, H.M.; Hung, K.K.; Vlastos, G.; Ames, F.C.; Ross, M.I.; Singletary, S.E. Prophylactic mastectomy. J. Am. Coll. Surg. 2000, 191, 322–330. [Google Scholar] [CrossRef]
- Euhus, D.M.; Diaz, J. Breast cancer prevention. Breast J. 2015, 21, 76–81. [Google Scholar] [CrossRef]
- Alaofi, R.K.; Nassif, M.O.; Al-Hajeili, M.R. Prophylactic mastectomy for the prevention of breast cancer: Review of the literature. Avicenna J. Med. 2018, 8, 67–77. [Google Scholar] [CrossRef]
- Tuttle, T.M.; Habermann, E.B.; Grund, E.H.; Morris, T.J.; Virnig, B.A. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: A trend toward more aggressive surgical treatment. J. Clin. Oncol. 2007, 25, 5203–5209. [Google Scholar] [CrossRef]
- Morrow, M.; Mehrara, B. Prophylactic mastectomy and the timing of breast reconstruction. Br. J. Surg. 2009, 96, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ticha, P.; Sukop, A. Patient-reported outcomes in bilateral prophylactic mastectomy with breast reconstruction: A narrative review. Breast 2023, 73, 103602. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Duxbury, P.; Murphy, J.; Foden, P.; Lalloo, F.; Clancy, T.; Wisely, J.; Kirwan, C.C.; Howell, A.; Evans, D.G. Patient reported outcome measures in a cohort of patients at high risk of breast cancer treated by bilateral risk reducing mastectomy and breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gahm, J.; Wickman, M.; Brandberg, Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer--prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast 2010, 19, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Gopie, J.P.; Mureau, M.A.; Seynaeve, C.; Ter Kuile, M.M.; Menke-Pluymers, M.B.; Timman, R.; Tibben, A. Body image issues after bilateral prophylactic mastectomy with breast reconstruction in healthy women at risk for hereditary breast cancer. Fam. Cancer 2013, 12, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Brandberg, Y.; Sandelin, K.; Erikson, S.; Jurell, G.; Liljegren, A.; Lindblom, A.; Linden, A.; von Wachenfeldt, A.; Wickman, M.; Arver, B. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: A prospective 1-year follow-up study. J. Clin. Oncol. 2008, 26, 3943–3949. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Hamill, J.B.; Kim, H.M.; Qi, J.; Wilkins, E.; Pusic, A.L. Impact of Bilateral Prophylactic Mastectomy and Immediate Reconstruction on Health-Related Quality of Life in Women at High Risk for Breast Carcinoma: Results of the Mastectomy Reconstruction Outcomes Consortium Study. Ann. Surg. Oncol. 2017, 24, 2502–2508. [Google Scholar] [CrossRef]
- Choi, Y.H.; Terry, M.B.; Daly, M.B.; MacInnis, R.J.; Hopper, J.L.; Colonna, S.; Buys, S.S.; Andrulis, I.L.; John, E.M.; Kurian, A.W.; et al. Association of Risk-Reducing Salpingo-Oophorectomy With Breast Cancer Risk in Women With BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2021, 7, 585–592. [Google Scholar] [CrossRef]
- Mavaddat, N.; Antoniou, A.C.; Mooij, T.M.; Hooning, M.J.; Heemskerk-Gerritsen, B.A.; GENEPSO; Nogues, C.; Gauthier-Villars, M.; Caron, O.; Gesta, P.; et al. Correction to: Risk-reducing salpingo-oophorectomy, natural menopause, and breast cancer risk: An international prospective cohort of BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2020, 22, 25. [Google Scholar] [CrossRef]
- Mai, P.L.; Miller, A.; Gail, M.H.; Skates, S.; Lu, K.; Sherman, M.E.; Ioffe, O.B.; Rodriguez, G.; Cohn, D.E.; Boggess, J.; et al. Risk-Reducing Salpingo-Oophorectomy and Breast Cancer Risk Reduction in the Gynecologic Oncology Group Protocol-0199 (GOG-0199). JNCI Cancer Spectr. 2020, 4, pkz075. [Google Scholar] [CrossRef]
- Modaffari, P.; Ponzone, R.; Ferrari, A.; Cipullo, I.; Liberale, V.; D’Alonzo, M.; Maggiorotto, F.; Biglia, N. Concerns and Expectations of Risk-Reducing Surgery in Women with Hereditary Breast and Ovarian Cancer Syndrome. J. Clin. Med. 2019, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Michaelson-Cohen, R.; Gabizon-Peretz, S.; Armon, S.; Srebnik-Moshe, N.; Mor, P.; Tomer, A.; Levy-Lahad, E.; Paluch-Shimon, S. Breast cancer risk and hormone replacement therapy among BRCA carriers after risk-reducing salpingo-oophorectomy. Eur. J. Cancer 2021, 148, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Gronwald, J.; Karlan, B.Y.; Huzarski, T.; Tung, N.; Moller, P.; Armel, S.; Lynch, H.T.; Senter, L.; Eisen, A.; et al. Hormone Replacement Therapy After Oophorectomy and Breast Cancer Risk Among BRCA1 Mutation Carriers. JAMA Oncol 2018, 4, 1059–1065. [Google Scholar] [CrossRef]
- Gordhandas, S.; Norquist, B.M.; Pennington, K.P.; Yung, R.L.; Laya, M.B.; Swisher, E.M. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol. Oncol. 2019, 153, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Breast Cancer Prevention: Current Approaches and Future Directions. Eur. J. Breast Health 2018, 14, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.S.; Vadlamudi, R.K. Role of estrogen receptor signaling in breast cancer metastasis. Int. J. Breast Cancer 2012, 2012, 654698. [Google Scholar]
