PRAME Is an Effective Tool for the Diagnosis of Nevus-Associated Cutaneous Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases Selection
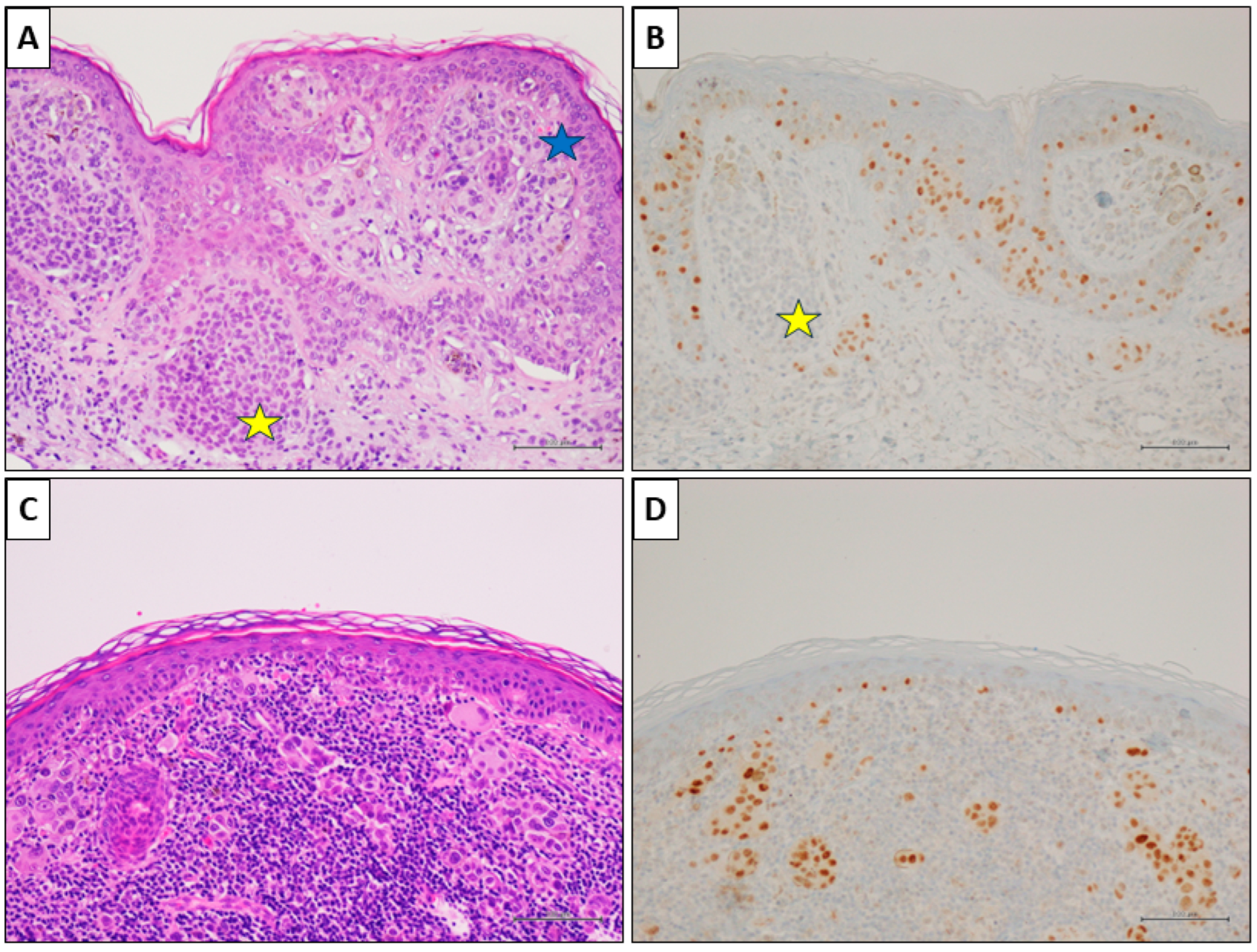
2.2. PRAME Immunohistochemistry
2.3. Statistical Analysis
2.4. Test Accuracy
3. Results
3.1. Clinical and Pathological Findings
3.2. PRAME Immunohistochemistry and Diagnostic Performance
3.3. Statistical Results
3.4. Test Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boothby-Shoemaker, W.; Guan, L.; Jones, B.; Chaffins, M.; Kohen, L.; Pimentel, J.; Veenstra, J.; Friedman, B.J. Real world validation of an adjunctive gene expression-profiling assay for melanoma diagnosis and correlation with clinical outcomes at an academic center. Hum. Pathol. 2023, 139, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Massaro, A.; Colagrande, A.; Lettini, T.; Cicco, S.; Parente, P.; Nacchiero, E.; Lospalluti, L.; Cascardi, E.; Giudice, G.; et al. Dermatopathology of malignant melanoma in the era of Artificial Intelligence: A single institutional experience. Diagnostics 2022, 12, 1972. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Pagliuca, F.; Zito Marino, F.; Argenziano, G.; Brancaccio, G.; Alfano, R.; Signoriello, G.; Moscarella, E.; Franco, R. Second diagnostic opinion by experienced dermatopathologists in the setting of a referral regional Melanoma Unit significantly improves the clinical management of patients with cutaneous melanoma. Front. Med. 2021, 7, 568946. [Google Scholar] [CrossRef] [PubMed]
- Massi, D.; Szumera-Ciećkiewicz, A.; Alos, L.; Simi, S.; Ugolini, F.; Palmieri, G.; Stanganelli, I.; Cook, M.G.; Mandalà, M. Impact of second opinion pathology review in the diagnosis and management of atypical melanocytic lesions: A prospective study of the Italian Melanoma Intergroup (IMI) and EORTC Melanoma Group. Eur. J. Cancer 2023, 189, 112921. [Google Scholar] [CrossRef] [PubMed]
- Bevona, C.; Goggins, W.; Quinn, T.; Fullerton, J.; Tsao, H. Cutaneous melanomas associated with nevi. Arch. Dermatol. 2003, 139, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Shitara, D.; Nascimento, M.M.; Puig, S.; Yamada, S.; Enokihara, M.M.; Michalany, N.; Bagatin, E. Nevus-associated melanomas: Clinicopathologic features. Am. J. Clin. Pathol. 2014, 142, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Pampena, R.; Kyrgidis, A.; Lallas, A.; Moscarella, E.; Argenziano, G.; Longo, C. A meta-analysis of nevus-associated melanoma: Prevalence and practical implications. J. Am. Acad. Dermatol. 2017, 77, 938–945.e4. [Google Scholar] [CrossRef]
- Yeh, I.; Bastian, B.C. Melanoma pathology: New approaches and classification. Br. J. Dermatol. 2021, 185, 282–293. [Google Scholar] [CrossRef]
