Promising Diagnostic and Therapeutic Approaches Based on VHHs for Cancer Management
Abstract
Simple Summary
Abstract
1. Introduction
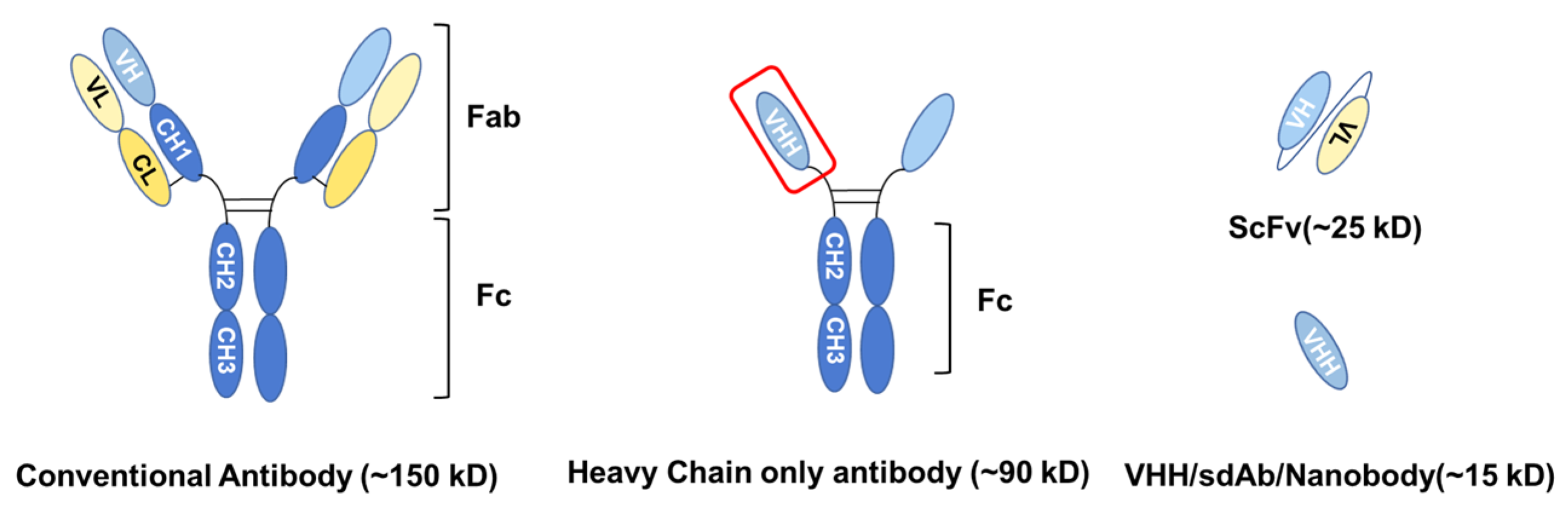
2. General Characteristics of VHH
2.1. Biochemical and Biophysical Characteristics
2.2. Low Immunogenicity
2.3. High Tissue Penetration and Fast Blood Clearance
3. The Generation of VHH
- The construction of a VHH gene library
- 2.
- The selection of specific VHH
- 3.
- The production of VHH
4. Applications of VHH
4.1. Molecular Imaging
4.2. VHHs in Anti-Cancer Therapies
4.2.1. Radioimmunotherapy
4.2.2. Photodynamic Therapy
4.2.3. VHH as Immune Checkpoint Inhibitor
4.2.4. Targeting Tumor-Specific Antigens
4.2.5. VHH-Drug Conjugates (VHH-DC)
4.2.6. VHH-Based CAR-T
4.3. Other Applications of VHHs
5. An Overview of Ongoing Clinical Trials of VHHs in Cancer Treatments
6. Discussion and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Streltsov, V.A.; Carmichael, J.A.; Nuttall, S.D. Structure of a shark IgNAR antibody variable domain and modeling of an early-developmental isotype. Protein Sci. 2005, 14, 2901–2909. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Desmyter, A.; Transue, T.R.; Ghahroudi, M.A.; Thi, M.H.; Poortmans, F.; Hamers, R.; Muyldermans, S.; Wyns, L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat. Struct. Biol. 1996, 3, 803–811. [Google Scholar] [CrossRef] [PubMed]
- De Groof, T.W.M.; Bobkov, V.; Heukers, R.; Smit, M.J. Nanobodies: New avenues for imaging, stabilizing and modulating GPCRs. Mol. Cell. Endocrinol. 2019, 484, 15–24. [Google Scholar] [CrossRef] [PubMed]
- De Groof, T.W.M.; Mashayekhi, V.; Fan, T.S.; Bergkamp, N.D.; Sastre Torano, J.; van Senten, J.R.; Heukers, R.; Smit, M.J.; Oliveira, S. Nanobody-Targeted Photodynamic Therapy Selectively Kills Viral GPCR-Expressing Glioblastoma Cells. Mol. Pharm. 2019, 16, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.G.; Choi, H.J.; Fung, J.J.; Pardon, E.; Casarosa, P.; Chae, P.S.; Devree, B.T.; Rosenbaum, D.M.; Thian, F.S.; Kobilka, T.S.; et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature 2011, 469, 175–180. [Google Scholar] [CrossRef]
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef]
- Kunz, P.; Zinner, K.; Mucke, N.; Bartoschik, T.; Muyldermans, S.; Hoheisel, J.D. The structural basis of nanobody unfolding reversibility and thermoresistance. Sci. Rep. 2018, 8, 7934. [Google Scholar] [CrossRef] [PubMed]
- Vosjan, M.J.; Perk, L.R.; Roovers, R.C.; Visser, G.W.; Stigter-van Walsum, M.; van Bergen En Henegouwen, P.M.; van Dongen, G.A. Facile labelling of an anti-epidermal growth factor receptor Nanobody with 68Ga via a novel bifunctional desferal chelate for immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 753–763. [Google Scholar] [CrossRef]
- Ebrahimizadeh, W.; Mousavi Gargari, S.L.; Javidan, Z.; Rajabibazl, M. Production of Novel VHH Nanobody Inhibiting Angiogenesis by Targeting Binding Site of VEGF. Appl. Biochem. Biotechnol. 2015, 176, 1985–1995. [Google Scholar] [CrossRef]
- Dumoulin, M.; Conrath, K.; Van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.R.; Liu, J.L.; Zabetakis, D.; Anderson, G.P. Enhancing Stability of Camelid and Shark Single Domain Antibodies: An Overview. Front. Immunol. 2017, 8, 865. [Google Scholar] [CrossRef]
- Arbabi-Ghahroudi, M. Camelid Single-Domain Antibodies: Promises and Challenges as Lifesaving Treatments. Int. J. Mol. Sci. 2022, 23, 5009. [Google Scholar] [CrossRef] [PubMed]
- Achour, I.; Cavelier, P.; Tichit, M.; Bouchier, C.; Lafaye, P.; Rougeon, F. Tetrameric and homodimeric camelid IgGs originate from the same IgH locus. J. Immunol. 2008, 181, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef]
- Schoen, P.; Jacobs, S.; Verschueren, K.; Ottevaere, I.; Sobry, S.; Holz, J.-B. Anti-RANKL nanobody ALX-0141 shows sustained biomarker inhibition in a phase I study in healthy postmenopausal women. In Bone Abstracts; Bioscientifica: Bristol, UK, 2013. [Google Scholar]
- Li, Y.; Zhou, C.; Li, J.; Liu, J.; Lin, L.; Li, L.; Cao, D.; Li, Q.; Wang, Z. Single domain based bispecific antibody, Muc1-Bi-1, and its humanized form, Muc1-Bi-2, induce potent cancer cell killing in muc1 positive tumor cells. PLoS ONE 2018, 13, e0191024. [Google Scholar] [CrossRef]
- Sulea, T. Humanization of Camelid Single-Domain Antibodies. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 299–312. [Google Scholar]
- Ackaert, C.; Smiejkowska, N.; Xavier, C.; Sterckx, Y.G.J.; Denies, S.; Stijlemans, B.; Elkrim, Y.; Devoogdt, N.; Caveliers, V.; Lahoutte, T.; et al. Immunogenicity Risk Profile of Nanobodies. Front. Immunol. 2021, 12, 632687. [Google Scholar] [CrossRef]
- Lwin, T.M.; Hernot, S.; Hollandsworth, H.; Amirfakhri, S.; Filemoni, F.; Debie, P.; Hoffman, R.M.; Bouvet, M. Tumor-specific near-infrared nanobody probe rapidly labels tumors in an orthotopic mouse model of pancreatic cancer. Surgery 2020, 168, 85–91. [Google Scholar] [CrossRef]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.B.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. (18)F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef]
- Broos, K.; Keyaerts, M.; Lecocq, Q.; Renmans, D.; Nguyen, T.; Escors, D.; Liston, A.; Raes, G.; Breckpot, K.; Devoogdt, N. Non-invasive assessment of murine PD-L1 levels in syngeneic tumor models by nuclear imaging with nanobody tracers. Oncotarget 2017, 8, 41932–41946. [Google Scholar] [CrossRef]
- Caljon, G.; Caveliers, V.; Lahoutte, T.; Stijlemans, B.; Ghassabeh, G.H.; Van Den Abbeele, J.; Smolders, I.; De Baetselier, P.; Michotte, Y.; Muyldermans, S.; et al. Using microdialysis to analyse the passage of monovalent nanobodies through the blood-brain barrier. Br. J. Pharmacol. 2012, 165, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, E.; Schuhmacher, A.J. Transportation of Single-Domain Antibodies through the Blood-Brain Barrier. Biomolecules 2021, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Jovcevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Chu, C.; Jablonska, A.; Behnam Azad, B.; Zwaenepoel, O.; Zawadzki, M.; Lisok, A.; Pomper, M.G.; Walczak, P.; Gettemans, J.; et al. PET imaging of distinct brain uptake of a nanobody and similarly-sized PAMAM dendrimers after intra-arterial administration. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1940–1951. [Google Scholar] [CrossRef]
- Custers, M.L.; Wouters, Y.; Jaspers, T.; De Bundel, D.; Dewilde, M.; Van Eeckhaut, A.; Smolders, I. Applicability of cerebral open flow microperfusion and microdialysis to quantify a brain-penetrating nanobody in mice. Anal. Chim. Acta 2021, 1178, 338803. [Google Scholar] [CrossRef]
- Heukers, R.; Fan, T.S.; de Wit, R.H.; van Senten, J.R.; De Groof, T.W.M.; Bebelman, M.P.; Lagerweij, T.; Vieira, J.; de Munnik, S.M.; Smits-de Vries, L.; et al. The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth. Oncogene 2018, 37, 4110–4121. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Cai, W. Nanobody: The “magic bullet” for molecular imaging? Theranostics 2014, 4, 386–398. [Google Scholar] [CrossRef]
