Aberrant DNA Methylation of NPTX2 as an Indicator of Malignant Behavior in Thymic Epithelial Tumors †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
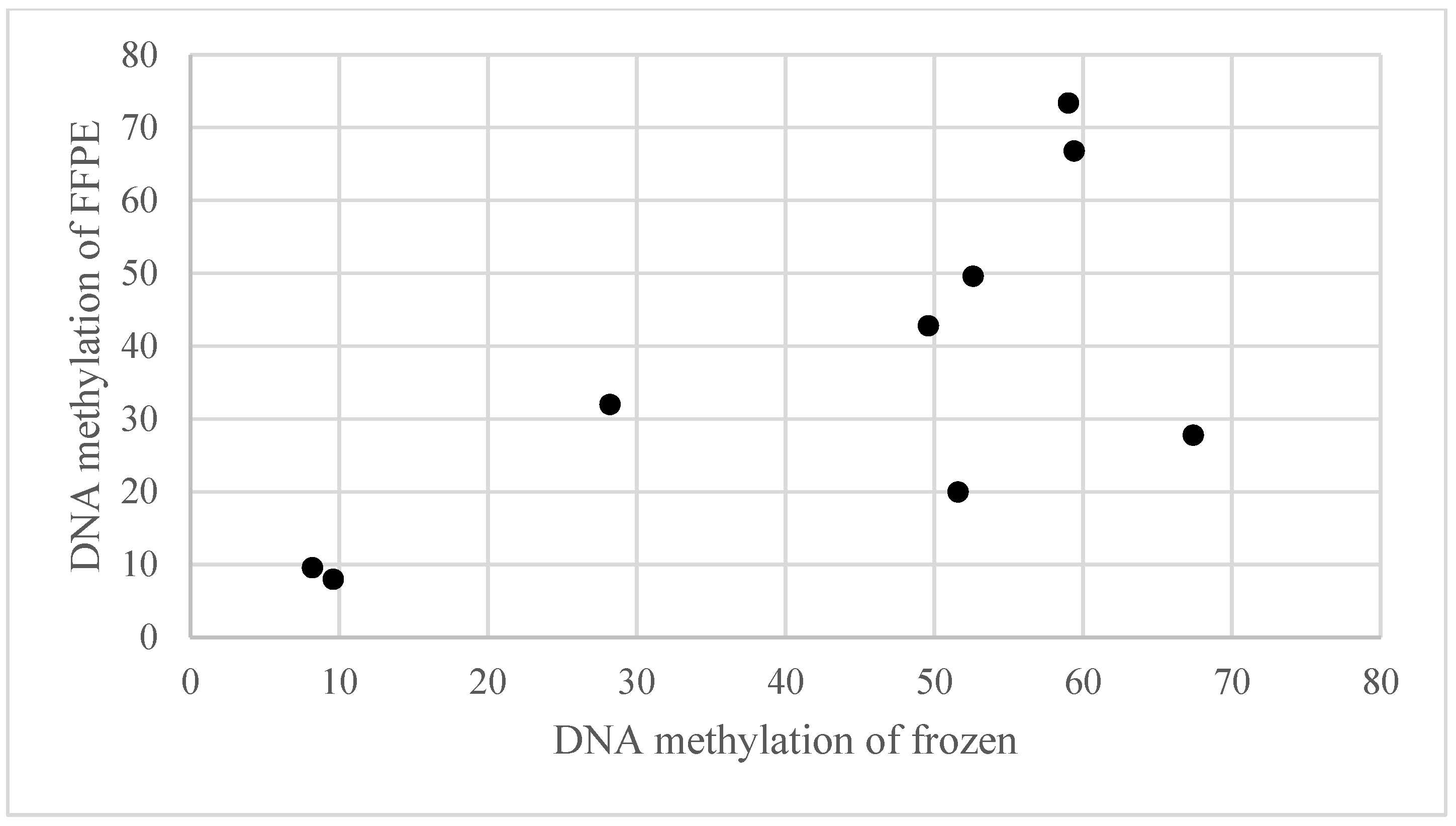
2.2. Global Methylation Analysis
2.3. DNA and RNA Extraction
2.4. Bisulfite Pyrosequencing
2.5. Reverse Transcription–Quantitative PCR (RT-qPCR)
2.6. IHC Staining
2.7. Statistical Analysis
3. Results
3.1. DNA Methylation Rate of the NPTX2 Gene in TET and Paired Normal Thymic Tissues
3.2. DNA Methylation Status of the NPTX2 Gene According to the WHO Histological Classification
3.3. DNA Methylation Status of the NPTX2 Gene According to the Masaoka–Koga Stage Classification
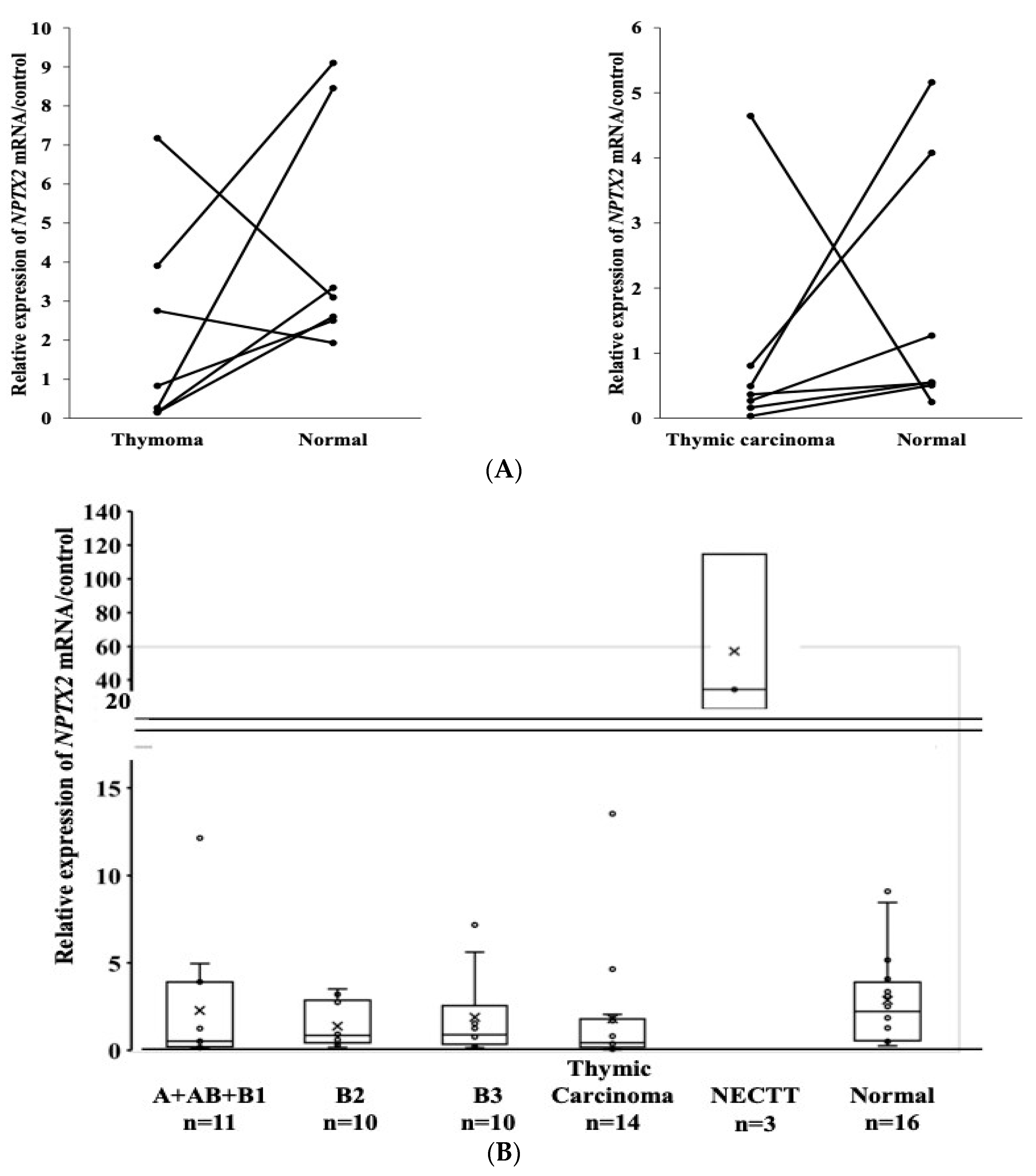
3.4. mRNA Expression of the NPTX2 Gene in TET and Paired Normal Thymic Tissues
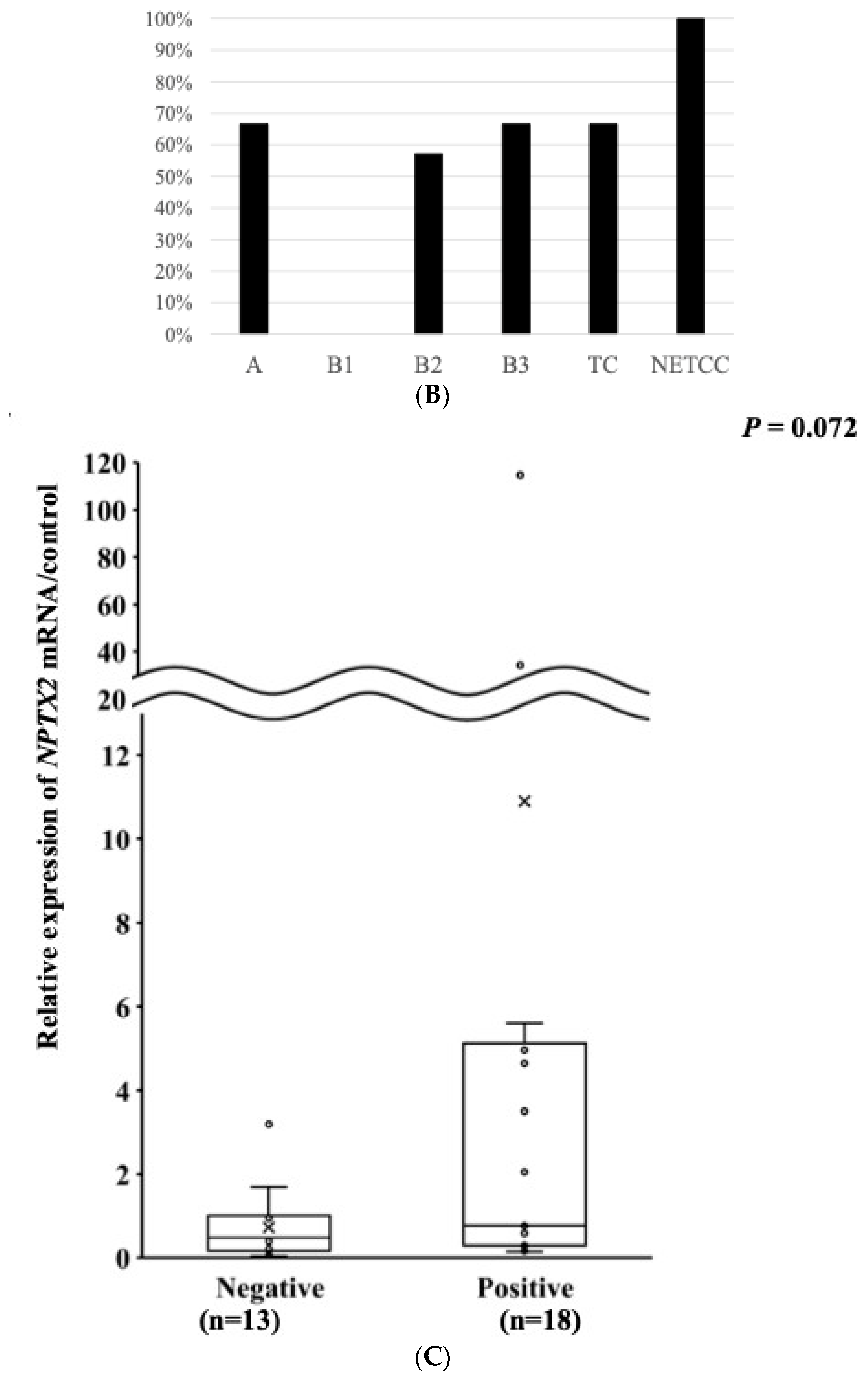
3.5. The mRNA Expression of the NPTX2 Gene According to the WHO Histological Classification
3.6. mRNA Expression of the NPTX2 Gene According to the Masaoka–Koga Staging System
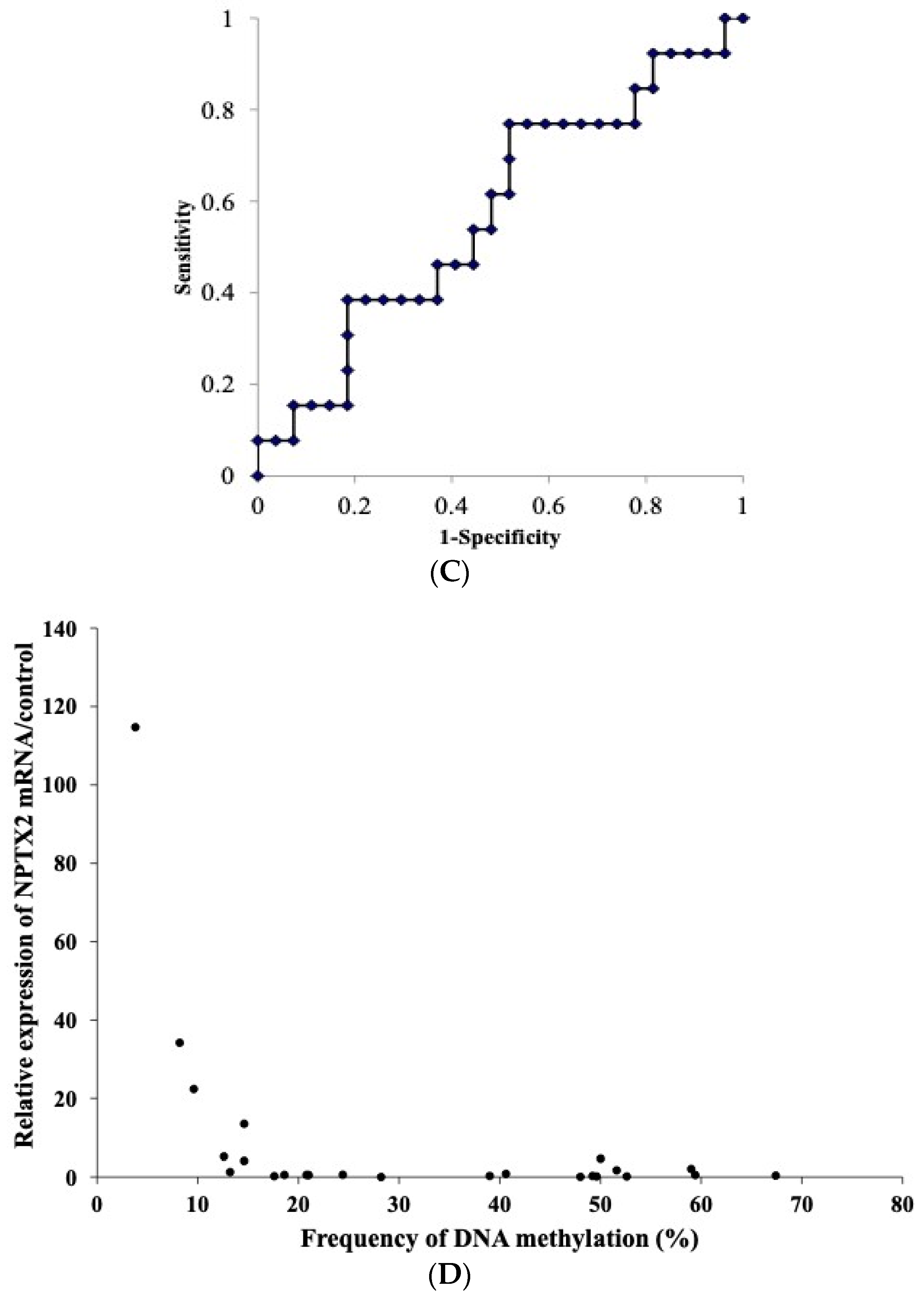
3.7. Relationship between the mRNA Expression and DNA Methylation of NPTX2 in TET
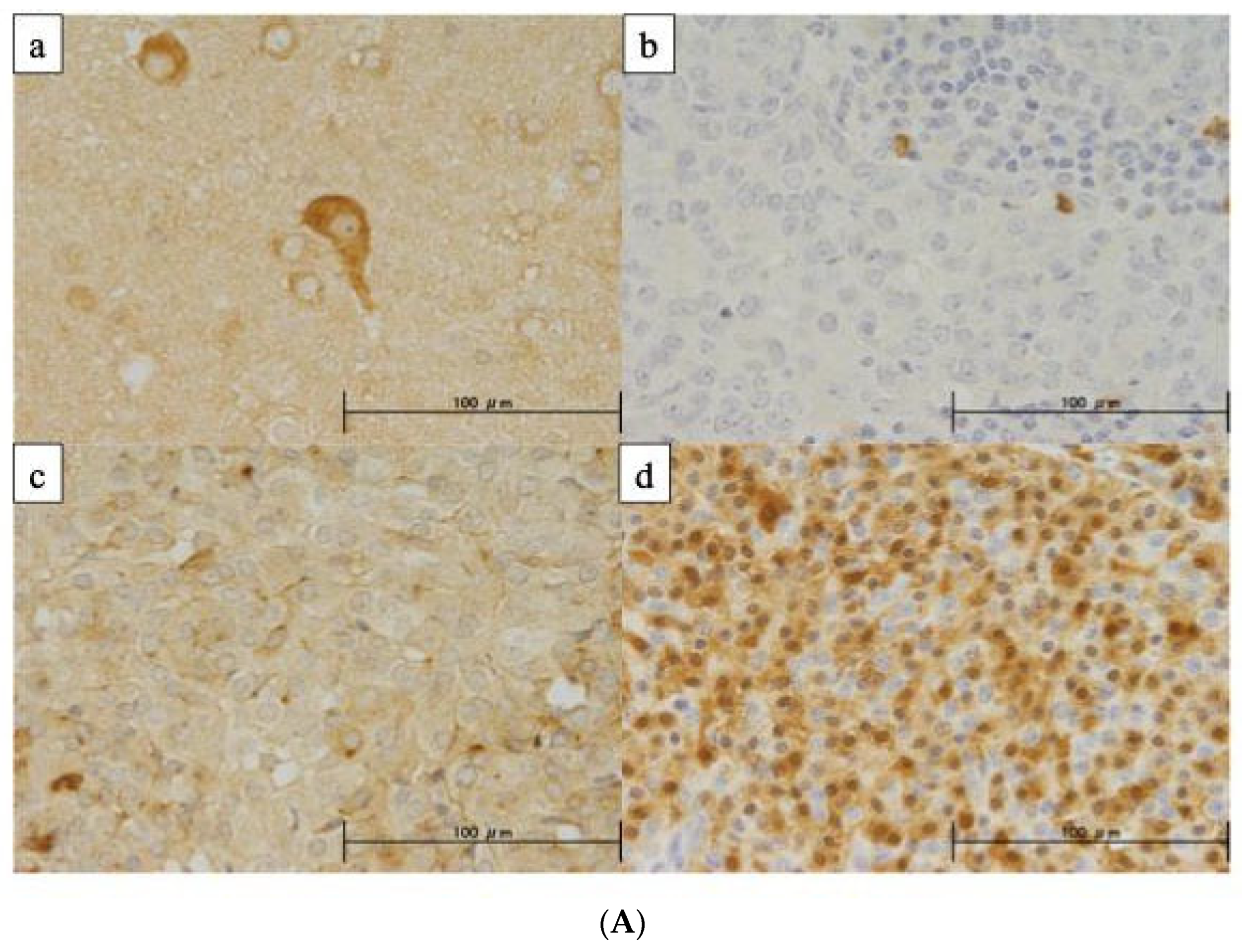
3.8. Protein Expression of NPTX2 Using IHC
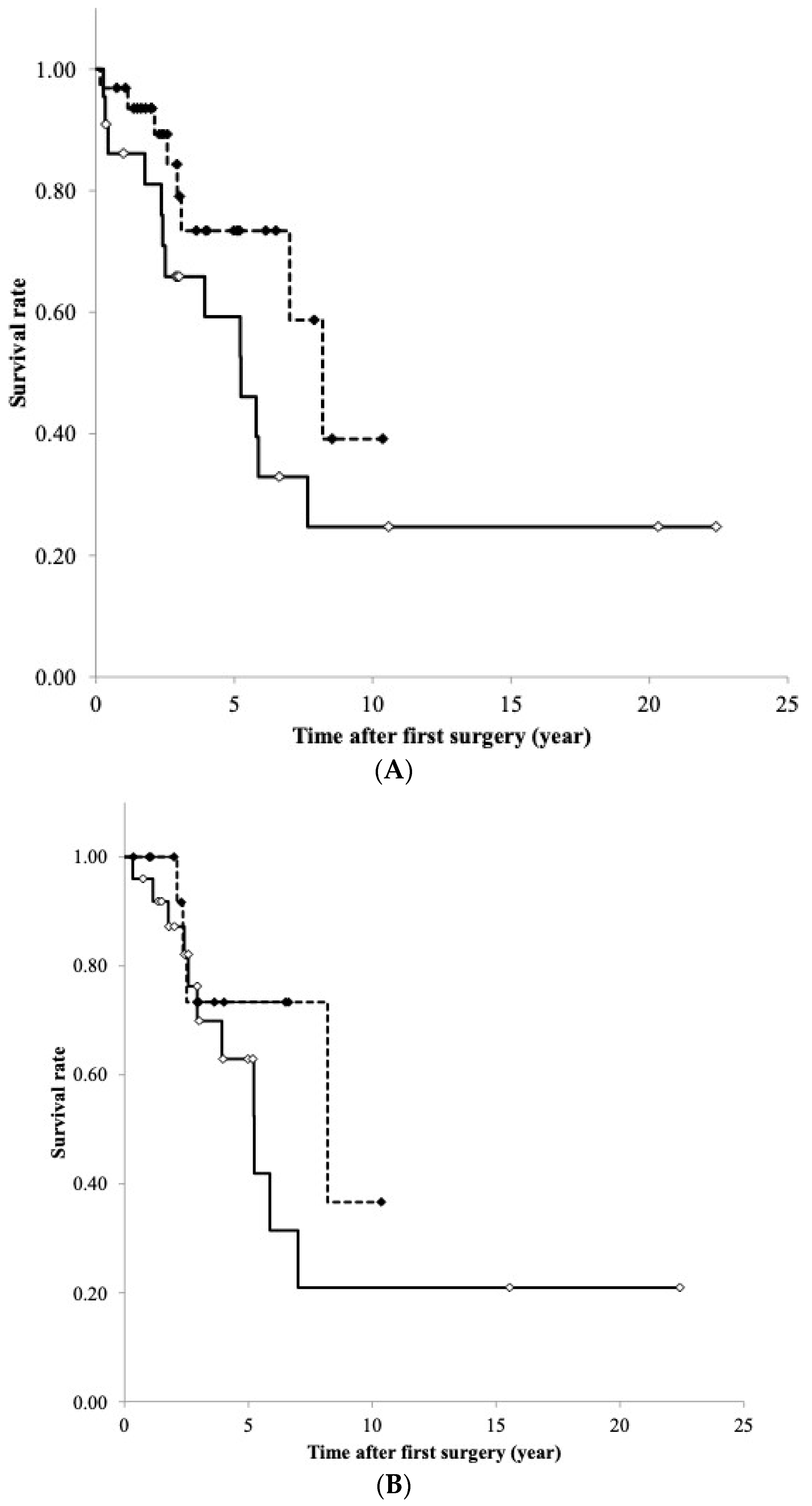
3.9. RFS Curve of TET According to DNA Methylation and mRNA Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample ID | WHO | Age | Gender | MG | Biospecimen | Masaoka–Koga Staging System | Histology/WHO Classification | Methylation (Pyrosequence) | Expression (RT-PCR) | Expression (IHC) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | N | T | N | |||||||||
| 1 | A | 50 | F | - | frozen | 1 | thymoma type A | ○ | ○ | |||
| 2 | A | 62 | M | - | frozen | 1 | thymoma type A | ○ | ○ | ○ | ||
| 3 | A | 80 | F | - | frozen | 1 | thymoma type A | ○ | ○ | |||
| 4 | A | 57 | F | + | frozen | 1 | thymoma type A | ○ | ○ | ○ | ○ | ○ |
| 5 | A | 84 | M | - | frozen | 4b | thymoma type A | ○ | ○ | ○ | ||
| 6 | A | 68 | M | - | frozen | 1 | thymoma type A | ○ | ○ | |||
| 7 | AB | 62 | F | - | frozen | 2 | thymoma type AB | ○ | ||||
| 8 | AB | 65 | F | - | frozen | 2 | thymoma type AB | ○ | ||||
| 9 | AB | 65 | F | - | frozen | 1 | thymoma type AB | ○ | ○ | |||
| 10 | B1 | 65 | F | - | frozen | 1 | thymoma type B1 | ○ | ○ | ○ | ||
| 11 | B1 | 51 | F | + | frozen | 2 | thymoma type B1 | ○ | ○ | ○ | ||