- Clark, W.H., Jr.; Elder, D.E.; Guerry, D.; Epstein, M.N.; Greene, M.H.; Van Horn, M. A study of tumor progression: The precursor lesions of superficial spreading and nodular melanoma. Hum. Pathol. 1984, 15, 1147–1165. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Parsons, P.G.; Green, A.C. p53 expression and risk factors for cutaneous melanoma: A case-control study. Int. J. Cancer 1998, 77, 843–848. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Oh, K.S.; Alexis, J. Potential diagnostic utility of PRAME and p16 immunohistochemistry in melanocytic nevi and malignant melanoma. J. Cutan. Pathol. 2023, 50, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Epping, M.T.; Wang, L.; Edel, M.J.; Carlée, L.; Hernandez, M.; Bernards, R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 2005, 122, 835–847. [Google Scholar] [CrossRef]
- Costessi, A.; Mahrour, N.; Tijchon, E.; Stunnenberg, R.; Stoel, M.A.; Jansen, P.W.; Sela, D.; Martin-Brown, S.; Washburn, M.P.; Florens, L.; et al. The tumour antigen PRAME is a subunit of a Cul2 ubiquitin ligase and associates with active NFY promoters. EMBO J. 2011, 30, 3786–3798. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, M.; Chłopek, M.; Kruczak, A.; Ryś, J.; Lasota, J.; Miettinen, M. PRAME expression in cancer. A systematic immunohistochemical study of >5800 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2022, 46, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.L.; De Pas, T.; Rittmeyer, A.; Vallières, E.; Kubisa, B.; Levchenko, E.; Wiesemann, S.; Masters, G.A.; Shen, R.; Tjulandin, S.A.; et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in patients with resected Non-Small Cell Lung Cancer: A phase I dose escalation study. J. Thorac. Oncol. 2016, 11, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Rivoltini, L.; Levchenko, E.; Testori, A.; Utikal, J.; Ascierto, P.A.; Demidov, L.; Grob, J.J.; Ridolfi, R.; Schadendorf, D.; et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: Results of a phase I dose escalation study. ESMO Open 2016, 1, e000068. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V. The cancer testis antigen PRAME as a biomarker for solid tumor cancer management. Biomark. Med. 2012, 6, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Grillini, M.; Ricci, C.; Pino, V.; Pedrini, S.; Fiorentino, M.; Corti, B. HMB45/PRAME, a novel double staining for the diagnosis of melanocytic neoplasms: Technical aspects, results, and comparison with other commercially available staining (PRAME and Melan a/PRAME). Appl. Immunohistochem. Mol. Morphol. 2022, 30, 14–18. [Google Scholar] [CrossRef]
- O’Connor, M.K.; Dai, H.; Fraga, G.R. PRAME immunohistochemistry for melanoma diagnosis: A STARD-compliant diagnostic accuracy study. J. Cutan. Pathol. 2022, 49, 780–786. [Google Scholar] [CrossRef]