- Patel, H.K.; Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Luca, A.; Avena, P.; De Amicis, F.; Casaburi, I.; Sirianni, R.; Pezzi, V. Estrogen Receptors-Mediated Apoptosis in Hormone-Dependent Cancers. Int. J. Mol. Sci. 2022, 23, 1242. [Google Scholar] [CrossRef]
- Das, S.K.S.; Singh, Y.; Kumar, P.; Thareja, S. Elective Estrogen Receptor Modulators (SERMs) for the treatment of ER+ breast cancer: An overview. J. Mol. Struct. 2022, 1270, 133853. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Cecchini, R.S.; Cronin, W.M.; Robidoux, A.; Bevers, T.B.; Kavanah, M.T.; Atkins, J.N.; Margolese, R.G.; et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 2005, 97, 1652–1662. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef]
- Cohen, I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol. Oncol. 2004, 94, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Bergman, L.; Beelen, M.L.; Gallee, M.P.; Hollema, H.; Benraadt, J.; van Leeuwen, F.E. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet 2000, 356, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Eeles, R.; Ashley, S.; Easton, D.; Chang, J.; Dowsett, M.; Tidy, A.; Viggers, J.; Davey, J. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 1998, 352, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Mariani, L.; Decensi, A.; Formelli, F.; Camerini, T.; Miceli, R.; Di Mauro, M.G.; Costa, A.; Marubini, E.; Sporn, M.B.; et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann. Oncol. 2006, 17, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Forbes, J.F.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A. International Breast Cancer Intervention Study II: Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J. Natl. Cancer Inst. 2007, 99, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.J.; Ashley, S.; Tidy, A.; Smith, I.E.; Dowsett, M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J. Natl. Cancer Inst. 2007, 99, 283–290. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; Forbes, J.F.; Investigators, I.-I. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Cummings, S.R.; Eckert, S.; Krueger, K.A.; Grady, D.; Powles, T.J.; Cauley, J.A.; Norton, L.; Nickelsen, T.; Bjarnason, N.H.; Morrow, M.; et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999, 281, 2189–2197. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Vogel, V.G.; Costantino, J.P.; Wickerham, D.L.; Cronin, W.M.; Cecchini, R.S.; Atkins, J.N.; Bevers, T.B.; Fehrenbacher, L.; Pajon, E.R.; Wade, J.L.; et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006, 295, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.G.; Costantino, J.P.; Wickerham, D.L.; Cronin, W.M.; Cecchini, R.S.; Atkins, J.N.; Bevers, T.B.; Fehrenbacher, L.; Pajon, E.R.; Wade, J.L., 3rd; et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev. Res. 2010, 3, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.; O’Brien, K.K.; Galvin, R.; Hardy, C.; McDonnell, R.; Joyce, D.; McDowell, R.D.; Aherne, E.; Keogh, C.; O’Sullivan, K.; et al. Quantifying patient preferences for symptomatic breast clinic referral: A decision analysis study. BMJ Open 2018, 8, e017286. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, K.; Nickel, B.; Moynihan, R.; Hersch, J.; Teixeira-Pinto, A.; Irwig, L.; Barratt, A. How different terminology for ductal carcinoma in situ impacts women’s concern and treatment preferences: A randomised comparison within a national community survey. BMJ Open 2015, 5, e008094. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.L.; Tan, S.; Keegan, T.H.; Clarke, C.A. Disparities in mammographic screening for Asian women in California: A cross-sectional analysis to identify meaningful groups for targeted intervention. BMC Cancer 2007, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Vadaparampil, S.T.; Miree, C.A.; Wilson, C.; Jacobsen, P.B. Psychosocial and behavioral impact of genetic counseling and testing. Breast Dis. 2006, 27, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, M.R.; Agahozo, M.C.; Koppert, L.B.; van Deurzen, C.H.M. A retrospective alternative for active surveillance trials for ductal carcinoma in situ of the breast. Int. J. Cancer 2020, 146, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Bäcklund, M.; Eriksson, M.; Gabrielson, M.; Hammarström, M.; Quay, S.; Bergqvist, J.; Hellgren, R.; Czene, K.; Hall, P. Topical Endoxifen for Mammographic Density Reduction-A Randomized Controlled Trial. Oncologist 2022, 27, e597–e600. [Google Scholar] [CrossRef]
- Lee, O.; Page, K.; Ivancic, D.; Helenowski, I.; Parini, V.; Sullivan, M.E.; Margenthaler, J.A.; Chatterton, R.T.; Jovanovic, B.; Dunn, B.K.; et al. A randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin. Cancer Res. 2014, 20, 3672–3682. [Google Scholar] [CrossRef]