| 12 | B1 | 72 | F | - | frozen | 1 | thymoma type B1 | ○ | ○ | ○ | ||
| 13 | B1 | 45 | F | + | frozen | 2 | thymoma type B1 | ○ | ○ | |||
| 14 | B1 | 65 | M | * | frozen | 3 | thymoma type B1 | ○ | ○ | ○ | ○ | |
| 15 | B1 | 37 | M | - | frozen | 2 | thymoma type B1 | ○ | ||||
| 16 | B2 | 74 | F | - | frozen | 2 | thymoma type B2 | ○ | ○ | ○ | ○ | |
| 17 | B2 | 65 | F | - | frozen | 2 | thymoma type B2 | ○ | ○ | ○ | ○ | |
| 18 | B2 | 43 | M | - | frozen | 4b | thymoma type B2 | ○ | ○ | ○ | ○ | |
| 19 | B2 | 40 | M | + | frozen | 2 | thymoma type B2 | ○ | ○ | ○ | ○ | |
| 20 | B2 | 75 | F | - | frozen | 2 | thymoma type B2 | ○ | ○ | ○ | ○ | |
| 21 | B2 | 40 | F | - | frozen | 2 | thymoma type B2 | ○ | ○ | ○ | ○ | |
| 22 | B2 | 52 | F | + | frozen | 2 | thymoma type B2 | ○ | ○ | ○ | ○ | |
| 23 | B2 | 60 | F | - | frozen | 1 | thymoma type B2 | ○ | ○ | ○ | ||
| 24 | B2 | 58 | F | - | frozen | 1 | thymoma type B2 | ○ | ||||
| 25 | B2 | 65 | M | + | frozen | 4a | thymoma type B2 | ○ | ○ | |||
| 26 | B2 | 38 | F | - | frozen | 3 | thymoma type B2 | ○ | ○ | ○ | ||
| 27 | B3 | 66 | M | - | frozen | 1 | thymoma type B3 | ○ | ○ | ○ | ||
| 28 | B3 | 28 | F | + | frozen | 4a | thymoma type B3 | ○ | ○ | ○ | ||
| 29 | B3 | 75 | F | - | frozen | 1 | thymoma type B3 | ○ | ○ | ○ | ||
| 30 | B3 | 64 | M | - | frozen | 2 | thymoma type B3 | ○ | ||||
| 31 | B3 | 36 | M | + | frozen | 4a | thymoma type B3 | ○ | ○ | |||
| 32 | B3 | 47 | M | - | frozen | 3 | thymoma type B3 | ○ | ||||
| 33 | B3 | 75 | M | - | frozen | 3 | thymoma type B3 | ○ | ○ | ○ | ○ | |
| 34 | B3 | 72 | M | - | frozen | 4a | thymoma type B3 | ○ | ○ | ○ | ||
| 35 | B3 | 68 | F | + | frozen | 2 | thymoma type B3 | ○ | ○ | ○ | ||
| 36 | B3 | 62 | M | + | frozen | 1 | thymoma type B3 | ○ | ||||
| 37 | B3 | 81 | F | - | frozen | 4a | thymoma type B3 | ○ | ○ | ○ | ○ | |
| 38 | B3 | 56 | M | + | frozen | 1 | thymoma type B3 | ○ | ○ | ○ | ○ | |
| 39 | carcinoma | 51 | F | - | frozen | 3 | thymic carcinoma | ○ | ○ | |||
| 40 | carcinoma | 55 | M | - | frozen | 2 | thymic carcinoma | ○ | ○ | ○ | ○ | |
| 41 | carcinoma | 51 | F | - | frozen | 2 | thymic carcinoma | ○ | ○ | |||
| 42 | carcinoma | 60 | M | - | frozen | 2 | thymic carcinoma | ○ | ○ | ○ | ○ | ○ |
| 43 | carcinoma | 58 | F | - | frozen | 4a | thymic carcinoma | ○ | ○ | ○ | ○ | ○ |
| 44 | carcinoma | 69 | F | - | frozen | 4b | thymic carcinoma | ○ | ○ | ○ | ||