- Alomari, A.K.; Tharp, A.W.; Umphress, B.; Kowal, R.P. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J. Cutan. Pathol. 2021, 48, 1115–1123. [Google Scholar] [CrossRef]
- Lohman, M.E.; Steen, A.J.; Grekin, R.C.; North, J.P. The utility of PRAME staining in identifying malignant transformation of melanocytic nevi. J. Cutan. Pathol. 2021, 48, 856–862. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME expression in melanocytic tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef]
- Kunc, M.; Żemierowska, N.; Skowronek, F.; Biernat, W. Diagnostic test accuracy meta-analysis of PRAME in distinguishing primary cutaneous melanomas from benign melanocytic lesions. Histopathology 2023, 83, 3–14. [Google Scholar] [CrossRef]
- Bosch, C.; Geller, A.C.; Stratigos, A.J. A review of nevus-associated melanoma: What is the evidence? J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1927–1936. [Google Scholar] [CrossRef]
- Bosch-Amate, X.; Podlipnik, S.; Riquelme-Mc Loughlin, C.; Carrera, C.; Barreiro-Capurro, A.; García-Herrera, A.; Alós, L.; Malvehy, J.; Puig, S. Clinicopathological, genetic and survival advantages of naevus-associated melanomas: A cohort study. Acta Derm. Venereal. 2021, 101, adv00425. [Google Scholar] [CrossRef]
- Dessinioti, C.; Geller, A.C.; Stergiopoulou, A.; Dimou, N.; Lo, S.; Keim, U.; Gershenwald, J.E.; Haydu, L.E.; Dummer, R.; Mangana, J.; et al. A multicentre study of naevus-associated melanoma vs. de novo melanoma, tumour thickness and body site differences. Br. J. Dermatol. 2021, 185, 101–109. [Google Scholar] [CrossRef]
- Tas, F.; Erturk, K. De novo and nevus-associated melanomas: Different histopathologic characteristics but similar survival rates. Pathol. Oncol. Res. 2020, 26, 2483–2487. [Google Scholar] [CrossRef]
- Manrique-Silva, E.; Reyes-García, D.; Folgado, B.; Martín-Gorgojo, A.; Traves, V.; Requena, C.; Nagore, E. The proportion of nevus-associated invasive melanoma differs with Breslow thickness: A cross-sectional study of 1087 cutaneous melanomas. J. Am. Acad. Dermatol. 2019, 81, 852–854. [Google Scholar] [CrossRef]
- Scalvenzi, M.; Megna, M.; Costa, C.; Fabbrocini, G.; Villani, A.; Greco, V. Cutaneous melanoma associated with naevi prevalence: A 15-year crosssectional retrospective study. Australas. J. Dermatol. 2020, 61, 39–42. [Google Scholar] [CrossRef]
- Alendar, T.; Kittler, H. Morphologic characteristics of nevi associated with melanoma: A clinical, dermatoscopic and histopathologic analysis. Dermatol. Pract. Concept. 2018, 8, 104–108. [Google Scholar] [CrossRef]
- Martin-Gorgojo, A.; Requena, C.; Garcia-Casado, Z.; Traves, V.; Kumar, R.; Nagore, E. Dysplastic vs. common naevus-associated vs. de novo melanomas: An observational retrospective study of 1021 patients. Acta Derm. Venereol. 2018, 98, 556–562. [Google Scholar] [CrossRef]
