Circulating Tumour Cells in the Prediction of Bone Metastasis
Abstract
:Simple Summary
Abstract
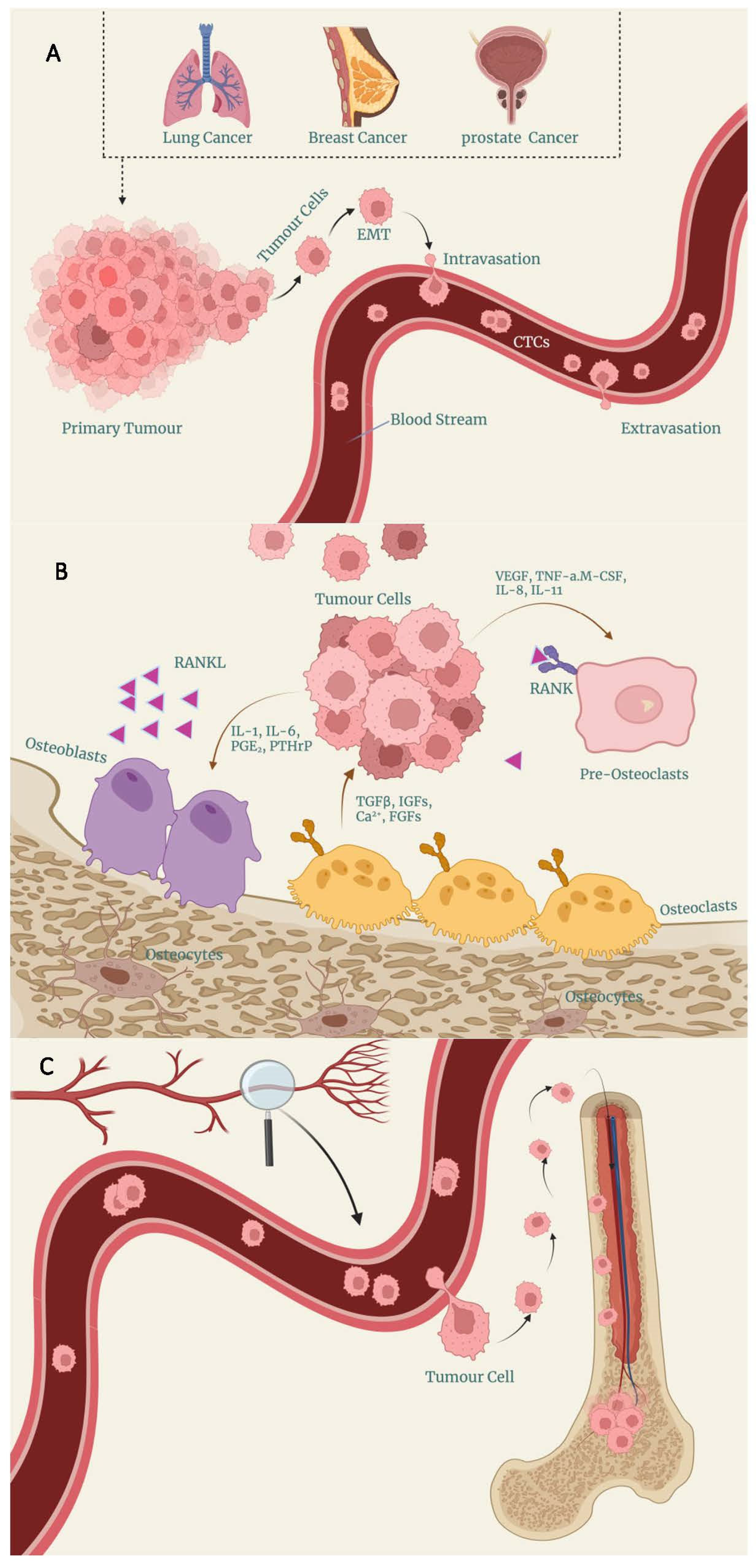
1. Background
1.1. Search Strategy and Eligibility Criteria
1.2. Latest Screening Techniques for CTCs in Patients Diagnosed with Bone Metastasis
1.3. Summary of Clinical Research Relating to CTCs Detection
1.4. CTCs as Prognostic Biomarkers of Recurrence and Therapeutic Targets for Bone Metastasis
1.4.1. Use of CTCs in Anti-Tumour Drug Screening
1.4.2. Gene Expression in CTCs
1.4.3. Changes in CTC Numbers Post Therapy
1.4.4. Use of CTCs in Combination with Other Biomarkers to Predict Bone Metastasis
1.5. Use of Artificial Intelligence to Identify CTCs
2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Tattersall, L.; Gagui, D.C.; Tippett, V.L.; Latif, N.B.A.; Shah, K.M.; Gartland, A. A Systematic Review of the Expression, Signalling and Function of P2 Receptors in Primary Bone Cancer. Front. Biosci. 2022, 27, 122. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Christiansen, C.F.; Ulrichsen, S.P.; Rørth, M.R.; Sørensen, H.T. Survival after bone metastasis by primary cancer type: A Danish population-based cohort study. BMJ Open 2017, 7, e016022. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers. 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, B.; Fan, Q. Mechanisms of breast cancer bone metastasis. Cancer Lett. 2010, 292, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.-T.; Smit, D.J.; Taipaleenmäki, H. The Endosteal Niche in Breast Cancer Bone Metastasis. Rev. Front. Oncol. 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Ariza, N.M.; Bible, K.C.; Clarke, B.L. Bone metastases in thyroid cancer. J. Bone Oncol. 2020, 21, 100282. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Adler, M.; Fazzari, M.; Tickoo, S.; Rosai, J.; Larson, S.M.; Robbins, R.J. Bone Metastases from Thyroid Carcinoma: Clinical Characteristics and Prognostic Variables in One Hundred Forty-Six Patients. Thyroid 2000, 10, 261–268. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Zekri, J.; Ahmed, N.; Coleman, R.E.; Hancock, B.W. The skeletal metastatic complications of renal cell carcinoma. Int. J. Oncol. 2001, 19, 379–382. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.-V.; Giaze, T.R.; Chin, K.Y.; Mohamed, N.; Ima-Nirwana, S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef]
- Morrissey, C.; Vessella, R.L. The role of tumor microenvironment in prostate cancer bone metastasis. J. Cell. Biochem. 2007, 101, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Metastasis: Quantitative analysis of distribution and fate of tumor emboli labeled with 125I-5-iodo-2′-deoxyuridine. J. Natl. Cancer Inst. 1970, 45, 773–782. [Google Scholar] [PubMed]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep Nature of Metastatic Inefficiency: Dormancy of Solitary Cells after Successful Extravasation and Limited Survival of Early Micrometastases. Am. J. Clin. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Sturgess, V.; Azubuike, U.F.; Tanner, K. Vascular regulation of disseminated tumor cells during metastatic spread. Biophys. Rev. 2023, 4, 011310. [Google Scholar] [CrossRef]