| 45 | carcinoma | 50 | F | + | FFPE | 3 | thymic carcinoma | ○ | ○ | |||
| 46 | carcinoma | 61 | M | - | frozen | 3 | thymic carcinoma | ○ | ○ | ○ | ||
| 47 | carcinoma | 62 | M | - | FFPE | 3 | thymic carcinoma | ○ | ○ | |||
| 48 | carcinoma | 67 | M | - | frozen | 4b | small cell carcinoma | ○ | ○ | ○ | ||
| 49 | carcinoma | 61 | F | - | frozen | 2 | thymic carcinoma | ○ | ○ | ○ | ||
| 50 | carcinoma | 68 | F | - | frozen | 2 | thymic carcinoma | ○ | ○ | ○ | ||
| 51 | carcinoma | 69 | M | - | frozen | 2 | thymic carcinoma | ○ | ○ | ○ | ○ | |
| 52 | carcinoma | 48 | F | - | frozen | 3 | thymic carcinoma | ○ | ○ | ○ | ○ | ○ |
| 53 | carcinoma | 66 | M | - | frozen | 2 | thymic carcinoma | ○ | ○ | ○ | ○ | ○ |
| 54 | carcinoma | 55 | F | - | frozen | 2 | thymic carcinoma | ○ | ○ | ○ | ○ | ○ |
| 55 | carcinoma | 68 | F | - | FFPE | 3 | thymic carcinoma | ○ | ○ | |||
| 56 | carcinoma | 74 | M | - | frozen | 1 | thymic carcinoma | ○ | ○ | ○ | ○ | |
| 57 | carcinoma | 73 | M | - | FFPE | 1 | thymic carcinoma | ○ | ○ | |||
| 58 | carcinoma | 81 | F | - | FFPE | 4b | thymic carcinoma | ○ | ||||
| 59 | carcinoma | 74 | M | - | FFPE | 4a | thymic carcinoma | ○ | ○ | |||
| 60 | carcinoma | 65 | M | - | FFPE | 3 | leiomyosarcoma | ○ | ||||
| 61 | carcinoma | 47 | F | - | FFPE | 4b | thymic carcinoma | ○ | ○ | |||
| 62 | carcinoma | 64 | F | - | FFPE | 4a | small cell carcinoma | ○ | ||||
| 63 | carcinoma | 70 | F | - | FFPE | 3 | thymic carcinoma | ○ | ||||
| 64 | NECTT | 61 | M | - | frozen | 1 | typical carcinoid | ○ | ○ | ○ | ||
| 65 | NECTT | 64 | M | - | frozen | 4b | atypical carcinoid | ○ | ○ | ○ | ||
| 66 | NECTT | 68 | M | - | frozen | 2 | typical carcinoid | ○ | ○ | ○ | ○ | |
| 67 | NECTT | 53 | M | - | FFPE | 3 | typical carcinoid | ○ | ||||
| 68 | NECTT | 71 | M | - | FFPE | 2 | typical carcinoid | ○ | ||||
| 69 | NECTT | 45 | F | - | FFPE | 4b | atypical carcinoid | ○ | ||||
| total | 64 | 24 | 48 | 16 | 39 | |||||||
| A. TaqMan Gene Expression Assay | |
|---|---|
| Gene/primer name | Sequence ID (BIO-RAD, Hercules, CA, USA) |
| NPTX2 | qHsaCED0034729 |
| GAPDH | qHsaCED0038674 |
| B. Pyrosequencing for NPTX2 | |
| primer for PCR (Forward) | 5′-GGGTGGAGGAGGGGTTATGGA-3′ |
| primer for PCR (Reverse) | [Biotin] 5′-AACCCCACCACCTCACTCT-3′ |
| primer for Pyrosequencing | 5′-AGGAGGGGTTATGGAG-3′ |