- Pandeya, N.; Kvaskoff, M.; Olsen, C.M.; Green, A.C.; Perry, S.; Baxter, C.; Davis, M.B.; Mortimore, R.; Westacott, L.; Wood, D.; et al. Factors related to nevus-associated cutaneous melanoma: A case-case study. J. Investig. Dermatol. 2018, 138, 1816–1824. [Google Scholar] [CrossRef]
- Pan, Y.; Adler, N.R.; Wolfe, R.; McLean, C.A.; Kelly, J.W. Nodular melanoma is less likely than superficial spreading melanoma to be histologically associated with a naevus. Med. J. Aust. 2017, 207, 333–338. [Google Scholar] [CrossRef]
- Sheen, Y.S.; Liao, Y.H.; Lin, M.H.; Chen, J.S.; Liau, J.Y.; Liang, C.W.; Chang, Y.L.; Chu, C.Y. Clinicopathological features and prognosis of patients with de novo versus nevus-associated melanoma in Taiwan. PLoS ONE 2017, 12, e0177126. [Google Scholar] [CrossRef]
- Cymerman, R.M.; Shao, Y.; Wang, K.; Zhang, Y.; Murzaku, E.C.; Penn, L.A.; Osman, I.; Polsky, D. De novo vs nevus-associated melanomas: Differences in associations with prognostic indicators and survival. J. Natl. Cancer Inst. 2016, 108, djw121. [Google Scholar] [CrossRef]
- Hacker, E.; Olsen, C.M.; Kvaskoff, M.; Pandeya, N.; Yeo, A.; Green, A.C.; Williamson, R.M.; Triscott, J.; Wood, D.; Mortimore, R.; et al. Histologic and phenotypic factors and MC1R status associated with BRAF(V600E), BRAF(V600K), and NRAS mutations in a community-based sample of 414 cutaneous melanomas. J. Investig. Dermatol. 2016, 136, 829–837. [Google Scholar] [CrossRef]
- Haenssle, H.A.; Mograby, N.; Ngassa, A.; Buhl, T.; Emmert, S.; Schön, M.P.; Rosenberger, A.; Bertsch, H.P. Association of patient risk factors and frequency of nevus-associated cutaneous melanomas. JAMA Dermatol. 2016, 152, 291–298. [Google Scholar] [CrossRef]
- Lin, W.M.; Luo, S.; Muzikansky, A.; Lobo, A.Z.; Tanabe, K.K.; Sober, A.J.; Cosimi, A.B.; Tsao, H.; Duncan, L.M. Outcome of patients with de novo versus nevus-associated melanoma. J. Am. Acad. Dermatol. 2015, 72, 54–58. [Google Scholar] [CrossRef]
- Manganoni, A.M.; Farisoglio, C.; Gavazzoni, F.; Facchetti, F.; Zanotti, F.; Calzavara-Pinton, P. Nodular melanomas associated with nevi. J. Am. Acad. Dermatol. 2010, 63, e97. [Google Scholar] [CrossRef]
- Purdue, M.P.; From, L.; Armstrong, B.K.; Kricker, A.; Gallagher, R.P.; McLaughlin, J.R.; Klar, N.S.; Marrett, L.D.; Genes, Environment, and Melanoma Study Group. Etiologic and other factors predicting nevus-associated cutaneous malignant melanoma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2015–2022. [Google Scholar] [CrossRef]