- Camerini, T.; Mariani, L.; De Palo, G.; Marubini, E.; Di Mauro, M.G.; Decensi, A.; Costa, A.; Veronesi, U. Safety of the synthetic retinoid fenretinide: Long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J. Clin. Oncol. 2001, 19, 1664–1670. [Google Scholar] [CrossRef]
- Russo, J. Significance of rat mammary tumors for human risk assessment. Toxicol. Pathol. 2015, 43, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Jaini, R.; Kesaraju, P.; Johnson, J.M.; Altuntas, C.Z.; Jane-Wit, D.; Tuohy, V.K. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat. Med. 2010, 16, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Palladini, A.; Thrane, S.; Janitzek, C.M.; Pihl, J.; Clemmensen, S.B.; de Jongh, W.A.; Clausen, T.M.; Nicoletti, G.; Landuzzi, L.; Penichet, M.L.; et al. Virus-like particle display of HER2 induces potent anti-cancer responses. Oncoimmunology 2018, 7, e1408749. [Google Scholar] [CrossRef] [PubMed]
- Bryson, P.D.; Han, X.; Truong, N.; Wang, P. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine 2017, 35, 5842–5849. [Google Scholar] [CrossRef] [PubMed]
- Burga, L.N.; Hu, H.; Juvekar, A.; Tung, N.M.; Troyan, S.L.; Hofstatter, E.W.; Wulf, G.M. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res. 2011, 13, R30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Leal, A.S.; Carapellucci, S.; Zydeck, K.; Sporn, M.B.; Liby, K.T. Chemoprevention of Preclinical Breast and Lung Cancer with the Bromodomain Inhibitor I-BET 762. Cancer Prev. Res. 2018, 11, 143–156. [Google Scholar] [CrossRef]
- Kim, E.H.; Deng, C.; Sporn, M.B.; Royce, D.B.; Risingsong, R.; Williams, C.R.; Liby, K.T. CDDO-methyl ester delays breast cancer development in BRCA1-mutated mice. Cancer Prev. Res. 2012, 5, 89–97. [Google Scholar] [CrossRef]
- Infante, M.; Fabi, A.; Cognetti, F.; Gorini, S.; Caprio, M.; Fabbri, A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 12. [Google Scholar] [CrossRef]
- Nolan, E.; Vaillant, F.; Branstetter, D.; Pal, B.; Giner, G.; Whitehead, L.; Lok, S.W.; Mann, G.B.; Rohrbach, K.; Huang, L.Y.; et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 2016, 22, 933–939. [Google Scholar] [CrossRef]
- Howe, L.R.; Subbaramaiah, K.; Patel, J.; Masferrer, J.L.; Deora, A.; Hudis, C.; Thaler, H.T.; Muller, W.J.; Du, B.; Brown, A.M.; et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002, 62, 5405–5407. [Google Scholar]
- Farhangi, B.; Alizadeh, A.M.; Khodayari, H.; Khodayari, S.; Dehghan, M.J.; Khori, V.; Heidarzadeh, A.; Khaniki, M.; Sadeghiezadeh, M.; Najafi, F. Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur. J. Pharmacol. 2015, 758, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Stachnik, A.; Yuen, T.; Iqbal, J.; Sgobba, M.; Gupta, Y.; Lu, P.; Colaianni, G.; Ji, Y.; Zhu, L.L.; Kim, S.M.; et al. Repurposing of bisphosphonates for the prevention and therapy of nonsmall cell lung and breast cancer. Proc. Natl. Acad. Sc.i USA 2014, 111, 17995–18000. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, Y.; Xu, X.C.; Hill, J.; Celestino, J.; Kim, H.T.; Mohsin, S.K.; Hilsenbeck, S.G.; Lamph, W.W.; Bissonette, R.; et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002, 62, 6376–6380. [Google Scholar] [PubMed]
- Li, Y.; Zhang, Y.; Hill, J.; Kim, H.T.; Shen, Q.; Bissonnette, R.P.; Lamph, W.W.; Brown, P.H. The rexinoid, bexarotene, prevents the development of premalignant lesions in MMTV-erbB2 mice. Br. J. Cancer 2008, 98, 1380–1388. [Google Scholar] [CrossRef]
- Sahin, K.; Yabas, M.; Orhan, C.; Tuzcu, M.; Sahin, T.K.; Ozercan, I.H.; Qazi, S.; Uckun, F.M. Prevention of DMBA-induced mammary gland tumors in mice by a dual-function inhibitor of JAK3 and EGF receptor tyrosine kinases. Expert Opin. Ther. Targets 2020, 24, 379–387. [Google Scholar] [CrossRef]
- Chun, Y.S.; Bisht, S.; Chenna, V.; Pramanik, D.; Yoshida, T.; Hong, S.M.; de Wilde, R.F.; Zhang, Z.; Huso, D.L.; Zhao, M.; et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis 2012, 33, 2242–2249. [Google Scholar] [CrossRef]
- Okugawa, H.; Yamamoto, D.; Uemura, Y.; Sakaida, N.; Tanano, A.; Tanaka, K.; Kamiyama, Y. Effect of perductal paclitaxel exposure on the development of MNU-induced mammary carcinoma in female S-D rats. Breast Cancer Res. Treat 2005, 91, 29–34. [Google Scholar] [CrossRef]
- Murata, S.; Kominsky, S.L.; Vali, M.; Zhang, Z.; Garrett-Mayer, E.; Korz, D.; Huso, D.; Baker, S.D.; Barber, J.; Jaffee, E.; et al. Ductal access for prevention and therapy of mammary tumors. Cancer Res. 2006, 66, 638–645. [Google Scholar] [CrossRef]
- Stearns, V.; Mori, T.; Jacobs, L.K.; Khouri, N.F.; Gabrielson, E.; Yoshida, T.; Kominsky, S.L.; Huso, D.L.; Jeter, S.; Powers, P.; et al. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Sci. Transl. Med. 2011, 3, 106ra108. [Google Scholar] [CrossRef]
- Gao, D.; Liu, J.; Yuan, J.; Wu, J.; Kuang, X.; Kong, D.; Zheng, W.; Wang, G.; Sukumar, S.; Tu, Y.; et al. Intraductal administration of N-methyl-N-nitrosourea as a novel rodent mammary tumor model. Ann. Transl. Med. 2021, 9, 576. [Google Scholar] [CrossRef]