| Scores | Observation |
|---|---|
| Proportion score | |
| 0 | 0% positive cells |
| 1 | <30% positive cells |
| 2 | 30–70% positive cells |
| 3 | >70% positive cells |
| Intensity score | |
| 0 | Weak |
| 1 | Moderate |
| 2 | Strong |


References
- Kondo, K. Therapy for thymic epithelial tumors. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 468–474. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Mary, A.; Nicholson, A.G. WHO classification of tumours of the lung, pleura, thymus and heart. In World Health Organization Classification of Tumours, 4th ed.; Bosman, F.T., Jaffe, E.S., Lakhani, S.R., Ohgaki, H., Eds.; IARC Press: Lyon, France, 2015; pp. 183–243. [Google Scholar]
- Shimosato, Y.; Mukai, K.; Matsuno, Y. Tumors of the mediastinum. In AFIP Atlas of Tumor Pathology, Series 4; Armed Forces Institute of Pathology: Washington, DC, USA, 2010. [Google Scholar]
- Zhang, Y.; Lin, D.; Aramini, B.; Yang, F.; Chen, X.; Wang, X.; Wu, L.; Huang, W.; Fan, J. Thymoma and Thymic Carcinoma: Surgical Resection and Multidisciplinary Treatment. Cancers 2023, 15, 1953. [Google Scholar] [CrossRef]
- Kondo, K.; Monden, Y. Therapy for thymic epithelial tumors: A clinical study of 1320 patients from Japan. Ann. Thorac. Surg. 2003, 76, 878–884; discussion 884–875. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Zeeshan, A. Thymoma: Current diagnosis and treatment. Chin. Med. J. 2013, 126, 2186–2191. [Google Scholar] [PubMed]
- Ruffini, E.; Filosso, P.L.; Guerrera, F.; Lausi, P.; Lyberis, P.; Oliaro, A. Optimal surgical approach to thymic malignancies: New trends challenging old dogmas. Lung Cancer 2018, 118, 161–170. [Google Scholar] [CrossRef]
- Krishnan, M.; Ganti, A.K. The role of targeted therapy in thymic carcinoma. J. Oncol. Pharm. Pract. 2019, 25, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Rajan, A.; Berman, A.; Tomita, Y.; Brzezniak, C.; Lee, M.J.; Lee, S.; Ling, A.; Spittler, A.J.; Carter, C.A.; et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: An open-label phase 2 trial. Lancet Oncol. 2015, 16, 177–186. [Google Scholar] [CrossRef]
- Giaccone, G.; Kim, C.; Thompson, J.; McGuire, C.; Kallakury, B.; Chahine, J.J.; Manning, M.; Mogg, R.; Blumenschein, W.M.; Tan, M.T.; et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol. 2018, 19, 347–355. [Google Scholar] [CrossRef]