- Gainkam, L.O.; Keyaerts, M.; Caveliers, V.; Devoogdt, N.; Vanhove, C.; Van Grunsven, L.; Muyldermans, S.; Lahoutte, T. Correlation between epidermal growth factor receptor-specific nanobody uptake and tumor burden: A tool for noninvasive monitoring of tumor response to therapy. Mol. Imaging Biol. 2011, 13, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Vaneycken, I.; D’Huyvetter, M.; Hernot, S.; De Vos, J.; Xavier, C.; Devoogdt, N.; Caveliers, V.; Lahoutte, T. Immuno-imaging using nanobodies. Curr. Opin. Biotechnol. 2011, 22, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, K.; Dreier, T.; Silence, K.; de Haard, H.; Lauwereys, M.; Casteels, P.; Beirnaert, E.; Jonckheere, H.; Van de Wiele, C.; Staelens, L.; et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006, 54, 1856–1866. [Google Scholar] [CrossRef]
- Iqbal, U.; Trojahn, U.; Albaghdadi, H.; Zhang, J.; O’Connor-McCourt, M.; Stanimirovic, D.; Tomanek, B.; Sutherland, G.; Abulrob, A. Kinetic analysis of novel mono- and multivalent VHH-fragments and their application for molecular imaging of brain tumours. Br. J. Pharmacol. 2010, 160, 1016–1028. [Google Scholar] [CrossRef]
- Altunay, B.; Morgenroth, A.; Beheshti, M.; Vogg, A.; Wong, N.C.L.; Ting, H.H.; Biersack, H.J.; Stickeler, E.; Mottaghy, F.M. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1371–1389. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Tang, M.; Zhao, J.; Zhu, X. Nanobody: A promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021, 11, 6. [Google Scholar] [CrossRef]
- Sheikhi, A.; Hojjat-Farsangi, M. An immunotherapeutic method for COVID-19 patients: A soluble ACE2-Anti-CD16 VHH to block SARS-CoV-2 Spike protein. Hum. Vaccines Immunother. 2021, 17, 92–97. [Google Scholar] [CrossRef]
- Sadeghi, A.; Behdani, M.; Muyldermans, S.; Habibi-Anbouhi, M.; Kazemi-Lomedasht, F. Development of a mono-specific anti-VEGF bivalent nanobody with extended plasma half-life for treatment of pathologic neovascularization. Drug Test. Anal. 2020, 12, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Hoefman, S.; Ottevaere, I.; Baumeister, J.; Sargentini-Maier, M.L. Pre-clinical intravenous serum pharmacokinetics of albumin binding and non-half-life extended Nanobodies®. Antibodies 2015, 4, 141–156. [Google Scholar] [CrossRef]
- Sleep, D.; Cameron, J.; Evans, L.R. Albumin as a versatile platform for drug half-life extension. BBA-Gen. Subj. 2013, 1830, 5526–5534. [Google Scholar] [CrossRef]
- Pan, H.; Liu, J.; Deng, W.; Xing, J.; Li, Q.; Wang, Z. Site-specific PEGylation of an anti-CEA/CD3 bispecific antibody improves its antitumor efficacy. Int. J. Nanomed. 2018, 13, 3189–3201. [Google Scholar] [CrossRef]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Xenaki, K.T.; Dorrestijn, B.; Muns, J.A.; Adamzek, K.; Doulkeridou, S.; Houthoff, H.; Oliveira, S.; van Bergen En Henegouwen, P.M. Homogeneous tumor targeting with a single dose of HER2-targeted albumin-binding domain-fused nanobody-drug conjugates results in long-lasting tumor remission in mice. Theranostics 2021, 11, 5525–5538. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.M.; Whiteheart, S.W.; Smiley, J.R.; Sharma, S.; Boaz, K.; Coleman, M.J.; Maynard, A.; Hersh, L.B.; Vander Kooi, C.W. Immunization of Alpacas (Lama pacos) with Protein Antigens and Production of Antigen-specific Single Domain Antibodies. J. Vis. Exp. 2019, 143, e58471. [Google Scholar] [CrossRef]
- Marturano, A.; Hendrickx, M.L.V.; Falcinelli, E.; Sebastiano, M.; Guglielmini, G.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S.; Declerck, P.J.; Gresele, P. Development of anti-matrix metalloproteinase-2 (MMP-2) nanobodies as potential therapeutic and diagnostic tools. Nanomedicine 2020, 24, 102103. [Google Scholar] [CrossRef]
- Jakobs, B.D.; Spannagel, L.; Purvanov, V.; Uetz-von Allmen, E.; Matti, C.; Legler, D.F. Engineering of Nanobodies Recognizing the Human Chemokine Receptor CCR7. Int. J. Mol. Sci. 2019, 20, 2597. [Google Scholar] [CrossRef]
- Baharlou, R.; Tajik, N.; Habibi-Anbouhi, M.; Shokrgozar, M.A.; Zarnani, A.H.; Shahhosseini, F.; Behdani, M. Generation and characterization of an anti-delta like ligand-4 Nanobody to induce non-productive angiogenesis. Anal. Biochem. 2018, 544, 34–41. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef]
- Evazalipour, M.; D’Huyvetter, M.; Tehrani, B.S.; Abolhassani, M.; Omidfar, K.; Abdoli, S.; Arezumand, R.; Morovvati, H.; Lahoutte, T.; Muyldermans, S.; et al. Generation and characterization of nanobodies targeting PSMA for molecular imaging of prostate cancer. Contrast Media Mol. Imaging 2014, 9, 211–220. [Google Scholar] [CrossRef]
- Ma, L.; Gu, K.; Zhang, C.H.; Chen, X.T.; Jiang, Y.; Melcher, K.; Zhang, J.; Wang, M.; Xu, H.E. Generation and characterization of a human nanobody against VEGFR-2. Acta Pharmacol. Sin. 2016, 37, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Wang, C.; Xu, Y.; Huang, W.; Yuan, Z.; Wang, T.; Dai, S.; Peng, S.; Pang, T.; Jiang, W.; et al. Generation of a safe and efficacious llama single-domain antibody fragment (vHH) targeting the membrane-proximal region of 4-1BB for engineering therapeutic bispecific antibodies for cancer. J. Immunother. Cancer 2021, 9, e002131. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, Y.; Yu, J.; Liu, W.; Li, F.; Xian, M.; Nian, R.; Song, H.; Feng, D. An efficient constitutive expression system for Anti-CEACAM5 nanobody production in the yeast Pichia pastoris. Protein Expr. Purif. 2019, 155, 43–47. [Google Scholar] [CrossRef]
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K.; et al. Generation and Characterization of ALX-0171, a Potent Novel Therapeutic Nanobody for the Treatment of Respiratory Syncytial Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 6–13. [Google Scholar] [CrossRef]
- Djender, S.; Schneider, A.; Beugnet, A.; Crepin, R.; Desrumeaux, K.E.; Romani, C.; Moutel, S.; Perez, F.; de Marco, A. Bacterial cytoplasm as an effective cell compartment for producing functional VHH-based affinity reagents and Camelidae IgG-like recombinant antibodies. Microb. Cell Fact. 2014, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, G.; Beaino, W.; Windhorst, A.D.; Zwezerijnen, G.J.C.; Oprea-Lager, D.E.; Hendrikse, N.H.; van Kuijk, C.; Boellaard, R.; Huisman, M.C.; Vugts, D.J. The Role of (89)Zr-Immuno-PET in Navigating and Derisking the Development of Biopharmaceuticals. J. Nucl. Med. 2021, 62, 438–445. [Google Scholar] [CrossRef]
- Hegi-Johnson, F.; Rudd, S.; Hicks, R.J.; De Ruysscher, D.; Trapani, J.A.; John, T.; Donnelly, P.; Blyth, B.; Hanna, G.; Everitt, S.; et al. Imaging immunity in patients with cancer using positron emission tomography. NPJ Precis. Oncol. 2022, 6, 24. [Google Scholar] [CrossRef]
- Kijanka, M.; Warnders, F.J.; El Khattabi, M.; Lub-de Hooge, M.; van Dam, G.M.; Ntziachristos, V.; de Vries, L.; Oliveira, S.; van Bergen En Henegouwen, P.M. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1718–1729. [Google Scholar] [CrossRef]
- Terwisscha van Scheltinga, A.G.; van Dam, G.M.; Nagengast, W.B.; Ntziachristos, V.; Hollema, H.; Herek, J.L.; Schröder, C.P.; Kosterink, J.G.; Lub-de Hoog, M.N.; de Vries, E.G. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J. Nucl. Med. 2011, 52, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Gondry, O.C.V.; Xavier, C.; Raes, L.; Vanhoeij, M.; Verfaillie, G.; Fontaine, C.; Glorieus, K.; De Grève, J.; Joris, S.; Luyten, I.; et al. Phase II trial to assess the repeatability and tumor uptake of [68Ga]Ga-HER2-sdAb PET/CT in patients with Breast Carcinoma. J. Nucl. Med. 2023, in press.