- de Groot, J.S.; van Diest, P.J.; van Amersfoort, M.; Vlug, E.J.; Pan, X.; Ter Hoeve, N.D.; Rosing, H.; Beijnen, J.H.; Youssef, S.A.; de Bruin, A.; et al. Intraductal cisplatin treatment in a BRCA-associated breast cancer mouse model attenuates tumor development but leads to systemic tumors in aged female mice. Oncotarget 2017, 8, 60750–60763. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Pai, P.; Korangath, P.; Sun, S.; Merino, V.F.; Yuan, J.; Li, S.; Nie, G.; Stearns, V.; et al. Intraductal fulvestrant for therapy of ERα-positive ductal carcinoma in situ of the breast: A preclinical study. Carcinogenesis 2019, 40, 903–913. [Google Scholar] [CrossRef]
- Park, J.; Thomas, S.; Zhong, A.Y.; Wolfe, A.R.; Krings, G.; Terranova-Barberio, M.; Pawlowska, N.; Benet, L.Z.; Munster, P.N. Local delivery of hormonal therapy with silastic tubing for prevention and treatment of breast cancer. Sci. Rep. 2018, 8, 92. [Google Scholar] [CrossRef]
- Sivaraman, L.; Gay, J.; Hilsenbeck, S.G.; Shine, H.D.; Conneely, O.M.; Medina, D.; O’Malley, B.W. Effect of selective ablation of proliferating mammary epithelial cells on MNU induced rat mammary tumorigenesis. Breast Cancer Res. Treat 2002, 73, 75–83. [Google Scholar] [CrossRef]
- Brock, A.; Krause, S.; Li, H.; Kowalski, M.; Goldberg, M.S.; Collins, J.J.; Ingber, D.E. Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice. Sci. Transl Med. 2014, 6, 217ra212. [Google Scholar] [CrossRef]
- Yoshida, T.; Jin, K.; Song, H.; Park, S.; Huso, D.L.; Zhang, Z.; Liangfeng, H.; Zhu, C.; Bruchertseifer, F.; Morgenstern, A.; et al. Effective treatment of ductal carcinoma in situ with a HER-2- targeted alpha-particle emitting radionuclide in a preclinical model of human breast cancer. Oncotarget 2016, 7, 33306–33315. [Google Scholar] [CrossRef]
- Wang, G.; Kumar, A.; Ding, W.; Korangath, P.; Bera, T.; Wei, J.; Pai, P.; Gabrielson, K.; Pastan, I.; Sukumar, S. Intraductal administration of transferrin receptor-targeted immunotoxin clears ductal carcinoma in situ in mouse models of breast cancer-a preclinical study. Proc. Natl. Acad. Sci. USA 2022, 119, e2200200119. [Google Scholar] [CrossRef]
- Kenyon, E.; Westerhuis, J.J.; Volk, M.; Hix, J.; Chakravarty, S.; Claucherty, E.; Zaluzec, E.; Ramsey, L.; Madaj, Z.; Hostetter, G.; et al. Ductal tree ablation by local delivery of ethanol prevents tumor formation in an aggressive mouse model of breast cancer. Breast Cancer Res. 2019, 21, 129. [Google Scholar] [CrossRef]
- Miller, W.R. Aromatase inhibitors: Mechanism of action and role in the treatment of breast cancer. Semin. Oncol. 2003, 30 (Suppl. 14), 3–11. [Google Scholar] [CrossRef]
- Maunsell, E.; Goss, P.E.; Chlebowski, R.T.; Ingle, J.N.; Alés-Martínez, J.E.; Sarto, G.E.; Fabian, C.J.; Pujol, P.; Ruiz, A.; Cooke, A.L.; et al. Quality of life in MAP.3 (Mammary Prevention 3): A randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer. J. Clin. Oncol. 2014, 32, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Ingle, J.N.; Alés-Martínez, J.E.; Cheung, A.M.; Chlebowski, R.T.; Wactawski-Wende, J.; McTiernan, A.; Robbins, J.; Johnson, K.C.; Martin, L.W.; et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 2011, 364, 2381–2391. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Cawthorn, S.; Mansel, R.E.; Loibl, S.; Bonanni, B.; Evans, D.G.; Howell, A.; et al. Use of anastrozole for breast cancer prevention (IBIS-II): Long-term results of a randomised controlled trial. Lancet 2020, 395, 117–122. [Google Scholar] [CrossRef]
- Formelli, F.; Clerici, M.; Campa, T.; Di Mauro, M.G.; Magni, A.; Mascotti, G.; Moglia, D.; De Palo, G.; Costa, A.; Veronesi, U. Five-year administration of fenretinide: Pharmacokinetics and effects on plasma retinol concentrations. J. Clin. Oncol. 1993, 11, 2036–2042. [Google Scholar] [CrossRef]
- Mehta, R.G.; Moon, R.C.; Hawthorne, M.; Formelli, F.; Costa, A. Distribution of fenretinide in the mammary gland of breast cancer patients. Eur. J. Cancer 1991, 27, 138–141. [Google Scholar] [CrossRef]
- Moon, R.C.; Thompson, H.J.; Becci, P.J.; Grubbs, C.J.; Gander, R.J.; Newton, D.L.; Smith, J.M.; Phillips, S.L.; Henderson, W.R.; Mullen, L.T.; et al. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979, 39, 1339–1346. [Google Scholar]
- Howe, L.R. Rexinoids and breast cancer prevention. Clin. Cancer Res. 2007, 13, 5983–5987. [Google Scholar] [CrossRef]
- Sukumar, J.; Gast, K.; Quiroga, D.; Lustberg, M.; Williams, N. Triple-negative breast cancer: Promising prognostic biomarkers currently in development. Expert Rev. Anticancer. Ther. 2021, 21, 135–148. [Google Scholar] [CrossRef]
- Toyama, T.; Yamashita, H.; Kondo, N.; Okuda, K.; Takahashi, S.; Sasaki, H.; Sugiura, H.; Iwase, H.; Fujii, Y. Frequently increased epidermal growth factor receptor (EGFR) copy numbers and decreased BRCA1 mRNA expression in Japanese triple-negative breast cancers. BMC Cancer 2008, 8, 309. [Google Scholar] [CrossRef]