- Sato, J.; Satouchi, M.; Itoh, S.; Okuma, Y.; Niho, S.; Mizugaki, H.; Murakami, H.; Fujisaka, Y.; Kozuki, T.; Nakamura, K.; et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): A multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 843–850. [Google Scholar] [CrossRef]
- Radovich, M.; Pickering, C.R.; Felau, I.; Ha, G.; Zhang, H.; Jo, H.; Hoadley, K.A.; Anur, P.; Zhang, J.; McLellan, M.; et al. Cancer Genome Atlas Network: The integrated genomic landscape of thymic epithelial tumors. Cancer Cell 2018, 33, 244–258. [Google Scholar] [CrossRef]
- Saito, M.; Fujiwara, Y.; Asao, T.; Honda, T.; Shimada, Y.; Kanai, Y.; Tsuta, K.; Kono, K.; Watanabe, S.; Ohe, Y.; et al. The genomic and epigenomic landscape in thymic carcinoma. Carcinogenesis 2017, 38, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Enkner, F.; Pichlhöfer, B.; Zaharie, A.T.; Krunic, M.; Holper, T.M.; Janik, S.; Moser, B.; Schlangen, K.; Neudert, B.; Walter, K.; et al. Molecular profiling of thymoma and thymic carcinoma: Genetic differences and potential novel therapeutic targets. Pathol. Oncol. Res. 2017, 23, 551–564. [Google Scholar] [CrossRef]
- Petrini, I.; Meltzer, P.S.; Kim, I.K.; Lucchi, M.; Park, K.S.; Fontanini, G.; Gao, J.; Zucali, P.A.; Calabrese, F.; Favaretto, A.; et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat. Genet. 2014, 46, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Shukuya, T.; Greenstein, R.A.; Kaplan, B.G.; Wakelee, H.; Ross, J.S.; Miura, K.; Furuta, K.; Kato, S.; Suh, J.; et al. Genomic characterization of thymic epithelial tumors in a real-world dataset. ESMO Open 2023, 11, 101627. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Kondo, K.; Takizawa, H.; Nagao, T.; Nakagawa, Y.; Fujino, H.; Toba, H.; Kenzaki, K.; Sakiyama, S.; Tangoku, A. Aberrant methylation of tumour-related genes in thymic epithelial tumours. Lung Cancer 2009, 64, 155–159. [Google Scholar] [CrossRef]
- Mokhtar, M.; Kondo, K.; Namura, T.; Ali, A.H.; Fujita, Y.; Takai, C.; Takizawa, H.; Nakagawa, Y.; Toba, H.; Kajiura, K.; et al. Methylation and expression profiles of MGMT gene in thymic epithelial tumors. Lung Cancer 2014, 83, 279–287. [Google Scholar] [CrossRef]
- Kajiura, K.; Takizawa, H.; Morimoto, Y.; Masuda, K.; Tsuboi, M.; Kishibuchi, R.; Wusiman, N.; Sawada, T.; Kawakita, N.; Toba, H.; et al. Frequent silencing of RASSF1A by DNA methylation in thymic neuroendocrine tumours. Lung Cancer 2017, 111, 116–123. [Google Scholar] [CrossRef]