- Krog, B.L.; Henry, M.D. Biomechanics of the Circulating Tumor Cell Microenvironment. Adv. Exp. Med. Biol. 2018, 1092, 209–233. [Google Scholar] [PubMed]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Coleman, R. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Lelekakis, M.; Moseley, J.M.; Martin, T.J.; Hards, D.; Williams, E.; Ho, P.; Lowen, D.; Javni, J.; Miller, F.R.; Slavin, J.; et al. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis 1999, 17, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Makuch, L.A.; Sosnoski, D.M.; Gay, C.V. Osteoblast-conditioned media influence the expression of E-selectin on bone-derived vascular endothelial cells. J. Cell. Biochem. 2006, 98, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, J.; Helwani, F.; Winkler, I. The endosteal ‘osteoblastic’niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 2010, 24, 1979–1992. [Google Scholar] [CrossRef] [PubMed]
- Vandendries, E.R.; Furie, B.C.; Furie, B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. J. Thromb. Haemost. 2004, 92, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, K.; Stegen, S.; Van Gastel, N.; Carmeliet, G. The vasculature: A vessel for bone metastasis. BoneKEy Rep. 2015, 4, 742. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Aravindan, B.K.; Prabhakar, J.; Somanathan, T.; Subhadra, L. The role of chemokine receptor 4 and its ligand stromal cell derived factor 1 in breast cancer. Ann. Transl. Med. 2015, 3, 23. [Google Scholar]
- Łukaszewski, B.; Nazar, J.; Goch, M.; Łukaszewska, M.; Stępiński, A.; Jurczyk, M.U. Diagnostic methods for detection of bone metastases. Contemp. Oncol. 2017, 21, 98–103. [Google Scholar] [CrossRef]
- De Giorgi, U.; Valero, V.; Rohren, E.; Mego, M.; Doyle, G.V.; Miller, M.C.; Ueno, N.T.; Handy, B.C.; Reuben, J.M.; Macapinlac, H.A.; et al. Circulating tumor cells and bone metastases as detected by FDG–PET/CT in patients with metastatic breast cancer. Ann. Oncol. 2010, 21, 33–39. [Google Scholar] [CrossRef]
- Basso, U.; Facchinetti, A.; Rossi, E.; Maruzzo, M.; Conteduca, V.; Aieta, M.; Massari, F.; Fraccon, A.P.; Mucciarini, C.; Sava, T.; et al. Prognostic Role of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma: A Large, Multicenter, prospective trial. J. Oncol. 2021, 26, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, J.; Li, J.; Jia, S.; Wang, Y.; Sun, Q.; Kang, Y.; Liu, Y.; Cao, Y.; Yu, J. Utility of Circulating Tumor Cells for Detection of Early-Stage Luminal A Breast Cancer. Am. J. Med. Sci. 2020, 360, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.M.; Vesely, C.; Childs, A.; Marafioti, T.; Khan, M.S.; Mandair, D.; Cives, M.; Ensell, L.; Lowe, H.; Akarca, A.U.; et al. Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br. J. Cancer 2019, 120, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients with Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.R.; Leversha, M.A.; Danila, D.C.; Lin, O.; Gonzalez-Espinoza, R.; Gu, B.; Anand, A.; Smith, K.; Maslak, P.; Doyle, G.V.; et al. Circulating Tumor Cell Analysis in Patients with Progressive Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2007, 13, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Wittner, B.S.; Donaldson, M.C.; O’Keefe, R.; Engstrom, A.; Bersani, F.; Zheng, Y.; Comaills, V.; Niederhoffer, K.; et al. AR Expression in Breast Cancer CTCs Associates with Bone Metastases. Mol. Cancer Res. 2018, 16, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Helo, P.; Cronin, A.M.; Danila, D.C.; Wenske, S.; Gonzalez-Espinoza, R.; Anand, A.; Koscuiszka, M.; Väänänen, R.M.; Pettersson, K.; Chun, F.K.; et al. Circulating Prostate Tumor Cells Detected by Reverse Transcription-PCR in Men with Localized or Castration-Refractory Prostate Cancer: Concordance with CellSearch Assay and Association with Bone Metastases and with Survival. Clin. Chem. 2009, 55, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.I.; et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694–710. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, L.; Yang, H.S.; Liu, G.F. Circulating tumor cells are associated with bone metastasis of lung cancer. Asian Pac. J. Cancer Prev. 2014, 15, 6369–6374. [Google Scholar] [CrossRef]
- Foroni, C.; Milan, M.; Strina, C.; Cappelletti, M.; Fumarola, C.; Bonelli, M.; Bertoni, R.; Ferrero, G.; Maldotti, M.; Takano, E.; et al. Pure anti-tumor effect of zoledronic acid in naïve bone-only metastatic and locally advanced breast cancer: Proof from the “biological window therapy”. Breast Cancer Res. Treat. 2014, 144, 113–121. [Google Scholar] [CrossRef]
- Smid, M.; Wang, Y.; Klijn, J.G.; Sieuwerts, A.M.; Zhang, Y.; Atkins, D.; Martens, J.W.; Foekens, J.A. Genes Associated With Breast Cancer Metastatic to Bone. Clin. Oncol. 2006, 24, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, A.; Larsson, K.; Månsson, M.; Björkman, J.; Rohlova, E.; Åhs, D.; Brisby, H.; Damber, J.E.; Welén, K. Circulating tumor cells mirror bone metastatic phenotype in prostate cancer. Oncotarget 2018, 9, 29403–29413. [Google Scholar] [CrossRef] [PubMed]
- Pantano, F.; Rossi, E.; Iuliani, M.; Facchinetti, A.; Simonetti, S.; Ribelli, G.; Zoccoli, A.; Vincenzi, B.; Tonini, G.; Zamarchi, R.; et al. Dynamic changes of Receptor activator of nuclear factor-κB expression in Circulating Tumor Cells during Denosumab predict treatment effectiveness in Metastatic Breast Cancer. Sci. Rep. 2020, 10, 1288. [Google Scholar] [CrossRef] [PubMed]
- Onken, J.S.; Fekonja, L.S.; Wehowsky, R.; Hubertus, V.; Vajkoczy, P. Metastatic dissemination patterns of different primary tumors to the spine and other bones. Clin. Exp. Metastasis 2019, 36, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Pierga, J.Y.; Hajage, D.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Diéras, V.; Rolland, E.; Mignot, L.; Mathiot, C.; et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann. Oncol. 2012, 23, 618–624. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Mego, M.; Rohren, E.M.; Rohren, E.M.; Liu, P.; Handy, B.C.; Reuben, J.M.; Macapinlac, H.A.; Hortobagyi, G.N.; Cristofanilli, M.; et al. 18F-FDG PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastases from breast cancer. J. Nucl. Med. 2010, 51, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Lovero, D.; D’Oronzo, S.; Palmirotta, R.; Cafforio, P.; Brown, J.; Wood, S.; Porta, C.; Lauricella, E.; Coleman, R.; Silvestris, F. Correlation between targeted RNAseq signature of breast cancer CTCs and onset of bone-only metastases. Br. J. Cancer 2022, 126, 419–429. [Google Scholar] [CrossRef]
- Bidard, F.C.; Vincent-Salomon, A.; Sigal-Zafrani, B.; Diéras, V.; Mathiot, C.; Mignot, L.; Thiery, J.P.; Sastre-Garau, X.; Pierga, J.Y. Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann. Oncol. 2008, 19, 496–500. [Google Scholar] [CrossRef]