- Arnes, J.B.; Begin, L.R.; Stefansson, I.; Brunet, J.S.; Nielsen, T.O.; Foulkes, W.D.; Akslen, L.A. Expression of epidermal growth factor receptor in relation to BRCA1 status, basal-like markers and prognosis in breast cancer. J. Clin. Pathol. 2009, 62, 139–146. [Google Scholar] [CrossRef]
- Sarnik, J.; Poplawski, T.; Tokarz, P. BET Proteins as Attractive Targets for Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 11102. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Mokbel, K. Chemoprevention of Breast Cancer With Vitamins and Micronutrients: A Concise Review. In Vivo 2019, 33, 983–997. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Cui, R.; Lin, J.; Ding, X. Curcumin in Treating Breast Cancer: A Review. J. Lab. Autom. 2016, 21, 723–731. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Therapy. 2007, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Kim, N.S.; Kim, H.J.; Koo, B.K.; Kwon, M.C.; Kim, Y.W.; Cho, Y.; Yokota, Y.; Penninger, J.M.; Kong, Y.Y. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell. Biol. 2006, 26, 1002–1013. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, E.; Jacob, A.P.; Jones, J.; Miller, R.; Roudier-Meyer, M.P.; Erwert, R.; Pinkas, J.; Branstetter, D.; Dougall, W.C. Rank ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 2010, 468, 103–107. [Google Scholar] [CrossRef]
- Tuohy, V.K.; Johnson, J.M.; Mazumder, S. Primary immunoprevention of adult onset cancers by vaccinating against retired tissue-specific self-proteins. Semin. Immunol. 2020, 47, 101392. [Google Scholar] [CrossRef]
- Crews, D.W.; Dombroski, J.A.; King, M.R. Prophylactic Cancer Vaccines Engineered to Elicit Specific Adaptive Immune Response. Front. Oncol. 2021, 11, 626463. [Google Scholar] [CrossRef]
- The Artemis Project® Plan to Develop a Breast Cancer Preventive Vaccine: Identification of Targets & Immune System Variations. Available online: https://www.stopbreastcancer.org/wp-content/uploads/2011/12/artemis-project-plan-saic-1.pdf (accessed on 30 December 2023).
- Artemis Project. Available online: https://www.stopbreastcancer.org/wp-content/uploads/2020/04/2015-artemis-preventive.pdf (accessed on 30 December 2023).
- Artemis Project. Available online: https://www.stopbreastcancer.org/wp-content/uploads/2021/01/2020_ArtemisReport-Final-PDF-for-Website-1.pdf (accessed on 30 December 2023).
- Nicolas-Morales, M.L.; Luisa-Sanjuan, A.; Gutierrez-Torres, M.; Vences-Velazquez, A.; Ortuno-Pineda, C.; Espinoza-Rojo, M.; Navarro-Tito, N.; Cortes-Sarabia, K. Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review. Vaccines 2022, 10, 1249. [Google Scholar] [CrossRef]
- Jaini, R.; Rayman, P.; Cohen, P.A.; Finke, J.H.; Tuohy, V.K. Combination of sunitinib with anti-tumor vaccination inhibits T cell priming and requires careful scheduling to achieve productive immunotherapy. Int. J. Cancer 2014, 134, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Goedegebuure, P.; Gillanders, W.E. Mammaglobin-A is a target for breast cancer vaccination. Oncoimmunology 2016, 5, e1069940. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Benshoff, N.; Fleming, T.P.; Dietz, J.R.; Gillanders, W.E.; Mohanakumar, T. Characterization of the role of CD8+T cells in breast cancer immunity following mammaglobin-A DNA vaccination using HLA-class-I tetramers. Breast Cancer Res. Treat. 2008, 110, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Jaramillo, A.; Benshoff, N.D.; Campbell, L.G.; Fleming, T.P.; Dietz, J.R.; Mohanakumar, T. Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J. Natl. Cancer Inst. 2004, 96, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Tiriveedhi, V.; Tucker, N.; Herndon, J.; Li, L.; Sturmoski, M.; Ellis, M.; Ma, C.; Naughton, M.; Lockhart, A.C.; Gao, F.; et al. Safety and preliminary evidence of biologic efficacy of a mammaglobin-a DNA vaccine in patients with stable metastatic breast cancer. Clin. Cancer Res. 2014, 20, 5964–5975. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes. Dev. 2009, 23, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Paine, I.S.; Lewis, M.T. The Terminal End Bud: The Little Engine that Could. J. Mammary Gland. Biol. Neoplasia. 2017, 22, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Henson, D.E.; Tarone, R.E.; Nsouli, H. Lobular involution: The physiological prevention of breast cancer. J. Natl. Cancer Inst. 2006, 98, 1589–1590. [Google Scholar] [CrossRef]
- Radisky, D.C.; Visscher, D.W.; Frank, R.D.; Vierkant, R.A.; Winham, S.; Stallings-Mann, M.; Hoskin, T.L.; Nassar, A.; Vachon, C.M.; Denison, L.A.; et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res. Treat 2016, 155, 423–430. [Google Scholar] [CrossRef]
- Ghosh, K.; Vachon, C.M.; Pankratz, V.S.; Vierkant, R.A.; Anderson, S.S.; Brandt, K.R.; Visscher, D.W.; Reynolds, C.; Frost, M.H.; Hartmann, L.C. Independent association of lobular involution and mammographic breast density with breast cancer risk. J. Natl. Cancer Inst. 2010, 102, 1716–1723. [Google Scholar] [CrossRef]