- Kishibuchi, R.; Kondo, K.; Soejima, S.; Tsuboi, M.; Kajiura, K.; Kawakami, Y.; Kawakita, N.; Sawada, T.; Toba, H.; Yoshida, M.; et al. DNA methylation of GHSR, GNG4, HOXD9 and SALL3 is a common epigenetic alteration in thymic carcinoma. Int. J. Oncol. 2020, 56, 315–326. [Google Scholar] [CrossRef]
- Soejima, S.; Kondo, K.; Tsuboi, M.; Muguruma, K.; Tegshee, B.; Kawakami, Y.; Kajiura, K.; Kawakita, N.; Toba, H.; Yoshida, M.; et al. GAD1 expression and its methylation as indicators of malignant behavior in thymic epithelial tumors. Oncol. Lett. 2021, 21, 483. [Google Scholar] [CrossRef]
- Breviarios, F.; d’Aniellos, E.M.; Golay, J.; Bottazzis, B.; Bairochll, A.; Sacconell, S.; Marzella, R.; Predazzi, V.; Rocchi, M.; Vallell, G.D.; et al. Interleukin-1-inducible genes in endothelial cells. J. Biol. Chem. 1992, 267, 22190–22197. [Google Scholar]
- Whitehead, A.S.; Zahedi, K.; Rits, M.; Mortensen, R.F.; Lelias, J.M. Mouse C-reactive protein. generation of cDNA clones, structural analysis and induction of MRNA during inflammation. Biochem. J. 1990, 266, 283–290. [Google Scholar] [CrossRef]
- Dowton, S.B.; McGrew, S.D. Rat serum amyloid P component. Biochem. J. 1990, 270, 571–585. [Google Scholar] [CrossRef]
- Lee, G.W.; Lee, T.H.; Vilcek, J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentraxin family of acute phase proteins. J. Immunol. 1993, 150, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Schlimgen, A.K.; Helms, J.A.; Vogel, H.; Perin, M.S. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron 1995, 14, 519–526. [Google Scholar] [CrossRef]
- Tsui, C.C.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Barnes, C.; Worley, P.F. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J. Neurosci. 1996, 16, 2463–2478. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.; Shanmugalingam, U.; Smith, P.D. The Role of Neuronal Pentraxin 2 (NP2) in Regulating Glutamatergic Signaling and Neuropathology. Front. Cell Neurosci. 2020, 8, 575. [Google Scholar] [CrossRef] [PubMed]
- Pelkey, K.A.; Barksdale, E.; Craig, M.T.; Yuan, X.; Sukumaran, M.; Vargish, C.A.; Mitchell, R.M.; Wyeth, M.S.; Petralia, R.S.; Chittajallu, R.; et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 2015, 85, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.B.; Hickey, L.; Michael, G.J.; Derkacs, M.; Christian, L.M.; Kalaitzakis, M.E.; Pearce, R.K.B.; Graeber, M.B. Neuronal pentraxin II is highly upregulated in Parkinson’s disease and a novel component of Lewy bodies. Acta Neuropathol. 2008, 115, 471–478. [Google Scholar] [CrossRef]