- Kaddu, S.; Smolle, J.; Zenahlik, P.; Hofmann-Wellenhof, R.; Kerl, H. Melanoma with benign melanocytic naevus components: Reappraisal of clinicopathological features and prognosis. Melanoma Res. 2002, 12, 271–278. [Google Scholar] [CrossRef]
- Ahmed, I.; Piepkorn, M.; Goldgar, D.E.; Cannon-Albright, L.A.; Meyer, L.J.; Skolnick, M.H.; Zone, J.J. HMB-45 staining of dysplastic melanocytic nevi in melanoma risk groups. J. Cutan. Pathol. 1991, 18, 257–260. [Google Scholar] [CrossRef]
- Gerami, P.; Busam, K.; Cochran, A.; Cook, M.G.; Duncan, L.M.; Elder, D.E.; Fullen, D.R.; Guitart, J.; LeBoit, P.E.; Mihm, M.C., Jr.; et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am. J. Surg. Pathol. 2014, 38, 934–940. [Google Scholar] [CrossRef]
- Duncan, L.M.; Berwick, M.; Bruijn, J.A.; Byers, H.R.; Mihm, M.C.; Barnhill, R.L. Histopathologic recognition and grading of dysplastic melanocytic nevi: An interobserver agreement study. J. Investig. Dermatol. 1993, 100, 318S–321S. [Google Scholar] [CrossRef]
- Duray, P.H.; DerSimonian, R.; Barnhill, R.; Stenn, K.; Ernstoff, M.S.; Fine, J.; Kirkwood, J.M. An analysis of interobserver recognition of the histopathologic features of dysplastic nevi from a mixed group of nevomelanocytic lesions. J. Am. Acad. Dermatol. 1992, 27, 741–749. [Google Scholar] [CrossRef]
- Corona, R.; Mele, A.; Amini, M.; De Rosa, G.; Coppola, G.; Piccardi, P.; Fucci, M.; Pasquini, P.; Faraggiana, T. Interobserver variability on the histopathologic diagnosis of cutaneous melanoma and other pigmented skin lesions. J. Clin. Oncol. 1996, 14, 1218–1223. [Google Scholar] [CrossRef]
- Elmore, J.G.; Barnhill, R.L.; Elder, D.E.; Longton, G.M.; Pepe, M.S.; Reisch, L.M.; Carney, P.A.; Titus, L.J.; Nelson, H.D.; Onega, T.; et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: Observer accuracy and reproducibility study. BMJ 2017, 357, j2813. [Google Scholar] [CrossRef]
- Gassenmaier, M.; Hahn, M.; Metzler, G.; Bauer, J.; Yazdi, A.S.; Keim, U.; Garbe, C.; Wagner, N.B.; Forchhammer, S. Diffuse PRAME expression is highly specific for thin melanomas in the distinction from severely dysplastic nevi but does not distinguish metastasizing from non-metastasizing thin melanomas. Cancers 2021, 13, 3864. [Google Scholar] [CrossRef]
- Koh, S.S.; Lau, S.K.; Scapa, J.V.; Cassarino, D.S. PRAME immunohistochemistry of spitzoid neoplasms. J. Cutan. Pathol. 2022, 49, 709–716. [Google Scholar] [CrossRef]
- Gerami, P.; Benton, S.; Zhao, J.; Zhang, B.; Lampley, N., 3rd; Roth, A.; Boutko, A.; Olivares, S.; Busam, K.J. PRAME expression correlates with genomic aberration and malignant diagnosis of spitzoid melanocytic neoplasms. Am. J. Dermatopathol. 2022, 44, 575–580. [Google Scholar] [CrossRef]