- Ried, K.; Tamanna, T.; Matthews, S.; Eng, P.; Sali, A. New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers. Original Research. Front. Oncol. 2020, 10, 582. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Lee, R.J.; Kalinich, M.; LiCausi, J.A.; Zheng, Y.; Chen, T.; Milner, J.D.; Emmons, E.; Ho, U.; Broderick, K.; et al. An RNA-Based Digital Circulating Tumor Cell Signature Is Predictive of Drug Response and Early Dissemination in Prostate Cancer. Cancer Discov. 2018, 8, 288–303. [Google Scholar] [CrossRef]
- Thalgott, M.; Rack, B.; Maurer, T.; Souvatzoglou, M.; Eiber, M.; Kreß, V.; Heck, M.M.; Andergassen, U.; Nawroth, R.; Gschwend, J.E.; et al. Detection of circulating tumor cells in different stages of prostate cancer. Cancer Res. Clin. Oncol. 2013, 139, 755–763. [Google Scholar] [CrossRef]
- Danila, D.C.; Heller, G.; Gignac, G.A.; Gonzalez-Espinoza, R.; Anand, A.; Tanaka, E.; Lilja, H.; Schwartz, L.; Larson, S.; Fleisher, M.; et al. Circulating Tumor Cell Number and Prognosis in Progressive Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2007, 13, 7053–7058. [Google Scholar] [CrossRef]
- Naito, T.; Tanaka, F.; Ono, A.; Yoneda, K.; Takahashi, T.; Murakami, H.; Nakamura, Y.; Tsuya, A.; Kenmotsu, H.; Shukuya, T.; et al. Prognostic Impact of Circulating Tumor Cells in Patients with Small Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 512–519. [Google Scholar] [CrossRef]
- Shimazu, K.; Fukuda, K.; Yoshida, T.; Inoue, M.; Shibata, H. High circulating tumor cell concentrations in a specific subtype of gastric cancer with diffuse bone metastasis at diagnosis. World J. Gastroenterol. 2016, 22, 6083–6088. [Google Scholar] [CrossRef]
- Li, S.; Yan, W.; Yang, X.; Chen, L.; Fan, L.; Liu, H.; Liu, K.; Zhang, Y.; Jiang, J. Less micrometastatic risk related to circulating tumor cells after endoscopic breast cancer surgery compared to open surgery. BMC Cancer 2019, 19, 1070. [Google Scholar] [CrossRef]
- Tamminga, M.; de Wit, S.; van de Wauwer, C.; van den Bos, H.; Swennenhuis, J.F.; Klinkenberg, T.J.; Hiltermann, T.J.N.; Andree, K.C.; Spierings, D.C.J.; Lansdorp, P.M.; et al. Analysis of released circulating tumor cells during surgery for non-small cell lung cancer. Clin. Cancer Res. 2020, 26, 1656–1666. [Google Scholar] [CrossRef]
- Matikas, A.; Kotsakis, A.; Apostolaki, S.; Politaki, H.; Perraki, M.; Kalbakis, K.; Nikolaou, M.; Economopoulou, P.; Hatzidaki, D.; Georgoulias, V. Detection of circulating tumour cells before and following adjuvant chemotherapy and long-term prognosis of early breast cancer. Br. J. Cancer 2022, 126, 1563–1569. [Google Scholar] [CrossRef]
- Tamminga, M.; de Wit, S.; Hiltermann, T.J.N.; Timens, W.; Schuuring, E.; Terstappen, L.W.M.M.; Groen, H.J.M. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 173. [Google Scholar] [CrossRef]
- Dotse, E.; Lim, K.H.; Wang, M.; Wijanarko, K.J.; Chow, K.T. An immunological perspective of circulating tumor cells as diagnostic biomarkers and therapeutic targets. Life 2022, 12, 323. [Google Scholar] [CrossRef]
- Jiang, C.; Hu, F.; Xia, X.; Guo, X. Prognostic value of alkaline phosphatase and bone-specific alkaline phosphatase in breast cancer: A systematic review and meta-analysis. Int. J. Biol. Markers 2023, 38, 25–36. [Google Scholar] [CrossRef]