- Rasul, M.F.; Hussen, B.M.; Salihi, A.; Ismael, B.S.; Jalal, P.J.; Zanichelli, A.; Jamali, E.; Baniahmad, A.; Ghafouri-Fard, S.; Basiri, A.; et al. Strategies to overcome the main challenges of the use of CRISPR/Cas9 as a replacement for cancer therapy. Mol. Cancer 2022, 21, 64. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y. Application of the CRISPR/Cas9 System to Drug Resistance in Breast Cancer. Adv. Sci. 2018, 5, 1700964. [Google Scholar] [CrossRef]
- Annunziato, S.; Kas, S.M.; Nethe, M.; Yücel, H.; Del Bravo, J.; Pritchard, C.; Bin Ali, R.; van Gerwen, B.; Siteur, B.; Drenth, A.P.; et al. Modeling invasive lobular breast carcinoma by CRISPR/Cas9-mediated somatic genome editing of the mammary gland. Genes. Dev. 2016, 30, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, S.; de Ruiter, J.R.; Henneman, L.; Brambillasca, C.S.; Lutz, C.; Vaillant, F.; Ferrante, F.; Drenth, A.P.; van der Burg, E.; Siteur, B.; et al. Comparative oncogenomics identifies combinations of driver genes and drug targets in BRCA1-mutated breast cancer. Nat. Commun. 2019, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, S.; Lutz, C.; Henneman, L.; Bhin, J.; Wong, K.; Siteur, B.; van Gerwen, B.; de Korte-Grimmerink, R.; Zafra, M.P.; Schatoff, E.M.; et al. In situ CRISPR-Cas9 base editing for the development of genetically engineered mouse models of breast cancer. EMBO J. 2020, 39, e102169. [Google Scholar] [CrossRef] [PubMed]
- Heitink, L.; Whittle, J.R.; Vaillant, F.; Capaldo, B.D.; Dekkers, J.F.; Dawson, C.A.; Milevskiy, M.J.G.; Surgenor, E.; Tsai, M.; Chen, H.R.; et al. In vivo genome-editing screen identifies tumor suppressor genes that cooperate with Trp53 loss during mammary tumorigenesis. Mol. Oncol. 2022, 16, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Pujol, H.; Girault, J.; Rouanet, P.; Fournier, S.; Grenier, J.; Simony, J.; Fourtillan, J.B.; Pujol, J.L. Phase I study of percutaneous 4-hydroxy-tamoxifen with analyses of 4-hydroxy-tamoxifen concentrations in breast cancer and normal breast tissue. Cancer Chemother. Pharmacol. 1995, 36, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, P.; Linares-Cruz, G.; Dravet, F.; Poujol, S.; Gourgou, S.; Simony-Lafontaine, J.; Grenier, J.; Kramar, A.; Girault, J.; Le Nestour, E.; et al. Neoadjuvant percutaneous 4-hydroxytamoxifen decreases breast tumoral cell proliferation: A prospective controlled randomized study comparing three doses of 4-hydroxytamoxifen gel to oral tamoxifen. J. Clin. Oncol. 2005, 23, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Wakeling, A.; Nicholson, R.I. Fulvestrant: An oestrogen receptor antagonist with a novel mechanism of action. Br. J. Cancer 2004, 90 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.S.; Dartora, V.; Cassone Salata, G.; Draszesski Malago, I.; Lopes, L.B. Contributions of nanotechnology to the intraductal drug delivery for local treatment and prevention of breast cancer. Int. J. Pharm. 2023, 635, 122681. [Google Scholar] [CrossRef]
- Love, S.M.; Zhang, W.; Gordon, E.J.; Rao, J.; Yang, H.; Li, J.; Zhang, B.; Wang, X.; Chen, G.; Zhang, B. A feasibility study of the intraductal administration of chemotherapy. Cancer Prev. Res. 2013, 6, 51–58. [Google Scholar] [CrossRef]
- Teo, W.W.; Sukumar, S. Combining the strength of genomics, nanoparticle technology, and direct intraductal delivery for breast cancer treatment. Breast Cancer Res. 2014, 16, 306. [Google Scholar] [CrossRef]
- Havaei, S.M.; Aucoin, M.G.; Jahanian-Najafabadi, A. Pseudomonas Exotoxin-Based Immunotoxins: Over Three Decades of Efforts on Targeting Cancer Cells With the Toxin. Front. Oncol. 2021, 11, 781800. [Google Scholar] [CrossRef]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. Alpha-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, L.; Hu, K.; Hanyu, M.; Zhang, Y.; Fujinaga, M.; Minegishi, K.; Ohkubo, T.; Nagatsu, K.; Jiang, C.; et al. A (211)At-labelled mGluR1 inhibitor induces cancer senescence to elicit long-lasting anti-tumor efficacy. Cell Rep. Med. 2023, 4, 100960. [Google Scholar] [CrossRef]
- Mark, C.; Lee, J.S.; Cui, X.; Yuan, Y. Antibody-Drug Conjugates in Breast Cancer: Current Status and Future Directions. Int. J. Mol. Sci. 2023, 24, 13726. [Google Scholar] [CrossRef]
- Chakravarty, S.; Hix, J.M.L.; Wiewiora, K.A.; Volk, M.C.; Kenyon, E.; Shuboni-Mulligan, D.D.; Blanco-Fernandez, B.; Kiupel, M.; Thomas, J.; Sempere, L.F.; et al. Tantalum oxide nanoparticles as versatile contrast agents for X-ray computed tomography. Nanoscale 2020, 12, 7720–7734. [Google Scholar] [CrossRef]
- Kenyon, E.; Zaluzec, E.; Powell, K.; Volk, M.; Chakravarty, S.; Hix, J.; Kiupel, M.; Shapiro, E.M.; Sempere, L.F. X-Ray Visualization of Intraductal Ethanol-Based Ablative Treatment for Prevention of Breast Cancer in Rat Models. J. Vis. Exp. 2022, 190, e64042. [Google Scholar]