- McAfee, J.L.; Scarborough, R.; Jia, X.S.; Azzato, E.M.; Astbury, C.; Ronen, S.; Andea, A.A.; Billings, S.D.; Ko, J.S. Combined utility of p16 and BRAF V600E in the evaluation of spitzoid tumors: Superiority to PRAME and correlation with FISH. J. Cutan. Pathol. 2023, 50, 155–168. [Google Scholar] [CrossRef]
- Umano, G.R.; Errico, M.E.; D’Onofrio, V.; Delehaye, G.; Trotta, L.; Spinelli, C.; Strambi, S.; Franco, R.; D’Abbronzo, G.; Ronchi, A.; et al. The challenge of melanocytic lesions in pediatric patients: Clinical-pathological findings and the diagnostic value of PRAME. Front. Oncol. 2021, 11, 688410. [Google Scholar] [CrossRef]
- Dimonitsas, E.; Liakea, A.; Sakellariou, S.; Thymara, I.; Giannopoulos, A.; Stratigos, A.; Soura, E.; Saetta, A.; Korkolopoulou, P. An update on molecular alterations in melanocytic tumors with emphasis on Spitzoid lesions. Ann. Transl. Med. 2018, 6, 249. [Google Scholar] [CrossRef]




| Score | Positive Cells (%) | Result |
|---|---|---|
| 0 | <1 | Negative |
| 1+ | 1–25 | Negative |
| 2+ | 26–50 | Intermediate |
| 3+ | 51–75 | Positive |
| 4+ | >75 | Positive |
| Age | |
| Mean age | 53 years |
| Median age | 51 years |
| Range | 27–88 years |
| Sex | |
| Males | 37 (53.6%) |
| Females | 32 (46.4%) |
| M:F | 1.16:1 |
| Topography | |
| Trunk | 41 (59.4%) |
| Inferior limbs | 12 (17.4%) |
| Superior limbs | 9 (13.0%) |
| Head and neck | 7 (10.2%) |
| Histological Diagnosis | |
| SSM | 44 (63.8%) |
| In situ CM | 22 (31.9%) |
| Spitzoid CM | 2 (2.9%) |
| Nodular CM | 1 (1.4%) |
| PRAME IHC Score in CM | |
| Score 0 | 11 (15.9%) |
| Score 1+ | 8 (11.6%) |
| Score 2+ | 9 (13.05%) |
| Score 3+ | 9 (13.05%) |
| Score 4+ | 32 (46.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronchi, A.; Cazzato, G.; Ingravallo, G.; D’Abbronzo, G.; Argenziano, G.; Moscarella, E.; Brancaccio, G.; Franco, R. PRAME Is an Effective Tool for the Diagnosis of Nevus-Associated Cutaneous Melanoma. Cancers 2024, 16, 278. https://doi.org/10.3390/cancers16020278
Ronchi A, Cazzato G, Ingravallo G, D’Abbronzo G, Argenziano G, Moscarella E, Brancaccio G, Franco R. PRAME Is an Effective Tool for the Diagnosis of Nevus-Associated Cutaneous Melanoma. Cancers. 2024; 16(2):278. https://doi.org/10.3390/cancers16020278
Chicago/Turabian StyleRonchi, Andrea, Gerardo Cazzato, Giuseppe Ingravallo, Giuseppe D’Abbronzo, Giuseppe Argenziano, Elvira Moscarella, Gabriella Brancaccio, and Renato Franco. 2024. "PRAME Is an Effective Tool for the Diagnosis of Nevus-Associated Cutaneous Melanoma" Cancers 16, no. 2: 278. https://doi.org/10.3390/cancers16020278
APA StyleRonchi, A., Cazzato, G., Ingravallo, G., D’Abbronzo, G., Argenziano, G., Moscarella, E., Brancaccio, G., & Franco, R. (2024). PRAME Is an Effective Tool for the Diagnosis of Nevus-Associated Cutaneous Melanoma. Cancers, 16(2), 278. https://doi.org/10.3390/cancers16020278