- Gondry, O.; Xavier, C.; Raes, L.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Breckpot, K.; Lecocq, Q.; Decoster, L.; Fontaine, C.; et al. Phase I Study of [68Ga]Ga-Anti-CD206-sdAb for PET/CT Assessment of Protumorigenic Macrophage Presence in Solid Tumors (MMR Phase I). J. Nucl. Med. 2023, 64, 1378–1384. [Google Scholar] [CrossRef]
- Rashidian, M.; Ingram, J.R.; Dougan, M.; Dongre, A.; Whang, K.A.; LeGall, C.; Cragnolini, J.J.; Bierie, B.; Gostissa, M.; Gorman, J.; et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 2017, 214, 2243–2255. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Davies, C.W.; Gill, H.; Kiefer, J.R.; Yin, J.; Ogasawara, A.; Urrutia, A.; Javinal, V.; Lin, Z.; Seshasayee, D.; et al. Development of an (18)F-labeled anti-human CD8 VHH for same-day immunoPET imaging. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Veldhoven-Zweistra, J.; Bolkestein, M.; Hoeben, S.; Koning, G.A.; Boerman, O.C.; de Jong, M.; van Weerden, W.M. A Novel (1)(1)(1)In-Labeled Anti-Prostate-Specific Membrane Antigen Nanobody for Targeted SPECT/CT Imaging of Prostate Cancer. J. Nucl. Med. 2015, 56, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Li, N.; Wang, S.; Zhou, S.; Li, R.; Yang, H.; Li, W.; Qu, J. Noninvasive Imaging of Tumor PD-L1 Expression Using [99mTc]Tc-Labeled KN035 with SPECT/CT. Mol. Pharm. 2023, 20, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Sun, X.; Qiu, L.; Sun, Y.; Li, K.; Liu, Q.; Zhao, Q.; Qin, S.; Lin, J. PET Imaging of Tumor PD-L1 Expression with a Highly Specific Nonblocking Single-Domain Antibody. J. Nucl. Med. 2020, 61, 117–122. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Li, K.; Li, H.; Lv, G.; Lin, J.; Qiu, L. Immuno-PET imaging of 68Ga-labeled nanobody Nb109 for dynamic monitoring the PD-L1 expression in cancers. Cancer Immunol. Immunother. 2021, 70, 1721–1733. [Google Scholar] [CrossRef]
- Movahedi, K.; Schoonooghe, S.; Laoui, D.; Houbracken, I.; Waelput, W.; Breckpot, K.; Bouwens, L.; Lahoutte, T.; De Baetselier, P.; Raes, G.; et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012, 72, 4165–4177. [Google Scholar] [CrossRef]
- Xavier, C.; Blykers, A.; Laoui, D.; Bolli, E.; Vaneyken, I.; Bridoux, J.; Baudhuin, H.; Raes, G.; Everaert, H.; Movahedi, K.; et al. Clinical Translation of [68Ga]Ga-NOTA-anti-MMR-sdAb for PET/CT Imaging of Protumorigenic Macrophages. Mol. Imaging Biol. 2019, 21, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; Zeven, K.; De Vlaeminck, Y.; Martens, S.; Massa, S.; Goyvaerts, C.; Raes, G.; Keyaerts, M.; Breckpot, K.; Devoogdt, N. Noninvasive Imaging of the Immune Checkpoint LAG-3 Using Nanobodies, from Development to Pre-Clinical Use. Biomolecules 2019, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; Awad, R.M.; De Vlaeminck, Y.; de Mey, W.; Ertveldt, T.; Goyvaerts, C.; Raes, G.; Thielemans, K.; Keyaerts, M.; Devoogdt, N.; et al. Single-Domain Antibody Nuclear Imaging Allows Noninvasive Quantification of LAG-3 Expression by Tumor-Infiltrating Leukocytes and Predicts Response of Immune Checkpoint Blockade. J. Nucl. Med. 2021, 62, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, T.; Shi, L.; Zhang, X.; Guo, X.; Hu, B.; Yao, M.; Zhu, H.; Yang, Z.; Jia, B.; et al. HER2-targeted dual radiotracer approach with clinical potential for noninvasive imaging of trastuzumab-resistance caused by epitope masking. Theranostics 2022, 12, 5551–5563. [Google Scholar] [CrossRef]
- An, S.; Zhang, D.; Zhang, Y.; Wang, C.; Shi, L.; Wei, W.; Huang, G.; Liu, J. GPC3-targeted immunoPET imaging of hepatocellular carcinomas. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2682–2692. [Google Scholar] [CrossRef]
- Liu, T.; Wu, Y.; Shi, L.; Li, L.; Hu, B.; Wang, Y.; Gao, H.; Yu, X.; Zhang, X.; Zhao, H.; et al. Preclinical evaluation of [99mTc]Tc-labeled anti-EpCAM nanobody for EpCAM receptor expression imaging by immuno-SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1810–1821. [Google Scholar] [CrossRef]
- Piramoon, M.; Hosseinimehr, S.J.; Omidfar, K.; Noaparast, Z.; Abedi, S.M. 99m Tc-anti-epidermal growth factor receptor nanobody for tumor imaging. Chem. Biol. Drug Des. 2017, 89, 498–504. [Google Scholar] [CrossRef]
- Hu, G.; Zhu, W.; Liu, Y.; Wang, Y.; Zhang, Z.; Zhu, S.; Duan, W.; Zhou, P.; Fu, C.; Li, F.; et al. Development and comparison of three (89)Zr-labeled anti-CLDN18.2 antibodies to noninvasively evaluate CLDN18.2 expression in gastric cancer: A preclinical study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2634–2644. [Google Scholar] [CrossRef]
- Wang, H.; Meng, A.M.; Li, S.H.; Zhou, X.L. A nanobody targeting carcinoembryonic antigen as a promising molecular probe for non-small cell lung cancer. Mol. Med. Rep. 2017, 16, 625–630. [Google Scholar] [CrossRef]
- van Lith, S.A.M.; Huizing, F.J.; Franssen, G.M.; Hoeben, B.A.W.; Lok, J.; Doulkeridou, S.; Boerman, O.C.; Gotthardt, M.; van Bergen En Henegouwen, P.M.P.; Bussink, J.; et al. Novel VHH-Based Tracers with Variable Plasma Half-Lives for Imaging of CAIX-Expressing Hypoxic Tumor Cells. Mol. Pharm. 2022, 19, 3511–3520. [Google Scholar] [CrossRef]
- Jailkhani, N.; Ingram, J.R.; Rashidian, M.; Rickelt, S.; Tian, C.; Mak, H.; Jiang, Z.; Ploegh, H.L.; Hynes, R.O. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl. Acad. Sci. USA 2019, 116, 14181–14190. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Declerck, N.B.; van Willigen, D.; Huygen, C.M.; De Sloovere, B.; Mateusiak, L.; Bridoux, J.; Puttemans, J.; Devoogdt, N.; van Leeuwen, F.W.B.; et al. The Design and Preclinical Evaluation of a Single-Label Bimodal Nanobody Tracer for Image-Guided Surgery. Biomolecules 2021, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted Nanobody-Based Molecular Tracers for Nuclear Imaging and Image-Guided Surgery. Surgery 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Vanhoeij, M.; Poortmans, N.; Puttemans, J.; Gillis, K.; Devoogdt, N.; Lahoutte, T.; Hernot, S. Improved Debulking of Peritoneal Tumor Implants by Near-Infrared Fluorescent Nanobody Image Guidance in an Experimental Mouse Model. Mol. Imaging Biol. 2018, 20, 361–367. [Google Scholar] [CrossRef] [PubMed]
- van Brussel, A.S.; Adams, A.; Oliveira, S.; Dorresteijn, B.; El Khattabi, M.; Vermeulen, J.F.; van der Wall, E.; Mali, W.P.; Derksen, P.W.; van Diest, P.J.; et al. Hypoxia-Targeting Fluorescent Nanobodies for Optical Molecular Imaging of Pre-Invasive Breast Cancer. Mol. Imaging Biol. 2016, 18, 535–544. [Google Scholar] [CrossRef]
- Huang, H.; Wu, T.; Shi, H.; Wu, Y.; Yang, H.; Zhong, K.; Wang, Y.; Liu, Y. Modular design of nanobody-drug conjugates for targeted-delivery of platinum anticancer drugs with an MRI contrast agent. Chem. Commun. 2019, 55, 5175–5178. [Google Scholar] [CrossRef]
- Prantner, A.M.; Yin, C.; Kamat, K.; Sharma, K.; Lowenthal, A.C.; Madrid, P.B.; Scholler, N. Molecular Imaging of Mesothelin-Expressing Ovarian Cancer with a Human and Mouse Cross-Reactive Nanobody. Mol. Pharm. 2018, 15, 1403–1411. [Google Scholar] [CrossRef]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef]
- Wiseman, G.A.; Kornmehl, E.; Leigh, B.; Erwin, W.D.; Podoloff, D.A.; Spies, S.; Sparks, R.B.; Stabin, M.G.; Witzig, T.; White, C.A. Radiation dosimetry results and safety correlations from 90Y-ibritumomab tiuxetan radioimmunotherapy for relapsed or refractory non-Hodgkin’s lymphoma: Combined data from 4 clinical trials. J. Nucl. Med. 2003, 44, 465–474. [Google Scholar]
- Rajendran, J.G.; Gopal, A.K.; Fisher, D.R.; Durack, L.D.; Gooley, T.A.; Press, O.W. Myeloablative 131I-tositumomab radioimmunotherapy in treating non-Hodgkin’s lymphoma: Comparison of dosimetry based on whole-body retention and dose to critical organ receiving the highest dose. J. Nucl. Med. 2008, 49, 837–844. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Dekempeneer, Y.M.S.; Santens, F.; Navarro, L.; Berdal, M.; Lucero, M.M.; Pombo Antunes, A.R.; Van Ginderachter, J.A.; Lahoutte, T.; Devoogdt, N.; D’Huyvetter, M. Preclinical evaluation of a radiotheranostic single-domain antibody against Fibroblast Activation Protein alpha. J. Nucl. Med. 2023, in press.