- Bartolini, A.; Paolo, D.D.; Noghero, A.; Murgia, D.; Sementa, A.R.; Cilli, M.; Pasqualini, R.; Arap, W.; Bussolino, F.; Ponzoni, M.; et al. The neuronal pentraxin-2 pathway is an unrecognized target in human neuroblastoma, which also offers prognostic value in patients. Cancer Res. 2015, 75, 4265–4271. [Google Scholar] [CrossRef]
- Von Roemeling, C.A.; Radisky, D.C.; Marlow, L.A.; Cooper, S.J.; Grebe, S.K.; Anastasiadis, P.Z.; Tun, H.W.; Copland, J.A. Neuronal pentraxin 2 supports clear cell renal cell carcinoma by activating the AMPA-selective glutamate receptor-4. Cancer Res. 2014, 74, 4796–4810. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Nicholson, A.G.; Kondo, K.; Van Schil, P.; Moran, C. The Masaoka-Koga stage classification for thymic malignancies: Clarification and definition of terms. J. Thorac. Oncol. 2011, 6 (Suppl. S3), S1710–S1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, J.; Li, Z.; Gong, Y. Neuronal pentraxin II (NPTX2) is frequently down-regulated by promoter hypermethylation in pancreatic cancers. Dig. Dis. Sci. 2012, 57, 2608–2614. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Li, L.; Li, Z.; Du, Y.; Gong, Y. The neuronal Pentraxin II gene (NPTX2) inhibit proliferation and invasion of pancreatic cancer cells in vitro. Mol. Biol. Rep. 2011, 38, 4903–4911. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Pia Patric, I.R.; Thinagararjan, S.; Srinivasan, S.; Mondal, B.; Hegde, A.S.; Chandramouli, B.A.; Santosh, V.; Arivazhagan, A.; Somasundaram, K.A. DNA methylation prognostic signature of glioblastoma: Identification of NPTX2-PTEN-NF-κB nexus. Cancer Res. 2013, 73, 6563–6573. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tian, G.; Jiang, C.; Xue, H.; Kuerbanjiang, M.; Sun, L.; Gu, L.; Zhou, H.; Liu, Y.; Zhang, Z.; et al. NPTX2 promotes colorectal cancer growth and liver metastasis by the activation of the canonical Wnt/β-catenin pathway via FZD6. Cell Death Dis. 2019, 10, 217. [Google Scholar] [CrossRef]
- Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000106236-NPTX2/pathology (accessed on 30 August 2018).