- Zhao, H.; Han, K.L.; Wang, Z.Y.; Chen, Y.; Li, H.T.; Zeng, J.L.; Shen, Z.; Yao, Y. Value of C-telopeptide-cross-linked type I collagen, osteocalcin, bone-specific alkaline phosphatase and procollagen Type I N-terminal propeptide in the diagnosis and prognosis of bone metastasis in patients with malignant tumors. Med. Sci. Monit. 2011, 17, 626–633. [Google Scholar] [CrossRef]
- Huang, P.; Lan, M.; Peng, A.F.; Yu, Q.F.; Chen, W.Z.; Liu, Z.L.; Liu, J.M.; Huang, S.H. Serum calcium, alkaline phosphotase and hemoglobin as risk factors for bone metastases in bladder cancer. PLoS ONE 2017, 12, e0183835. [Google Scholar] [CrossRef]
- Li, L.; Shen, X.; Liang, Y.; Li, B.; Si, Y.; Ma, R. N-telopeptide as a potential diagnostic and prognostic marker for bone metastasis in human cancers: A meta-analysis. Heliyon 2023, 9, e15980. [Google Scholar] [CrossRef]
- Lyubimova, N.V.; Pashkov, M.; Tyulyandin, S.A.; Gol’dberg, V.E.; Kushlinskii, N.E. Tartrate-resistant acid phosphatase as a marker of bone metastases in patients with breast cancer and prostate cancer. Bull. Exp. Biol. Med. 2004, 138, 77–79. [Google Scholar] [CrossRef]
- Wolff, J.M.; Bares, R.; Jung, P.K.; Buell, U.; Jakse, G. Prostate-specific antigen as a marker of bone metastasis in patients with prostate cancer. Urol. Int. 1996, 56, 169–173. [Google Scholar] [CrossRef]
- Thomsen, F.B.; Westerberg, M.; Garmo, H.; Robinson, D.; Holmberg, L.; Ulmert, H.D.; Stattin, P. Prediction of metastatic prostate cancer by prostate-specific antigen in combination with T stage and Gleason Grade: Nationwide, population-based register study. PLoS ONE 2020, 15, e0228447. [Google Scholar] [CrossRef]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A review of the role of carcinoembryonic antigen in clinical practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef]
- Li, A.A.; Cao, Z.Y.; Liu, J.M.; Huang, S.H.; Liu, Z.L. The risk factors for bone metastases in patients with colorectal cancer. Medicine 2018, 97, e12694. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Dang, L.; Liang, C.; Lu, A.; Zhang, G. RANKL/RANK System-Based Mechanism for Breast Cancer Bone Metastasis and Related Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 76. [Google Scholar] [CrossRef]
- Dell’Aquila, E.; Armento, G.; Iuliani, M.; Simonetti, S.; D’Onofrio, L.; Zeppola, T.; Madaudo, C.; Russano, M.; Citarella, F.; Ribelli, G.; et al. Denosumab for cancer-related bone loss. Expert Opin. Biol. Ther. 2020, 20, 1261–1274. [Google Scholar] [CrossRef]
- Akashi, T.; Okumura, T.; Terabayashi, K.; Yoshino, Y.; Tanaka, H.; Yamazaki, T.; Numata, Y.; Fukuda, T.; Manabe, T.; Baba, H.; et al. The use of an artificial intelligence algorithm for circulating tumor cell detection in patients with esophageal cancer. Oncol. Lett. 2023, 26, 320. [Google Scholar] [CrossRef]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to machine learning, neural networks, and deep learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar]
- He, B.; Lu, Q.; Lang, J.; Yu, H.; Peng, C.; Bing, P.; Li, S.; Zhou, Q.; Liang, Y.; Tian, G. A new method for CTC images recognition based on machine learning. Front. Bioeng. Biotechnol. 2020, 8, 897. [Google Scholar] [CrossRef] [PubMed]