- Xu, J.; Li, S.; Xu, S.; Dai, J.; Luo, Z.; Cui, J.; Cai, F.; Geng, C.; Wang, Z.; Tang, X. Screening and Preclinical Evaluation of Novel Radiolabeled Anti-Fibroblast Activation Protein-α Recombinant Antibodies. Cancer Biother. Radiopharm. 2023, 38, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Bolli, E.; D’Huyvetter, M.; Murgaski, A.; Berus, D.; Stange, G.; Clappaert, E.J.; Arnouk, S.; Pombo Antunes, A.R.; Krasniqi, A.; Lahoutte, T.; et al. Stromal-targeting radioimmunotherapy mitigates the progression of therapy-resistant tumors. J. Control Release 2019, 314, 1–11. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. (131)I-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef]
- Feng, Y.; Meshaw, R.; McDougald, D.; Zhou, Z.; Zhao, X.G.; Jannetti, S.A.; Reiman, R.E.; Pippen, E.; Marjoram, R.; Schaal, J.L.; et al. Evaluation of an (131)I-labeled HER2-specific single domain antibody fragment for the radiopharmaceutical therapy of HER2-expressing cancers. Sci. Rep. 2022, 12, 3020. [Google Scholar] [CrossRef]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med. 2014, 55, 650–656. [Google Scholar] [CrossRef]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl. Med. Biol. 2018, 56, 10–20. [Google Scholar] [CrossRef]
- Feng, Y.; Meshaw, R.; Zhao, X.G.; Jannetti, S.; Vaidyanathan, G.; Zalutsky, M.R. Effective Treatment of Human Breast Carcinoma Xenografts with Single-Dose (211)At-Labeled Anti-HER2 Single-Domain Antibody Fragment. J. Nucl. Med. 2023, 64, 124–130. [Google Scholar] [CrossRef]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an Anti-HER2 Nanobody Labeled with (225)Ac for Targeted α-Particle Therapy of Cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Collado Camps, E.; Canovas, C.; Kip, A.; Gotthardt, M.; Rijpkema, M.; Denat, F.; Goncalves, V.; van Lith, S.A.M. Site-Specific Dual-Labeling of a VHH with a Chelator and a Photosensitizer for Nuclear Imaging and Targeted Photodynamic Therapy of EGFR-Positive Tumors. Cancers 2021, 13, 428. [Google Scholar] [CrossRef]
- Deken, M.M.; Kijanka, M.M.; Beltran Hernandez, I.; Slooter, M.D.; de Bruijn, H.S.; van Diest, P.J.; van Bergen En Henegouwen, P.M.P.; Lowik, C.; Robinson, D.J.; Vahrmeijer, A.L.; et al. Nanobody-targeted photodynamic therapy induces significant tumor regression of trastuzumab-resistant HER2-positive breast cancer, after a single treatment session. J. Control Release 2020, 323, 269–281. [Google Scholar] [CrossRef] [PubMed]
- van Driel, P.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.A.; de Bruijn, H.S.; van Diest, P.J.; Vahrmeijer, A.L.; van Bergen En Henegouwen, P.M.P.; et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control Release 2016, 229, 93–105. [Google Scholar] [CrossRef] [PubMed]
- van Driel, P.B.; van der Vorst, J.R.; Verbeek, F.P.; Oliveira, S.; Snoeks, T.J.; Keereweer, S.; Chan, B.; Boonstra, M.C.; Frangioni, J.V.; van Bergen en Henegouwen, P.M.; et al. Intraoperative fluorescence delineation of head and neck cancer with a fluorescent anti-epidermal growth factor receptor nanobody. Int. J. Cancer 2014, 134, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Hernández, I.; Angelier, M.L.; Del Buono D’Ondes, T.; Di Maggio, A.; Yu, Y.; Oliveira, S. The Potential of Nanobody-Targeted Photodynamic Therapy to Trigger Immune Responses. Cancers 2020, 12, 978. [Google Scholar] [CrossRef] [PubMed]
- Heukers, R.; Mashayekhi, V.; Ramirez-Escudero, M.; de Haard, H.; Verrips, T.C.; van Bergen En Henegouwen, P.M.P.; Oliveira, S. VHH-Photosensitizer Conjugates for Targeted Photodynamic Therapy of Met-Overexpressing Tumor Cells. Antibodies 2019, 8, 26. [Google Scholar] [CrossRef]
- Mashayekhi, V.; Xenaki, K.T.; van Bergen En Henegouwen, P.M.P.; Oliveira, S. Dual Targeting of Endothelial and Cancer Cells Potentiates In Vitro Nanobody-Targeted Photodynamic Therapy. Cancers 2020, 12, 2732. [Google Scholar] [CrossRef]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef]
- Petit, P.F.; Bombart, R.; Desimpel, P.H.; Naulaerts, S.; Thouvenel, L.; Collet, J.F.; Van den Eynde, B.J.; Zhu, J. T Cell-Mediated Targeted Delivery of Anti-PD-L1 Nanobody Overcomes Poor Antibody Penetration and Improves PD-L1 Blocking at the Tumor Site. Cancer Immunol. Res. 2022, 10, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Casson, C.N.; Matsuda, A.; Kim, J.I.; Shi, J.Q.; Iwasaki, S.; Chen, S.; Modrell, B.; Chan, C.; Tavares, D.; et al. Fc-independent functions of anti-CTLA-4 antibodies contribute to anti-tumor efficacy. Cancer Immunol. Immunother. 2022, 71, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, L.; Xu, X.; Qi, M.; Zhang, J.; He, S.; Tian, Q.; Song, S. Engineering a Smart Agent for Enhanced Immunotherapy Effect by Simultaneously Blocking PD-L1 and CTLA-4. Adv. Sci. 2021, 8, e2102500. [Google Scholar] [CrossRef]
- Ma, L.; Gai, J.; Qiao, P.; Li, Y.; Li, X.; Zhu, M.; Li, G.; Wan, Y. A novel bispecific nanobody with PD-L1/TIGIT dual immune checkpoint blockade. Biochem. Biophys. Res. Commun. 2020, 531, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, e1514–e1525. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Zhang, W.; Zhou, A.P.; Guo, W.; Yang, J.; Yuan, Y.; Zhu, L.; Qin, S.; Xiang, S.; et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J. Hematol. Oncol. 2021, 14, 95. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakajima, T.E.; Yamamoto, N.; Yonemori, K.; Koyama, T.; Kondo, S.; Sunakawa, Y.; Izawa, N.; Horie, Y.; Xiang, S.; et al. Phase I study of envafolimab (KN035), a novel subcutaneous single-domain anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors. Investig. New Drugs 2022, 40, 1021–1031. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Liu, Y.; Ma, S.; Huang, B.; Feng, X.; Wang, H. A novel multifunctional anti-PD-L1-CD16a-IL15 induces potent cancer cell killing in PD-L1-positive tumour cells. Transl. Oncol. 2022, 21, 101424. [Google Scholar] [CrossRef]
- Fang, T.; Li, R.; Li, Z.; Cho, J.; Guzman, J.S.; Kamm, R.D.; Ploegh, H.L. Remodeling of the Tumor Microenvironment by a Chemokine/Anti-PD-L1 Nanobody Fusion Protein. Mol. Pharm. 2019, 16, 2838–2844. [Google Scholar] [CrossRef]
- Awad, R.M.; Lecocq, Q.; Zeven, K.; Ertveldt, T.; De Beck, L.; Ceuppens, H.; Broos, K.; De Vlaeminck, Y.; Goyvaerts, C.; Verdonck, M.; et al. Formatting and gene-based delivery of a human PD-L1 single domain antibody for immune checkpoint blockade. Mol. Ther. Methods Clin. Dev. 2021, 22, 172–182. [Google Scholar] [CrossRef]
- Broos, K.; Lecocq, Q.; Xavier, C.; Bridoux, J.; Nguyen, T.T.; Corthals, J.; Schoonooghe, S.; Lion, E.; Raes, G.; Keyaerts, M.; et al. Evaluating a Single Domain Antibody Targeting Human PD-L1 as a Nuclear Imaging and Therapeutic Agent. Cancers 2019, 11, 872. [Google Scholar] [CrossRef]
- Broos, K.; Lecocq, Q.; Keersmaecker, B.; Raes, G.; Corthals, J.; Lion, E.; Thielemans, K.; Devoogdt, N.; Keyaerts, M.; Breckpot, K. Single Domain Antibody-Mediated Blockade of Programmed Death-Ligand 1 on Dendritic Cells Enhances CD8 T-cell Activation and Cytokine Production. Vaccines 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhao, L.; Wang, Y.; Qiu, C.; Xu, Z.; Huang, K.; Zhu, H.; Li, Z.; Li, H. Nanobodies targeting the interaction interface of programmed death receptor 1 (PD-1)/PD-1 ligand 1 (PD-1/PD-L1). Prep. Biochem. Biotechnol. 2020, 50, 252–259. [Google Scholar] [CrossRef]
- Noelia, S.-P.; Eva, M.; María Cristina, B.-B.; Sandra, H.-S.; Noelia, C.; Gualberto, G.-S.; Cristian, S.; Lucia, V. Long-Term Systemic Expression of a Novel PD-1 Blocking Nanobody from an AAV Vector Provides Antitumor Activity without Toxicity. Biomedicines 2020, 8, 562. [Google Scholar]
- Wan, R.; Liu, A.; Hou, X.; Lai, Z.; Li, J.; Yang, N.; Tan, J.; Mo, F.; Hu, Z.; Yang, X.; et al. Screening and antitumor effect of an anti-CTLA-4 nanobody. Oncol. Rep. 2018, 39, 511–518. [Google Scholar] [CrossRef]
- Gainkam, L.O.; Huang, L.; Caveliers, V.; Keyaerts, M.; Hernot, S.; Vaneycken, I.; Vanhove, C.; Revets, H.; De Baetselier, P.; Lahoutte, T. Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. J. Nucl. Med. 2008, 49, 788–795. [Google Scholar] [CrossRef]