- Kenyon, E.; Zaluzec, E.K.; Powell, K.; Volk, M.; Chakravarty, S.; Hix, J.; Arora, R.; Westerhuis, J.J.; Kiupel, M.; Shapiro, E.M.; et al. Intraductal Delivery and X-ray Visualization of Ethanol-Based Ablative Solution for Prevention and Local Treatment of Breast Cancer in Mouse Models. J. Vis. Exp. 2022, 182, e63457. [Google Scholar]
- Robertson, N.; Sempere, L.; Kenyon, E.; Mallet, C.; Smith, K.; Hix, J.; Halim, A.; Fan, J.; Moore, A. Omniparticle Contrast Agent for Multimodal Imaging: Synthesis and Characterization in an Animal Model. Mol. Imaging Biol. 2023, 25, 401–412. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Birenbaum-Carmeli, D.; Lubinski, J.; Gronwald, J.; Lynch, H.; Moller, P.; Ghadirian, P.; Foulkes, W.D.; Klijn, J.; Friedman, E.; et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int. J. Cancer 2008, 122, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Lu, M.D.; Xie, X.Y.; Xu, H.X.; Xu, Z.F.; Liu, G.J.; Yin, X.Y.; Huang, J.F.; Lencioni, R. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology 2009, 253, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Andersson, R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J. Gastroenterol. 2012, 18, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Li, Z.S.; Jin, Z.D. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J. Gastroenterol. 2013, 19, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.L.; Morhard, R.; DeSoto, M.; Chelales, E.; Yang, J.; Nief, C.; Crouch, B.; Everitt, J.; Previs, R.; Katz, D.; et al. Optimizing ethyl cellulose-ethanol delivery towards enabling ablation of cervical dysplasia. Sci. Rep. 2021, 11, 16869. [Google Scholar] [CrossRef] [PubMed]
- Nief, C.; Morhard, R.; Chelales, E.; Adrianzen Alvarez, D.; Bourla Bs, I.; Lam, C.T.; Sag, A.A.; Crouch, B.T.; Mueller, J.L.; Katz, D.; et al. Polymer-assisted intratumoral delivery of ethanol: Preclinical investigation of safety and efficacy in a murine breast cancer model. PLoS ONE 2021, 16, e0234535. [Google Scholar] [CrossRef] [PubMed]
- Chelales, E.; Morhard, R.; Nief, C.; Crouch, B.; Everitt, J.I.; Sag, A.A.; Ramanujam, N. Radiologic-pathologic analysis of increased ethanol localization and ablative extent achieved by ethyl cellulose. Sci. Rep. 2021, 11, 20700. [Google Scholar] [CrossRef] [PubMed]
- Morhard, R.; Nief, C.; Barrero Castedo, C.; Hu, F.; Madonna, M.; Mueller, J.L.; Dewhirst, M.W.; Katz, D.F.; Ramanujam, N. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci. Rep. 2017, 7, 8750. [Google Scholar] [CrossRef] [PubMed]
- Morhard, R.; Mueller, J.L.; Tang, Q.; Nief, C.; Chelales, E.; Lam, C.T.; Alvarez, D.A.; Rubinstein, M.; Katz, D.F.; Ramanujam, N. Understanding Factors Governing Distribution Volume of Ethyl Cellulose-Ethanol to Optimize Ablative Therapy in the Liver. IEEE Trans. Biomed. Eng. 2020, 67, 2337–2348. [Google Scholar] [CrossRef]
- Sannier, K.; Dompmartin, A.; Theron, J.; Labbe, D.; Barrellier, M.T.; Leroyer, R.; Toure, P.; Leroy, D. A new sclerosing agent in the treatment of venous malformations. Study on 23 cases. Interv. Neuroradiol. 2004, 10, 113–127. [Google Scholar] [CrossRef]
- Dompmartin, A.; Blaizot, X.; Theron, J.; Hammer, F.; Chene, Y.; Labbe, D.; Barrellier, M.T.; Gaillard, C.; Leroyer, R.; Chedru, V.; et al. Radio-opaque ethylcellulose-ethanol is a safe and efficient sclerosing agent for venous malformations. Eur. Radiol. 2011, 21, 2647–2656. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.B.; Zhou, S.Y.; Chen, K.S.; Niu, C.Q.; Tan, X.Y.; Jiang, Y.Z.; Lin, Q.Q. Comparison between absolute ethanol and bleomycin for the treatment of venous malformation in children. Exp. Ther. Med. 2013, 6, 305–309. [Google Scholar] [CrossRef]
- Wohlgemuth, W.A.; Muller-Wille, R.; Teusch, V.; Hammer, S.; Wildgruber, M.; Uller, W. Ethanolgel sclerotherapy of venous malformations improves health-related quality-of-life in adults and children-results of a prospective study. Eur. Radiol. 2017, 27, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.; FitzJohn, T.; Tan, S.T. Ethanol sclerotherapy for venous malformation. ANZ J. Surg. 2016, 86, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Chen, C.L.; Chang, K.; Lee, J.; Samarasena, J. Ethanol Ablation of a Peripheral Nerve Sheath Tumor Presenting as a Small Bowel Obstruction. ACG Case Rep. J. 2015, 3, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Gueng, M.K.; Chou, Y.H.; Tiu, C.M.; Chiou, S.Y.; Cheng, Y.F. Pseudoaneurysm of the Breast Treated with Percutaneous Ethanol Injection. J. Med. Ultrasound 2014, 22, 114–116. [Google Scholar] [CrossRef]
- Chun, Y.S.; Yoshida, T.; Mori, T.; Huso, D.L.; Zhang, Z.; Stearns, V.; Perkins, B.; Jones, R.J.; Sukumar, S. Intraductally administered pegylated liposomal doxorubicin reduces mammary stem cell function in the mammary gland but in the long term, induces malignant tumors. Breast Cancer Res. Treat 2012, 135, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Consumption of Alcoholic Beverages. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100E-11.pdf (accessed on 30 December 2023).