- Da Col, G.; Del Ben, F.; Bulfoni, M.; Turetta, M.; Gerratana, L.; Bertozzi, S.; Beltrami, A.P.; Cesselli, D. Image analysis of circulating tumor cells and leukocytes predicts survival and metastatic pattern in breast cancer patients. Front. Oncol. 2022, 12, 725318. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, J.; Jin, Y.; Cheng, S.; Huang, S.; Zhang, N.; Wang, Y. Artificial intelligence based on blood biomarkers including CTCs predicts outcomes in epithelial ovarian cancer: A prospective study. Onco Targets Ther. 2021, 14, 3267–3280. [Google Scholar] [CrossRef]
- Zeune, L.L.; Boink, Y.E.; van Dalum, G.; Nanou, A.; de Wit, S.; Andree, K.C.; Swennenhuis, J.F.; van Gils, S.A.; Terstappen, L.W.M.M.; Brune, C. Deep learning of circulating tumour cells. Nat. Mach. Intell. 2020, 2, 124–133. [Google Scholar] [CrossRef]
| CTC Screening Technique | Enrichment Method | Blood Volume (mL) | Clinical Value | Primary Tumour Type (Study Year) |
|---|---|---|---|---|
| CellSearchTM System | Immunomagnetic | 7.5 | ≥5 CTCs in 90% of patients with bone metastases | Breast (2010) [30] |
| Total CTCs associated with Time-to-Bone-Metastasis-Progression | Breast (2021) [31] | |||
| ≥1 CTCs in 93.65% of patients with bone metastasis | Lung (2014) [32] | |||
| Significant association between bone metastases and CTC expressing CXCR4 | Neuroendocrine (2019) [33] | |||
| CTC number ≥2 significantly associated with liver or bone metastases | Lung (2011) [34] | |||
| Diagnosis and prognosis of bone metastasis | Prostate (2007) [35] | |||
| CTC iChip | Immunomagnetic | 10 | Role of androgen receptor signalling in CTCs in breast cancer bone metastasis | Breast (2018) [36] |
| RT-PCR | Density gradient centrifugation | 2.5 | CTCs closely associated with clinical evidence of bone metastases | Prostate (2009) [37] |
| Author, Year | Primary Cancer Site | Patient Recruitment Period | No. of Patients | Technology | Study Details |
|---|---|---|---|---|---|
| De Giorgi, Valero et al., 2010 [30] | Breast cancer | 2004–2008 | 195 | CellSearchTM | The relationship between CTCs level and bone metastases |
| Basso, Facchinetti et al., 2021 [31] | Renal cancer | 2008–2010 | 195 | CellSearchTM | Drug trial with sunitinib (77.5%) or pazopanib (21%) |
| Cheng, Liu et al., 2014 [32] | Lung cancer | 2009–2013 | 97 | CellSearchTM | Relationship between increased CTCs and MRI-detected metastasis in bone in patients diagnosed with progressive lung cancer. |
| Rizzo, Vesely et al., 2019 [33] | Neuroendocrine cancer | 2009–2017 | 254 | CellSearchTM | Expression of CXCR4 on CTCs as a potential predictor of skeletal invasion. |
| Matthew G, Krebs et al., 2011 [34] | Lung cancer | 2007–2009 | 109 | CellSearchTM | Determine prevalence and clinical significance of CTCs in patients with non–small-cell lung cancer. |
| Shaffer, David R et al., 2007 [35] | Prostate cancer | 2009 | 63 | CellSearchTM | CTCs from peripheral blood of patients with advanced prostate cancer using immunomagnetic trapping techniques. |
| Aceto, Bardia et al., 2018 [36] | Breast cancer | 2018 | 32 | CTC-iChip | RNA sequencing of CTCs from with metastatic oestrogen receptor (ER)+ breast cancer, comparing cases with progression in bone versus visceral organs. |
| Helo, Cronin et al., 2009 [37] | Prostate cancer | 2009 | 180 | RT-PCR | CTCs in patients with localised prostate cancer or CRPC by real-time RT-PCR of KLK3 and KLK2 mRNAs. |
| Foroni, Milan et al., 2014 [40] | Breast cancer | 2014 | 33 | CellSearchTM | Anti-tumour effect of zoledronic acid (ZA) |