- Huang, L.; Gainkam, L.O.; Caveliers, V.; Vanhove, C.; Keyaerts, M.; De Baetselier, P.; Bossuyt, A.; Revets, H.; Lahoutte, T. SPECT imaging with 99mTc-labeled EGFR-specific nanobody for in vivo monitoring of EGFR expression. Mol. Imaging Biol. 2008, 10, 167–175. [Google Scholar] [CrossRef]
- Maryam, Q.; Mahdi, B.; Mohammad Ali, S.; Vahid, M.-K.; Homa, M.-K.; Mahdi, H.-A. Construction and expression of an anti-VEGFR2 Nanobody-Fc fusionbody in NS0 host cell. Protein Expr. Purif. 2016, 123, 19–25. [Google Scholar]
- Van Impe, K.; Bethuyne, J.; Cool, S.; Impens, F.; Ruano-Gallego, D.; De Wever, O.; Vanloo, B.; Van Troys, M.; Lambein, K.; Boucherie, C.; et al. A nanobody targeting the F-actin capping protein CapG restrains breast cancer metastasis. Breast Cancer Res. 2013, 15, R116. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qi, S.; Unger, M.; Hou, Y.N.; Deng, Q.W.; Liu, J.; Lam, C.M.C.; Wang, X.W.; Xin, D.; Zhang, P.; et al. Immuno-targeting the multifunctional CD38 using nanobody. Sci. Rep. 2016, 6, 27055. [Google Scholar] [CrossRef]
- Ingram, J.R.; Blomberg, O.S.; Sockolosky, J.T.; Ali, L.; Schmidt, F.I.; Pishesha, N.; Espinosa, C.; Dougan, S.K.; Garcia, K.C.; Ploegh, H.L.; et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc. Natl. Acad. Sci. USA 2017, 114, 10184–10189. [Google Scholar] [CrossRef] [PubMed]
- Maussang, D.; Mujic-Delic, A.; Descamps, F.J.; Stortelers, C.; Vanlandschoot, P.; Stigter-van Walsum, M.; Vischer, H.F.; van Roy, M.; Vosjan, M.; Gonzalez-Pajuelo, M.; et al. Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo. J. Biol. Chem. 2013, 288, 29562–29572. [Google Scholar] [CrossRef]
- Sadeghnezhad, G.; Romão, E.; Bernedo-Navarro, R.; Massa, S.; Khajeh, K.; Muyldermans, S.; Hassania, S. Identification of New DR5 Agonistic Nanobodies and Generation of Multivalent Nanobody Constructs for Cancer Treatment. Int. J. Mol. Sci. 2019, 20, 4818. [Google Scholar] [CrossRef]
- Zottel, A.; Jovčevska, I.; Šamec, N.; Mlakar, J.; Šribar, J.; Križaj, I.; Skoblar Vidmar, M.; Komel, R. Anti-vimentin, anti-TUFM, anti-NAP1L1 and anti-DPYSL2 nanobodies display cytotoxic effect and reduce glioblastoma cell migration. Ther. Adv. Med. Oncol. 2020, 12, 1758835920915302. [Google Scholar] [CrossRef] [PubMed]
- Tabtimmai, L.; Suphakun, P.; Srisook, P.; Kiriwan, D.; Phanthong, S.; Kiatwuthinon, P.; Chaicumpa, W.; Choowongkomon, K. Cell-penetrable nanobodies (transbodies) that inhibit the tyrosine kinase activity of EGFR leading to the impediment of human lung adenocarcinoma cell motility and survival. J. Cell. Biochem. 2019, 120, 18077–18087. [Google Scholar] [CrossRef]
- Chen, T.; Liu, X.; Hong, H.; Wei, H. Novel single-domain antibodies against the EGFR domain III epitope exhibit the anti-tumor effect. J. Transl. Med. 2020, 18, 376. [Google Scholar] [CrossRef]
- Lamtha, T.; Krobthong, S.; Yingchutrakul, Y.; Samutrtai, P.; Gerner, C.; Tabtimmai, L.; Choowongkomon, K. A novel nanobody as therapeutics target for EGFR-positive colorectal cancer therapy: Exploring the effects of the nanobody on SW480 cells using proteomics approach. Proteome Sci. 2022, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Han, Y.; Sun, Q.; Wang, X.; Xu, T.; Xie, W.; Huang, X. Anti-MET VHH Pool Overcomes MET-Targeted Cancer Therapeutic Resistance. Mol. Cancer Ther. 2019, 18, 100–111. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Q.; Xia, H.; Xu, H.; Bi, F. Identification and characterization of inhibitory nanobody against p38delta. Biochem. Biophys. Res. Commun. 2022, 600, 60–66. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, H.; Zheng, W.; Wang, T.; Ma, X. Design and screening of a chimeric survivin-specific nanobody and its anticancer activities in vitro. Anticancer. Drugs 2016, 27, 839–847. [Google Scholar] [CrossRef]
- Meltzer, M.; Eliash, N.; Azoulay, Z.; Hadad, U.; Papo, N. In vitro inhibition of cancer angiogenesis and migration by a nanobody that targets the orphan receptor Tie1. Cell. Mol. Life Sci. 2022, 79, 312. [Google Scholar] [CrossRef]
- Teicher, B.A. Antibody-drug conjugate targets. Curr. Cancer Drug Targets 2009, 9, 982–1004. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Xing, Y.; Meng, T.; Ma, L.; Pei, J.; Cong, Y.; Zhang, X.; Ren, Z.; Wang, X.; et al. Dual Mechanisms of Novel CD73-Targeted Antibody and Antibody-Drug Conjugate in Inhibiting Lung Tumor Growth and Promoting Antitumor Immune-Effector Function. Mol. Cancer Ther. 2020, 19, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Sharkey, R.M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert. Opin. Biol. Ther. 2020, 20, 871–885. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Kong, Y.; Wang, Z.; Lei, C.; Li, J.; Ding, L.; Wang, C.; Cheng, Y.; Wei, Y.; et al. A highly stable human single-domain antibody-drug conjugate exhibits superior penetration and treatment of solid tumors. Mol. Ther. 2022, 30, 2785–2799. [Google Scholar] [CrossRef]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs 2021, 13, 1951427. [Google Scholar] [CrossRef] [PubMed]
- Espelin, C.W.; Leonard, S.C.; Geretti, E.; Wickham, T.J.; Hendriks, B.S. Dual HER2 Targeting with Trastuzumab and Liposomal-Encapsulated Doxorubicin (MM-302) Demonstrates Synergistic Antitumor Activity in Breast and Gastric Cancer. Cancer Res. 2016, 76, 1517–1527. [Google Scholar] [CrossRef]
- Rosenfeld, L.; Sananes, A.; Zur, Y.; Cohen, S.; Dhara, K.; Gelkop, S.; Ben Zeev, E.; Shahar, A.; Lobel, L.; Akabayov, B.; et al. Nanobodies Targeting Prostate-Specific Membrane Antigen for the Imaging and Therapy of Prostate Cancer. J. Med. Chem. 2020, 63, 7601–7615. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Farajollahi, M.M.; Behdani, M.; Tarighi, P. Production and Conjugation of Truncated Recombinant Diphtheria Toxin to VEGFR-2 Specific Nanobody and Evaluation of its Cytotoxic Effect on PC-3 Cell Line. Mol. Biotechnol. 2022, 64, 1218–1226. [Google Scholar] [CrossRef]
- Li, R.; Zhu, X.; Zhou, P.; Qiao, Y.; Li, Y.; Xu, Y.; Shi, X. Generation of a High-Affinity Nanobody Against CD147 for Tumor Targeting and Therapeutic Efficacy Through Conjugating Doxorubicin. Front. Immunol. 2022, 13, 852700. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Golubovskaya, V. CAR-T Cells Targeting Immune Checkpoint Pathway Players. Front. Biosci. 2022, 27, 121. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Petukhov, A.; Rakhmatullina, A.; Miftakhova, R.; Fainshtein, M.; Rizvanov, A.; Bulatov, E. Adoptive Immunotherapy beyond CAR T-Cells. Cancers 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Wagner, D.L.; Fritsche, E.; Pulsipher, M.A.; Ahmed, N.; Hamieh, M.; Hegde, M.; Ruella, M.; Savoldo, B.; Shah, N.N.; Turtle, C.J.; et al. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Delgoffe, G.M.; Xu, C.; Mackall, C.L.; Green, M.R.; Gottschalk, S.; Speiser, D.E.; Zehn, D.; Beavis, P.A. The role of exhaustion in CAR T cell therapy. Cancer Cell 2021, 39, 885–888. [Google Scholar] [CrossRef]
- Lynn, R.C.; Weber, E.W.; Sotillo, E.; Gennert, D.; Xu, P.; Good, Z.; Anbunathan, H.; Lattin, J.; Jones, R.; Tieu, V.; et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 2019, 576, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Dougan, M.; Jailkhani, N.; Ingram, J.; Fang, T.; Kummer, L.; Momin, N.; Pishesha, N.; Rickelt, S.; Hynes, R.O.; et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl. Acad. Sci. USA 2019, 116, 7624–7631. [Google Scholar] [CrossRef]
- Xie, Y.J.; Dougan, M.; Ingram, J.R.; Pishesha, N.; Fang, T.; Momin, N.; Ploegh, H.L. Improved Antitumor Efficacy of Chimeric Antigen Receptor T Cells that Secrete Single-Domain Antibody Fragments. Cancer Immunol. Res. 2020, 8, 518–529. [Google Scholar] [CrossRef]
- Rajabzadeh, A.; Rahbarizadeh, F.; Ahmadvand, D.; Kabir Salmani, M.; Hamidieh, A.A. A VHH-Based Anti-MUC1 Chimeric Antigen Receptor for Specific Retargeting of Human Primary T Cells to MUC1-Positive Cancer Cells. Cell J. 2021, 22, 502–513. [Google Scholar] [CrossRef]