| Total | Pyrosequencing Analysis | RT-PCR Analysis | IHC Analysis | ||
|---|---|---|---|---|---|
| Gender | |||||
| male | 31 (46.3%) | 29 (45.3%) | 23 (47.9%) | 17 (43.6%) | |
| female | 36 (53.7%) | 35 (54.7%) | 25 (52.1%) | 22 (56.4%) | |
| Age (year) | 61.2 ± 11.9 # | 61.1 ± 12.1 | 60.6 ± 12.2 | 60.7 ± 11.5 | |
| (28~84) ! | (28~84) | (28~84) | (28~80) | ||
| WHO histological classification | |||||
| Thymoma | 36 (53.7%) | 33 (51.6%) | 31 (64.6%) | 18 (46.2%) | |
| A | 6 (9.0%) | 5 (7.8%) | 5 (10.4%) | 3 (7.7%) | |
| AB | 3 (4.5%) | 2 (3.1%) | 1 (2.1%) | 0 (0.0%) | |
| B1 | 5 (7.5%) | 5 (7.8%) | 5 (10.4%) | 2 (5.1%) | |
| B2 | 10 (14.9%) | 10 (15.6%) | 10 (20.8%) | 7 (17.9%) | |
| B3 | 12 (17.9%) | 11 (17.2%) | 10 (20.8%) | 6 (15.4%) | |
| Thymic carcinoma * | 25 (37.3%) | 25 (39.1%) | 14 (29.2%) | 18 (46.2%) | |
| NECTT | 6 (9.0%) | 6 (9.4%) | 3 (6.3%) | 3 (7.7%) | |
| typical carcinoid | 4 (6.0%) | 4 (6.3%) | 2 (4.2%) | 2 (5.1%) | |
| atypical carcinoid | 2 (3.0%) | 2 (3.1%) | 1 (2.1%) | 1 (2.6%) | |
| Masaoka–Koga staging system | |||||
| I | 16 (23.9%) | 14 (21.9%) | 13 (27.1%) | 9 (23.1%) | |
| II | 22 (32.8%) | 21 (32.8%) | 18 (37.5%) | 15 (38.5%) | |
| III | 13 (19.4%) | 13 (20.3%) | 6 (12.5%) | 6 (15.4%) | |
| IVA | 8 (11.9%) | 8 (12.5%) | 6 (12.5%) | 4 (10.3%) | |
| IVB | 8 (11.9%) | 8 (12.5%) | 5 (10.4%) | 5 (12.8%) | |
| Myasthenia gravis | |||||
| - | 55 (82.1%) | 53 (82.8%) | 37 (77.1%) | 32 (82.1%) | |
| + | 12 (17.9%) | 11 (17.2%) | 11 (22.9%) | 7 (17.9%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, K.; Muguruma, K.; Soejima, S.; Takai, C.; Kenzaki, K.; Kawakita, N.; Toba, H.; Takizawa, H. Aberrant DNA Methylation of NPTX2 as an Indicator of Malignant Behavior in Thymic Epithelial Tumors. Cancers 2024, 16, 329. https://doi.org/10.3390/cancers16020329
Kondo K, Muguruma K, Soejima S, Takai C, Kenzaki K, Kawakita N, Toba H, Takizawa H. Aberrant DNA Methylation of NPTX2 as an Indicator of Malignant Behavior in Thymic Epithelial Tumors. Cancers. 2024; 16(2):329. https://doi.org/10.3390/cancers16020329
Chicago/Turabian StyleKondo, Kazuya, Kyoka Muguruma, Shiho Soejima, Chikako Takai, Koichiro Kenzaki, Naoya Kawakita, Hiroaki Toba, and Hiromitsu Takizawa. 2024. "Aberrant DNA Methylation of NPTX2 as an Indicator of Malignant Behavior in Thymic Epithelial Tumors" Cancers 16, no. 2: 329. https://doi.org/10.3390/cancers16020329