- Garaycoechea, J.I.; Crossan, G.P.; Langevin, F.; Mulderrig, L.; Louzada, S.; Yang, F.; Guilbaud, G.; Park, N.; Roerink, S.; Nik-Zainal, S.; et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature 2018, 553, 171–177. [Google Scholar] [CrossRef]
- Patel, S.; Sparman, N.Z.R.; Arneson, D.; Alvarsson, A.; Santos, L.C.; Duesman, S.J.; Centonze, A.; Hathaway, E.; Ahn, I.S.; Diamante, G.; et al. Mammary duct luminal epithelium controls adipocyte thermogenic programme. Nature 2023, 620, 192–199. [Google Scholar] [CrossRef]
- Markiewicz, E.; Fan, X.; Mustafi, D.; Zamora, M.; Conzen, S.D.; Karczmar, G.S. MRI ductography of contrast agent distribution and leakage in normal mouse mammary ducts and ducts with in situ cancer. Magn. Reson. Imaging 2017, 40, 48–52. [Google Scholar] [CrossRef]
- Markiewicz, E.; Fan, X.; Mustafi, D.; Zamora, M.; Roman, B.B.; Jansen, S.A.; Macleod, K.; Conzen, S.D.; Karczmar, G.S. High resolution 3D MRI of mouse mammary glands with intra-ductal injection of contrast media. Magn. Reson. Imaging 2015, 33, 161–165. [Google Scholar] [CrossRef]
- Clark, A.; Bird, N.K.; Brock, A. Intraductal Delivery to the Rabbit Mammary Gland. J. Vis. Exp. 2017, 121, e55209. [Google Scholar]
- Lai, Y.E.; Morhard, R.; Ramanujam, N.; Nolan, M.W. Minimally invasive ethyl cellulose ethanol ablation in domesticated cats with naturally occurring head and neck cancers: Six cats. Vet Comp. Oncol. 2021, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, F. Minimally-invasive thermal ablation of early-stage breast cancer: A systemic review. Eur. J. Surg. Oncol. 2010, 36, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Lee, J.Y.; Kim, H.R.; Kim, B.R.; Park, E.J.; Kim, H.S.; Han, J.K.; Choi, B.I. Therapeutic Effects of Microbubbles Added to Combined High-Intensity Focused Ultrasound and Chemotherapy in a Pancreatic Cancer Xenograft Model. Korean J. Radiol. 2016, 17, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials 2019, 9, 1195. [Google Scholar] [CrossRef]
- Wild, C.P. The global cancer burden: Necessity is the mother of prevention. Nat. Rev. Cancer 2019, 19, 123–124. [Google Scholar] [CrossRef]
- Hughes, K. Comparative mammary gland postnatal development and tumourigenesis in the sheep, cow, cat and rabbit: Exploring the menagerie. Semin. Cell Dev. Biol. 2021, 114, 186–195. [Google Scholar] [CrossRef]
- Schoniger, S.; Degner, S.; Jasani, B.; Schoon, H.A. A Review on Mammary Tumors in Rabbits: Translation of Pathology into Medical Care. Animals 2019, 9, 762. [Google Scholar] [CrossRef]
- Bourne, R.A.; Bryant, J.A.; Falconer, I.R. Stimulation of DNA synthesis by prolactin in rabbit mammary tissue. J. Cell Sci. 1974, 14, 105–111. [Google Scholar] [CrossRef]
- Falconer, I.R. The distribution of 131 I- or 125 I-labelled prolactin in rabbit mammary tissue after intravenous or intraductal injection. J. Endocrinol. 1972, 53, 58–59. [Google Scholar]
- Fiddler, T.J.; Birkinshaw, M.; Falconer, I.R. Effects of intraductal prolactin on some aspects of the ultrastructure and biochemistry of mammary tissue in the pseudopregnant rabbit. J. Endocrinol. 1971, 49, 459–469. [Google Scholar] [CrossRef]
- Fiddler, T.J.; Falconer, I.R. The effect of intraductal prolactin on protein and nucleic acid biosynthesis in the rabbit mammary gland. Biochem. J. 1969, 115, 58P–59P. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, A. Detection and Assay of Prolactin by the Local Lactogenic Response in the Rabbit. J. Endocrinol. 1963, 27, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Slawson, S.H.; Johnson, B.A. Ductography: How to and what if? Radiographics 2001, 21, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Sheiman, L.S.; Levesque, P.H. The In’s and Out’s of Ductography: A Comprehensive Review. Curr. Probl. Diagn. Radiol. 2016, 45, 61–70. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaluzec, E.K.; Sempere, L.F. Systemic and Local Strategies for Primary Prevention of Breast Cancer. Cancers 2024, 16, 248. https://doi.org/10.3390/cancers16020248
Zaluzec EK, Sempere LF. Systemic and Local Strategies for Primary Prevention of Breast Cancer. Cancers. 2024; 16(2):248. https://doi.org/10.3390/cancers16020248
Chicago/Turabian StyleZaluzec, Erin K., and Lorenzo F. Sempere. 2024. "Systemic and Local Strategies for Primary Prevention of Breast Cancer" Cancers 16, no. 2: 248. https://doi.org/10.3390/cancers16020248