- Hanssens, H.; Meeus, F.; De Veirman, K.; Breckpot, K.; Devoogdt, N. The antigen-binding moiety in the driver’s seat of CARs. Med. Res. Rev. 2022, 42, 306–342. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Z.; Li, X.; Yan, Z.; Yang, Y.; Qiao, Z.; Liu, Y. Neutralization of TNFα in tumor with a novel nanobody potentiates paclitaxel-therapy and inhibits metastasis in breast cancer. Cancer Lett. 2017, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Karami, E.; Sabatier, J.M.; Behdani, M.; Irani, S.; Kazemi-Lomedasht, F. A nanobody-derived mimotope against VEGF inhibits cancer angiogenesis. J. Enzym. Inhib. Med. Chem. 2020, 35, 1233–1239. [Google Scholar] [CrossRef]
- Farajpour, Z.; Rahbarizadeh, F.; Kazemi, B.; Ahmadvand, D. A nanobody directed to a functional epitope on VEGF, as a novel strategy for cancer treatment. Biochem. Biophys. Res. Commun. 2014, 446, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Xiong, J.; Gu, X.; Chen, X.; Wu, S.; Wang, Z.; Wang, D.; Tu, J.; Xie, J. Novel recombinant immunotoxin of EGFR specific nanobody fused with cucurmosin, construction and antitumor efficiency in vitro. Oncotarget 2017, 8, 38568–38580. [Google Scholar] [CrossRef]
- Mohammadlou, M.; Salehi, S.; Baharlou, R. Development of anti DLL4 Nanobody fused to truncated form of Pseudomonas exotoxin: As a novel immunotoxin to inhibit of cell proliferation and neovascularization. Anal. Biochem. 2022, 653, 114776. [Google Scholar] [CrossRef]
- Ji, F.; Sha, H.; Meng, F.; Zhu, A.; Ding, N.; Zhang, H.; Xu, H.; Qian, H.; Yu, L.; Liu, Q.; et al. Tumor-penetrating peptide fused EGFR single-domain antibody enhances radiation responses following EGFR inhibition in gastric cancer. Oncol. Rep. 2018, 40, 1583–1591. [Google Scholar] [CrossRef]
- Dougan, M.; Ingram, J.R.; Jeong, H.J.; Mosaheb, M.M.; Bruck, P.T.; Ali, L.; Pishesha, N.; Blomberg, O.; Tyler, P.M.; Servos, M.M.; et al. Targeting Cytokine Therapy to the Pancreatic Tumor Microenvironment Using PD-L1-Specific VHHs. Cancer Immunol. Res. 2018, 6, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Han, T.; Kang, N.; Huang, S.; Liu, Y. Preparation of RGD4C fused anti-TNFα nanobody and inhibitory activity on triple-negative breast cancer in vivo. Life Sci. 2020, 260, 118274. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yu, X.; Kang, X.; Zhao, Y.; Zhao, P.; Jin, H.; Fu, X.; Wan, Y.; Peng, C.; Huang, Y. Remodeling Tumor-Associated Macrophages and Neovascularization Overcomes EGFR(T790M) -Associated Drug Resistance by PD-L1 Nanobody-Mediated Codelivery. Small 2018, 14, e1802372. [Google Scholar] [CrossRef] [PubMed]
- Woodham, A.W.; Cheloha, R.W.; Ling, J.; Rashidian, M.; Kolifrath, S.C.; Mesyngier, M.; Duarte, J.N.; Bader, J.M.; Skeate, J.G.; Da Silva, D.M.; et al. Nanobody-Antigen Conjugates Elicit HPV-Specific Antitumor Immune Responses. Cancer Immunol. Res. 2018, 6, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shu, Y.; Hu, S.; Qi, Z.; Chen, Y.; Ma, J.; Wang, Y.; Cheng, P. In Situ Tumor Vaccine Expressing Anti-CD47 Antibody Enhances Antitumor Immunity. Front. Oncol. 2022, 12, 897561. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Jiang, F. Prospects of Neutralizing Nanobodies Against SARS-CoV-2. Front. Immunol. 2021, 12, 690742. [Google Scholar] [CrossRef]
- Güttler, T.; Aksu, M.; Dickmanns, A.; Stegmann, K.M.; Gregor, K.; Rees, R.; Taxer, W.; Rymarenko, O.; Schünemann, J.; Dienemann, C.; et al. Neutralization of SARS-CoV-2 by highly potent, hyperthermostable, and mutation-tolerant nanobodies. EMBO J. 2021, 40, e107985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, C.; Xing, Y.; He, J.; O’Doherty, J.; Huang, W.; Zhao, J. Development of a 99mTc-Labeled Single-Domain Antibody for SPECT/CT Assessment of HER2 Expression in Breast Cancer. Mol. Pharm. 2021, 18, 3616–3622. [Google Scholar] [CrossRef]
- Wong, N.C.; Cai, Y.; Meszaros, L.K.; Biersack, H.J.; Cook, G.J.; Ting, H.H.; Mottaghy, F.M. Preclinical development and characterisation of 99mTc-NM-01 for SPECT/CT imaging of human PD-L1. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 154–166. [Google Scholar]
- Xing, Y.; Chand, G.; Liu, C.; Cook, G.J.R.; O’Doherty, J.; Zhao, L.; Wong, N.C.L.; Meszaros, L.K.; Ting, H.H.; Zhao, J. Early Phase I Study of a 99mTc-Labeled Anti-Programmed Death Ligand-1 (PD-L1) Single-Domain Antibody in SPECT/CT Assessment of PD-L1 Expression in Non-Small Cell Lung Cancer. J. Nucl. Med. 2019, 60, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, T.; Li, L.; Shi, L.; Yao, M.; Li, C.; Ma, X.; Zhu, H.; Jia, B.; Wang, F. IgG-Binding Nanobody Capable of Prolonging Nanobody-Based Radiotracer Plasma Half-Life and Enhancing the Efficacy of Tumor-Targeted Radionuclide Therapy. Bioconjug. Chem. 2022, 33, 1328–1339. [Google Scholar] [CrossRef]
- Alauddin, M.M.; Khawli, L.A. Advances in Immuno-PET for the Detection of Cancer and Assessment of Response to Therapy. Curr. Med. Chem. 2021, 28, 647–672. [Google Scholar] [CrossRef]
- Varasteh, Z.; Braeuer, M.; Mohanta, S.; Steinsiek, A.L.; Habenicht, A.; Omidvari, N.; Topping, G.J.; Rischpler, C.; Weber, W.A.; Sager, H.B.; et al. In vivo Visualization of M2 Macrophages in the Myocardium After Myocardial Infarction (MI) Using (68) Ga-NOTA-Anti-MMR Nb: Targeting Mannose Receptor (MR, CD206) on M2 Macrophages. Front. Cardiovasc. Med. 2022, 9, 889963. [Google Scholar] [CrossRef]
- Varasteh, Z.; Mohanta, S.; Li, Y.; López Armbruster, N.; Braeuer, M.; Nekolla, S.G.; Habenicht, A.; Sager, H.B.; Raes, G.; Weber, W. Targeting mannose receptor expression on macrophages in atherosclerotic plaques of apolipoprotein E-knockout mice using 68Ga-NOTA-anti-MMR nanobody: Non-invasive imaging of atherosclerotic plaques. EJNMMI Res. 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Kanojia, D.; Rashidi, A.; Ahmed, A.U.; Balyasnikova, I.V.; Lesniak, M.S. Diagnostic Clinical Trials in Breast Cancer Brain Metastases: Barriers and Innovations. Clin. Breast Cancer 2019, 19, 383–391. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; Aa, F.V.; et al. Phase I Trial of 131I-GMIB-Anti-HER2-VHH1, a New Promising Candidate for HER2-Targeted Radionuclide Therapy in Breast Cancer Patients. J. Nucl. Med. 2021, 62, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Arasanz, H.; Fernández-Rubio, L.; Blanco, E.; Echaide, M.; Bocanegra, A.; Teijeira, L.; Garnica, M.; Morilla, I.; Martínez-Aguillo, M.; et al. CAR-T Cells for the Treatment of Lung Cancer. Life 2022, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Lacal, P.M.; Martire, M.; Navarra, P.; Graziani, G. Clinical experience with CTLA-4 blockade for cancer immunotherapy: From the monospecific monoclonal antibody ipilimumab to probodies and bispecific molecules targeting the tumor microenvironment. Pharmacol. Res. 2022, 175, 105997. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.T.-w.; Dai, V.; Greenberg, J.; Ballman, K.V.; Li, L.; Garcia, C.A.; Scheff, R.J.; Saxena, A.; Giaccone, G. Phase II study of KN046 in patients with thymic carcinoma who failed immune checkpoint inhibitors. J. Clin. Oncol. 2022, 40. [Google Scholar] [CrossRef]
- Zhang, F. Structural Biology of anti-PD-L1 and anti-CTLA4 Antibodies in Immune Therapies for Cancer Treatments. Ph.D. Thesis, Shanghai Jiaotong University, Shanghai, China, 2018. [Google Scholar]
- Papadopoulos, K.P.; Harb, W.; Lu, N.; Ma, X.; He, Y.; Yuan, L.; Fu, M.; Lin, Y.; Xu, W.; Wang, X.; et al. Phase I study of KN035, a novel fusion Anti-PD-L1 antibody administered subcutaneously in patients with advanced solid tumors in the USA. Ann. Oncol. 2018, 29, viii405. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, H.; Wang, X.; Bai, Y.; Wang, P.; Wu, J.; Jiang, X.; Wang, Y.; Cai, H.; Xu, T.; et al. Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell Discov. 2017, 3, 17004. [Google Scholar] [CrossRef]
- Hong, D.; SciMentum, S.; Tanyi, J.; MacMullen, L.; Jalbert, L.; Muzithras, V.; Zikaras, K.; Cao, L.; O’Cearbhaill, R.; Quintás-Cardama, A. 959O Gavocabtagene autoleucel (gavo-cel, TC-210) dose escalation in refractory mesothelin-expressing solid tumors. Ann. Oncol. 2021, 32, S830. [Google Scholar] [CrossRef]
- Markham, A. Envafolimab: First Approval. Drugs 2022, 82, 235–240. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, C.; Xing, Y.; He, J.; O’Doherty, J.; Huang, W.; Zhao, J. First-in-Human Study of a 99mTc-Labeled Single-Domain Antibody for SPECT/CT Assessment of HER2 Expression in Breast Cancer. 2021. Available online: https://www.researchsquare.com/article/rs-356098/v1 (accessed on 15 April 2022).