| Smid, Wang et al., 2006 [41] | Breast cancer | 2016 | 107 | - | Genes associated with breast cancer metastatic to bone |
| Josefsson, Larsson et al., 2018 [42] | Prostate cancer | 2013–2016 | 25 | Capturing CTCs on EpCAM- and HER2 antibody-conjugated magnetic beads | Comparing expression profiles of 41 prostate cancer-related genes between paired CTC and spinal column metastasis patients. Gene expression (EpCAM, GAPDH, GUSB, CD45, CD44) |
| Pantano, Rossi et al., 2020 [43] | Breast cancer | 2012–2015 | 42 | CellSearchTM | Novel CTC assay by using an anti-RANK monoclonal antibody in conjunction with CellSearchTM platform |
| Onken, Fekonja et al., 2019 [44] | Lung cancer, prostate cancer, breast cancer, kidney cancer, lower gastrointestinal tract, and malignant melanoma | 2005–2015 | 507 | - | Investigating metastatic dissemination of tumour cells to spinal bone and other osseous organs |
| Pierga, Hajage et al., 2012 [45] | Breast cancer | 2007–2009 | 267 | CellSearchTM | First-line chemotherapy |
| De Giorgi, Mego et al., 2010 [46] | Breast cancer | 2004–2008 | 55 | CellSearchTM | Determining the predictive significance of CTC counts and 18F-FDG PET/CT findings in patients with bone metastases from breast cancer treated with standard systemic therapies |
| Lovero, D’Oronzo et al., 2022 [47] | Breast cancer | 2022 | 30 | - | Developing a targeted RNAseq assay to screen a genes critically involved in the metastatic cascade |
| Bidard, Vincent-Salomon, A et al., 2008 [48] | Breast cancer | 1998–2005 | 837 | - | Study of clinical outcomes in metastatic breast cancer according CTC status. |
| Ried, Tamanna et al., 2020 [49] | Renal cancer | 2014–2019 | 49 | ISET®-CTC | The cytology-based ISET®-CTC Test |
| Miyamoto, Lee et al., 2018 [50] | Prostate cancer | 2018 | 61 | CTC-iChip | A novel assay for detection of liver-derived CTCs |
| Mark, Rack et al., 2013 [51] | Prostate cancer | 2008–2010 | 90 | CellSearchTM | Explore CTC counts in different stages of prostate cancer in association with tumour burden |
| Danila, Heller et al., 2007 [52] | Prostate cancer | 2007 | 120 | CellSearchTM | Various hormonal and cytotoxic therapies. |
| Naito, Tanaka et al., 2012 [53] | Lung cancer | 2009–2010 | 51 | CellSearchTM | Evaluating relationship of CTCs to disease prognosis |
| Shimazu, Fukuda et al., 2016 [54] | Gastric cancer | 2014–2015 | 39 | CellSearchTM | Clarify the biological features contributing to gastric cancer with diffuse bone metastases at diagnosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-W.; Sun, A.K.; Cheung, J.P.-Y.; Ho, J.C.-Y. Circulating Tumour Cells in the Prediction of Bone Metastasis. Cancers 2024, 16, 252. https://doi.org/10.3390/cancers16020252
Choi S-W, Sun AK, Cheung JP-Y, Ho JC-Y. Circulating Tumour Cells in the Prediction of Bone Metastasis. Cancers. 2024; 16(2):252. https://doi.org/10.3390/cancers16020252
Chicago/Turabian StyleChoi, Siu-Wai, Aria Kaiyuan Sun, Jason Pui-Yin Cheung, and Jemmi Ching-Ying Ho. 2024. "Circulating Tumour Cells in the Prediction of Bone Metastasis" Cancers 16, no. 2: 252. https://doi.org/10.3390/cancers16020252
APA StyleChoi, S.-W., Sun, A. K., Cheung, J. P.-Y., & Ho, J. C.-Y. (2024). Circulating Tumour Cells in the Prediction of Bone Metastasis. Cancers, 16(2), 252. https://doi.org/10.3390/cancers16020252