- D’Angelo, S.P.; Robinson, S.I.; Lam, J.; Adams, B.J.; Freddo, J.L.; Theuer, C.P.; Maki, R.G. ENVASARC: A pivotal trial of envafolimab, and envafolimab in combination with ipilimumab, in patients with advanced or metastatic undifferentiated pleomorphic sarcoma or myxofibrosarcoma who have progressed on prior chemotherapy. J. Clin. Oncol. 2021, 39, TPS11581. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Huang, M.; Qin, S.; Zhao, J.; Sang, S.; Zheng, M.; Bian, Y.; Huang, C.; Zhang, H.; et al. Pilot study of a novel nanobody (68) Ga-NODAGA-SNA006 for instant PET imaging of CD8(+) T cells. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4394–4405. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.; Yao, G.F.; Zhong, W.X.; Duan, W.W.; Zhou, Z.X.; Wen, X.F.; Chen, Y.L.; Fang, J.J.; Wang, Y.W.; Jiang, W.Q.; et al. DR30303, a SMART-VHHBody powered anti-CLDN18.2 VHH-Fc with enhanced ADCC activity for the treatment of gastric and pancreatic cancers. Cancer Res. 2022, 82, 2857. [Google Scholar] [CrossRef]

| Target | Conjugation | Cancer Models | Main Findings | Reference |
|---|---|---|---|---|
| CD8 | 89Zr | BrCa | The CD8+ T cells in solid tumors were monitored by 89Zr-labeled anti-CD8-VHH, which signal positively corresponded with ICI treatment response. | [66] |
| CD8 | 18F | ALL | Imaging with the 18F-VHH enabled rapid visualization of CD8+ T cells within 1 h, while no visible tumor uptake was observed with the control VHH. | [67] |
| PSMA | 111In | CRPC | Renal uptake was efficiently reduced by co-injection of gelofusine and lysine. Replacing the c-myc-his tag with the cysteine reduced renal uptake without loss of targeting. | [68] |
| PD-L1 | 99mTc | TC-1 (immortalized murine lung epithelial cell) | VHH accumulation correlated with the levels of PD-L1 in tumors, even if PD-L1 expression was low. | [26] |
| PD-L1 | 99mTc | NSCLC | [99mTc]Tc-HYNIC-KN035 displayed a high PD-L1 specificity both in vitro and in vivo, that was positively correlated with the expression of PD-L1. | [69] |
| PD-L1 | 68Ga | SKCM, BrCa | 68Ga-NOTA-Nb109 specifically accumulated in tumors with a maximum uptake of 5 ± 0.35% injected dose/g at 1 h. | [70] |
| PD-L1 | 68Ga | GBM, CRC, NSCLC | Tumor-to-muscle ratio (TMR) reached its peak at 40 min post-injection. The heart uptake was almost fully cleared at 35 min post-injection. | [71] |
| MMR | 99mTc | TS/A (murine mammary adenocarcinoma), 3LL-R (Lewis Lung carcinoma) | Anti-MMR VHH targeted pro-angiogenic MMR-expressing TAMs with tumor uptake correlating with the amount of TAMs in the tumor. | [72] |
| MMR | 68Ga | 3LL-R | TMR was determined while no treatment-related toxicologically relevant changes or acute immunological reactions were observed. The tolerated dose was established to be >1.68 mg/kg body weight. The dosimetry levels for humans were calculated by using the data in mice. | [73] |
| LAG-3 | 99mTc | TC-1 (immortalized murine lung epithelial cell) | The tumor uptake of VHHs 3132 and 3206 targeting LAG-3 was comparable with high contrast at 1 h post-injection. | [74] |
| LAG-3 | 99mTc | MC38 (murine CRC), MO4 (murine melanoma), and TC-1 | The radiolabeled anti-LAG-3 VHH detected LAG-3 expressing TILs 1 h post tracer injection. | [75] |
| HER2 | 68Ga | HER2+ cancer | A high tumor-to-organ ratio was measured at 1 h post-injection with increased uptake upon increasing the injected dose. | [76] |
| HER2 | 18F | OvCa | The tumor-to-organ ratio at 1 h post-injection showed excellent specificity. | [25] |
| HER2 | 99mTc | BrCa | The tumor had significant radiotracer uptake at 0.5 h after injection. | [77] |
| Glypican-3 | 68Ga, 18F | HCC | The fusion of VHH to an albumin-binding domain increased the tumor uptake and decreased kidney accumulation of the radiotracer (1 h to 6 h). | [78] |
| EpCAM | 99mTc | EpCAM driven cancer | The uptake value in tumors was increased about two times from 0.5 h till 12 h after injection, while it could clearly image tumor-draining lymph nodes. | [79] |
| EGFR | 99mTc | EGFR+ cancer, A431 (epidermoid carcinoma) | VHH uptake correlated with tumor burden and tumor response to EGFR inhibitor (erlotinib). | [34] |
| EGFR | 99mTc | A431 | In vivo, the study demonstrated that OA-cb6 labeled with 99mTc showed an approximately 2.7-fold tumor-muscle ratio at 4 h post-injection. | [80] |
| CLDN18.2 | 89Zr | STAD | The VHH had good tumor uptake to evaluate the expression of CLDN18.2 in gastric cancer for patient selection. | [81] |
| CEACAM5 | 99mTc | NSCLC | The high ratio of the signal in the tumor compared with the background confirmed that the VHH can be used as a molecular probe for imaging CEACAM5-expressing tumors. | [82] |
| CAIX | 111In | HNSCC | The anti-CAIX VHH targeted hypoxia regions in solid tumors. | [83] |
| * EDB of FN | 64Cu | pan-cancer | Targeted the extracellular matrix to image tumor progression, metastasis, and fibrosis. | [84] |
| Targets | Conjugates | Cancer Models | References |
|---|---|---|---|
| HER2 | 131I (β/γ) | HER2+ cancer | [98,99] |
| 125I, 131I-SGMIB (β/γ) | BrCa | [100] | |
| 177Lu (β) | OvCa | [94] | |
| 211At (α) | HER2+ cancer | [101,102] | |
| 225Ac (α) | SKOV3, BrCa | [103] | |
| 211At (α) | BrCa | [102] | |
| FAPα * | 89Zr (γ), 177Lu(β) | FAPα+ cancer | [96] |
| 131I-SGMIB (β/γ) | FAPα+ cancer | [95] | |
| 225Ac (α) | |||
| MMR | 177Lu (β), 111In(γ) | TS/A | [97] |
| Targets | Conjugates | Cancer Models | Main Findings | References |
|---|---|---|---|---|
| HER2 | IRDye700DX | SK-BR-3 (HER2+, sensitive), HCC1954, JIMT1, HCC1419 (HER2+, resistant), MCF7 (HER2 low), MDA-MB-231 (HER2−) | Anti-HER2 VHH-PS could potently and selectively induce cell death in HER2-positive cells regardless of its sensitivity to trastuzumab. | [106] |
| EGFR | IRDye700DX | A431 | The PS was conjugated with 111In-VHH in a site-specific way, which resulted in light-induced toxicity via cellular internalization. | [105] |
| EGFR | IRDye700DX | Cell lines with different EGFR expression | The anti-EGFR VHH-PS led to approx. 90% tumor necrosis and almost no toxicity in healthy tissue 24 h after PDT. | [107,108] |
| EGFR | IRDye700DX | A431, SCC-U8 | VHH-PS induced the release of DAMPs (HSP70, ATP) and the pro-inflammatory cytokines of moDCs by incubating it with a conditioned medium, which stimulates the immune system. | [109] |
| MET | IRDye700DX | MKN45 | The anti-MET VHH-PS had a nanomolar affinity and led to cell death at nanomolar concentration with illumination. | [110] |
| US28 | IRDye700DX | U251-iUS28 | The anti-US28 VHH-PS was the first example using GPCR as a target for VHH-directed PDT, which selectively killed US28-expressing glioblastoma cells. | [7,32] |
| EGFR/VEGFR2 | IRDye700DX | OSCC | The dual-targeting VHH-PS showed improved efficacy in co-culture of endothelial and cancer cells. | [111] |
| Targets | Cancer Models | Main Findings | References |
|---|---|---|---|
| PD-L1+CD16a+IL15 | PD-L1+ cancer | The fusion promoted cell growth in vitro, while it attenuated tumor growth in vivo. | [120] |
| PD-L1 | PaCa | The VHH-CCL21 fusion could target PD-L1 positive TME and promote recruiting effector cells. | [121] |
| PD-L1 | MC38 | VHH outperformed conventional antibodies in inhibiting tumor growth due to VHH’s higher tumor penetration in the MC38 tumor. | [113] |
| PD-L1 | PD-L1+ cancer | Monovalent, bivalent, and trivalent agents enhanced TCR signaling in PD-L1 positive cancer cells, to result in CD8+ T cell activation and cytokine production to attenuate cancer progression. | [122,123,124] |
| PD-L1+TIGIT | MC38 | The multivalent bispecific VHH could synergistically enhance T cell activity by inhibiting tumor growth in vitro. | [116] |
| PD-1 | A549, BxPC3 | The VHH could block the PD-1/PD-L1 interaction. | [125] |
| PD-1 | MC38 | Long-term systemic expression of VHH by AAV vector provided anti-tumor activity without toxicity. | [126] |
| CTLA-4 | Melanoma | The anti-CTLA4 VHH delayed melanoma growth and prolonged the survival time in mice. | [127] |
| CTLA-4 | MC38, H22 | The half-life-extended version of VHH exhibited therapeutic efficacy in a Fc independent manner. | [114] |
| 4-1BB+PD-L1 | CT-26-huPD-L1, MC-38-huPD-L1 | The bispecific VHH showed anti-tumor efficacy with negligible hepatotoxicity. | [55] |
| Target | Cancer Models | Main Findings | Reference |
|---|---|---|---|
| CapG | MDA-MB-231 | Anti-CapG VHH prevented the formation of lung metastasis. | [131] |
| CD38 | Melanoma | Anti-CD38 VHH Pseudomonas exotoxin A (PE38) showed highly selective cytotoxicity. The effectiveness could be increased by retinoid acid. | [132] |
| CD47 | Melanoma | Anti-CTLA4 VHH synergized with other immune therapies when CD47 in TME was near-completely blocked. | [133] |
| CEACAM5/CD3 | LS174T, SKOV3 | The in vivo half-life of the bispecific VHH was increased 12-fold via the PEGylation strategy, accompanied by more potent tumor inhibition. | [45] |
| CXCR7 | HNSCC | The anti-CXCR7 VHH inhibited tumor growth by reducing the secretion of CXCL1 in vitro and inhibiting angiogenesis in vivo. | [134] |
| DLL4 | MKN, HEK293 | The DLL4 could bind on the surface of MKN cells, and gastric carcinoma tissue and inhibit the maturation of capillary-like structures in HUVECs. | [51] |
| DR5 | Hela, Colo205 | Multivalent anti-DR5 VHHs had higher apoptotic capacity than the monovalent form that could mimic the activity of the natural TRAIL ligand. | [135] |
| DPYSL2, TUFM, Vimentin, NAP1-L1 | GBM | The anti-TUFM VHH showed a cytotoxic effect on GBM CSCs, while other VHHs were shown to target mature GBM cells. | [136] |
| EGFR | LUAD | VHH was linked with the cell-penetrating peptide nonaarginine. The VHH inhibited intracellular signaling by binding EGFR resulting in reduced cell migration. | [137] |
| EGFR | A549, DU145, MCF-7 | The anti-EGFR extracellular domain III VHH showed an anti-tumor effect both in vitro and in vivo. | [138] |
| EGFR | SW480 | VHH could inhibit cancer cell viability by altering proteins involved in the DNA-damage checkpoint process. | [139] |
| MET | HepG2, SK-HEP-1, HCC827, NIH3T3 | Anti-MET VHH pool that acts against the whole ectodomain of MET could overcome MET targeted treatment resistance by promoting MET degradation and blocking the kinase activity of MET. The anti-MET VHH treatment could suppress cancer proliferation, viability, and colony formation in vitro and tumorigenesis in vivo. | [140] |
| p38δ | Hela | The VHH inhibited the target kinase activity and tumor growth. | [141] |
| Survivin | HepG2 | The VHH targeted survivin and blocked the signaling pathway resulting in apoptosis. | [142] |
| Tie1 | U87MG | Targeting Tie1 with specific VHH triggered Tie1-dependent inhibition of RTK phosphorylation and angiogenesis in endothelial cells and suppressed GBM viability and migration. | [143] |
| Target | Cargo | Cancer Models | Main Findings | Reference |
|---|---|---|---|---|
| EGFR | Mal-Pt | A375, A431 | The VHH-DC could be specifically internalized into EGFR-positive cancer cells, resulting in higher therapeutic effects and lower side effects compared with cisplatin alone. | [89] |
| PSMA | Doxorubicin | PC3-PIP, PC3-flu * | An in vivo study showed that a 42-fold lower amount of VHH-DC could result in similar tumor growth inhibition compared with commercial doxorubicin treatment. | [150] |
| HER2 | Doxorubicin | BT474-M3, NCI-N87 | VHH-DC could simultaneously bind with the HER2 target on cancer cells with trastuzumab, which results in synergistic antitumor activity. | [149] |
| HER2 | Auristatin F | BT474, MDA-MB-231 | VHH-DC-albumin fusion overcame the rapid renal clearance, which resulted in long-lasting tumor remission. | [47] |
| VEGFR2 | Diphtheria Toxin | PC3 | Coupling toxin with immune “carrier” resulted in cancer cell growth inhibition, while toxin alone was ineffective. | [151] |
| CD147 | Doxorubicin | Hela, 4T1, U87, 293T(low), SMMC-7721 | In vitro studies showed the VHH-DC could inhibit tumor cell proliferation and induce cell apoptosis. The VHH-DC had a synergistic effect in inhibiting the growth of tumors in vivo, as compared with the treatment of doxorubicin or VHH monotherapy. | [152] |
| 5T4 | SN38 | BxPC-3, Huh-7 | N501-SN38 showed deeper tumor penetration, higher tumor uptake, and faster accumulation at the tumor site than conventional ADC and exhibited effective antitumor activity both in vitro and in vivo. | [147] |
| Agent | Target | Cancer Type | Study Identifier | Phase | Status | Primary Purpose | Related Publication |
|---|---|---|---|---|---|---|---|
| 99mTc-NM-02 | HER2 | Breast cancer | NCT04040686 | Early Phase I | Recruiting | Diagnostic | [177] |
| 99mTc-NM-01 | PD-L1 | Non-Small Cell Lung Cancer | NCT02978196 | Early Phase I | Recruiting | Diagnostic | [178,179] |
| 99mTc-MIRC208 | HER2 | HER2 positive cancer | NCT04591652 | Not Applicable | Recruiting | Diagnostic | [180] |
| 89Zr-KN035 | PD-L1 | PD-L1 positive solid tumor | NCT04977128 | Not Applicable | Recruiting | Diagnostic | [181] |
| 68Ga-THP-APN09 | PD-L1 | Lung cancer Melanoma | NCT05156515 | Not Applicable | Recruiting | Diagnostic | [181] |
| 68Ga-NOTA-Anti-MMR-VHH2 | MMR | Breast cancer Head and Neck cancer Melanoma (skin) | NCT04168528 | Phase I/IIa | Recruiting | Diagnostic | [73,182,183] |
| 68Ga-NOTA-Anti-MMR-VHH2 | MMR | Breast cancer Pancreatic cancer Salivary gland cancer Gastric cancer Endometrial cancer Uterine cancer Non-Small Cell Lung Cancer Biliary tract cancer Cholangiocarcinoma Colorectal cancer Urothelial carcinoma Prostate cancer | NCT03924466 | Phase II | Recruiting | Diagnostic | [73,182,183] |
| 68Ga-NOTA-Anti-HER2 VHH1 | HER2 | Breast cancer | NCT03924466 | Phase II | Recruiting | Diagnostic | [18,64,184] |
| 68Ga-NOTA-Anti-HER2 VHH1 | HER2 | Breast cancer | NCT03331601 | Phase II | Recruiting | Diagnostic | [18,64,184] |
| 99mTc-NM01 | PD-L1 | Non-Small Cell Lung Cancer | NCT04992715 | Phase II | Recruiting | Diagnostic | [178,179] |
| 131I-SGMIB Anti-HER2 VHH1 | HER2 | Breast cancer | NCT02683083 | Phase I | Completed | Diagnostic | [185] |
| 68Ga-ACN376 | CLDN18.2 | Solid tumor | NCT05436093 | Not Applicable | Recruiting | Screening | |
| αPD1-MSLN- CAR-T Cells | PD-1 | Solid tumor | NCT05373147 | Early Phase I | Recruiting | Treatment | [186] |
| αPD1-MSLN- CAR-T Cells | PD-1 | Colorectal cancer Ovarian cancer | NCT04503980 | Early Phase I | Recruiting | Treatment | [186] |
| αPD1-MSLN- CAR-T Cells | PD-1 | Non-small-cell Lung Cancer Mesothelioma | NCT04489862 | Early Phase I | Recruiting | Treatment | [186] |
| αPD1-MSLN- CAR-T Cells | PD-1 | Colorectal cancer | NCT05089266 | Phase I | Not yet recruiting | Treatment | [186] |
| KN046+Axitinib | PD-L1/CTLA4 Bispecific | Advanced Non-small Cell Lung cancer | NCT05420220 | Phase II | Not yet recruiting | Treatment | [187] |
| KN046 | PD-L1/CTLA4 Bispecific | Thymic carcinoma | NCT04469725 | Phase II | Recruiting | Treatment | [188] |
| KN044 | CTLA4 | Advanced solid tumor | NCT04126590 | Phase I | Recruiting | Treatment | [189] |
| KN035 | PD-L1 | Solid tumor | NCT03101488 | Phase I | Completed | Treatment | [190,191] |
| KN035 | PD-L1 | Advanced or metastatic solid tumor | NCT03248843 | Phase I | Completed | Treatment | [190,191] |
| JS014 (fusion with IL-21) + Pembrolizumab | Human Serum Albumin | Malignant neoplasm Experimental solid tumor Adult lymphoma | NCT05296772 | Phase I | Active, not recruiting | Treatment | |
| Gavocabtagene autoleucel (gavo-cel; TC-210) | Mesothelin | Mesothelioma | NCT03907852 | Phase I Phase II | Recruiting | Treatment | [192] |
| Envofolimab (KN035)+Gemcitabine and Cisplatin | PD-L1 | Biliary tract cancer | NCT04910386 | Phase II | Not yet recruiting | Treatment | [193] |
| 99mTc-NM-02, 188Re-NM-02 | HER2 | Breast cancer | NCT04674722 | Early Phase I | Recruiting | Treatment | [177,194] |
| Envafolimab (+Ipilimumab) | PD-L1 | Pleomorphic sarcoma Myxofibrosarcoma | NCT04480502 | Phase II | Recruiting | Treatment | [195] |
| 68Ga-NODAGA-SNA006 | CD8α | Solid tumors | NCT05126927 | Early Phase I | Recruiting | Diagnostic | [196] |
| DR30303-IgG1Fc | CLDN18.2 | Malignant neoplasm of the digestive system | NCT05639153 | Phase I | Recruiting | Treatment | [197] |
| [99mTc]-NM-01 | PD-L1 | Non-small cell lung cancer, malignant melanoma | NCT04436406 | Not Applicable | Recruiting | Diagnostic | |
| 68Ga-PD-L2 | PD-L2 | Colorectal cancer, Lung cancer | NCT05803746 | Not Applicable | Recruiting | Diagnostic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, Y.; Devoogdt, N.; Lambin, P.; Dubois, L.J.; Yaromina, A. Promising Diagnostic and Therapeutic Approaches Based on VHHs for Cancer Management. Cancers 2024, 16, 371. https://doi.org/10.3390/cancers16020371
Cong Y, Devoogdt N, Lambin P, Dubois LJ, Yaromina A. Promising Diagnostic and Therapeutic Approaches Based on VHHs for Cancer Management. Cancers. 2024; 16(2):371. https://doi.org/10.3390/cancers16020371
Chicago/Turabian StyleCong, Ying, Nick Devoogdt, Philippe Lambin, Ludwig J. Dubois, and Ala Yaromina. 2024. "Promising Diagnostic and Therapeutic Approaches Based on VHHs for Cancer Management" Cancers 16, no. 2: 371. https://doi.org/10.3390/cancers16020371
APA StyleCong, Y., Devoogdt, N., Lambin, P., Dubois, L. J., & Yaromina, A. (2024). Promising Diagnostic and Therapeutic Approaches Based on VHHs for Cancer Management. Cancers, 16(2), 371. https://doi.org/10.3390/cancers16020371









