Retrospective Evaluation of GEC-ESTRO Constraints for Definitive Radiochemotherapy with Brachytherapy and Correlation with Oncologic Outcome in Cervical Cancer: A Monocenter Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
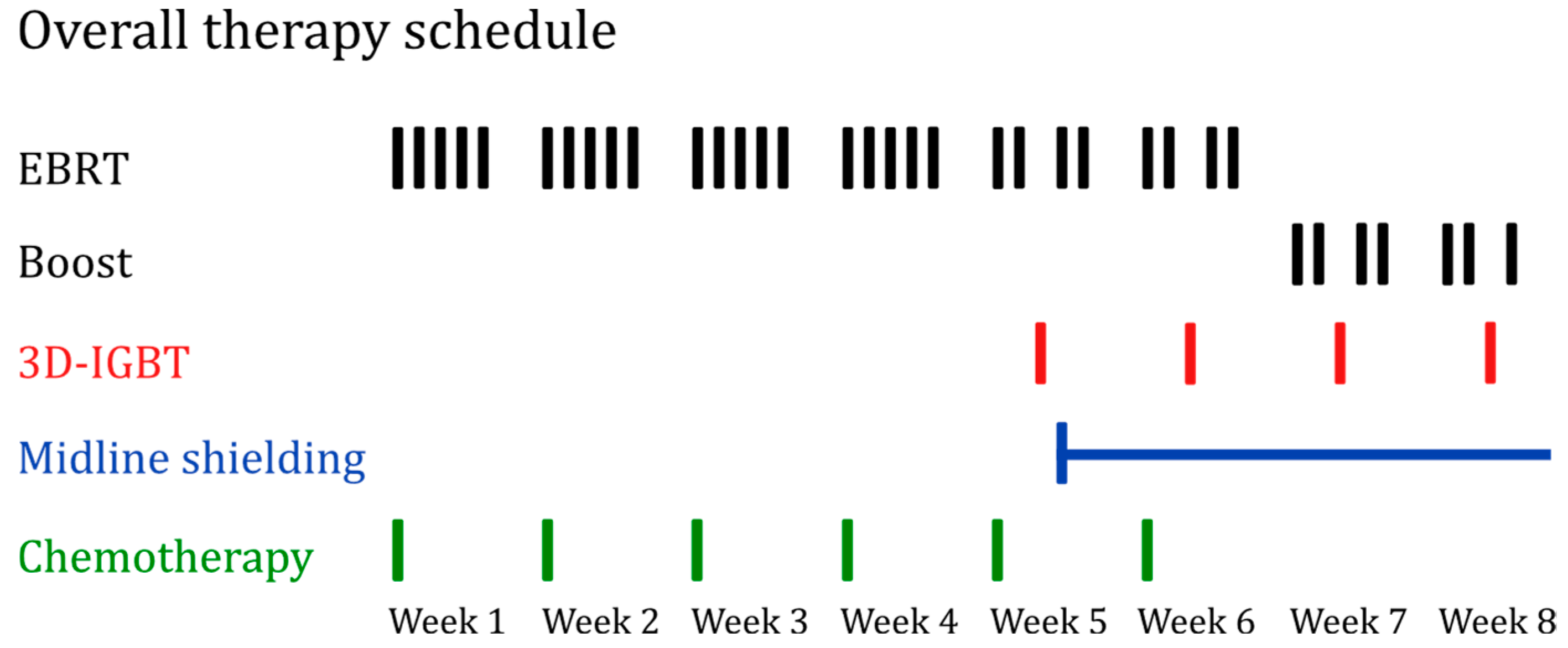
2.1. Patients and Treatment
2.2. Technical Details
2.3. Recorded Data
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics and Follow-Up
3.2. Treatment
3.2.1. Imaging and Completion of Therapy
3.2.2. Dose–Volume Parameters
3.3. Organs at Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, H.B.; Pifer, P.M.; Mohindra, P.; Albuquerque, K.; Beriwal, S. Advances in management of locally advanced cervical cancer. Indian J. Med. Res. 2021, 154, 248–261. [Google Scholar] [CrossRef]
- World Health Organization. Cervical Cancer: Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 15 August 2024).
- Mayadev, J.S.; Ke, G.; Mahantshetty, U.; Pereira, M.D.; Tarnawski, R.; Toita, T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Krebsgesellschaft; Deutsche Krebshilfe; AWMF. S3-Leitlinie Diagnostik, Therapie und Nachsorge der Patientin mit Zervixkarzinom, Langversion, 2.2, 2022: AWMF-Registernummer: 032/033OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/zervixkarzinom (accessed on 15 June 2024).
- Chargari, C.; Peignaux, K.; Escande, A.; Renard, S.; Lafond, C.; Petit, A.; Lam Cham Kee, D.; Durdux, C.; Haie-Méder, C. Radiotherapy of cervical cancer. Cancer Radiother. 2022, 26, 298–308. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef]
- Cibula, D.; Rosaria Raspollini, M.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Radiother. Oncol. 2023, 184, 109682. [Google Scholar] [CrossRef]
- Pötter, R.; Haie-Meder, C.; van Limbergen, E.; Barillot, I.; de Brabandere, M.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother. Oncol. 2006, 78, 67–77. [Google Scholar] [CrossRef]
- Haie-Meder, C.; Pötter, R.; van Limbergen, E.; Briot, E.; de Brabandere, M.; Dimopoulos, J.; Dumas, I.; Hellebust, T.P.; Kirisits, C.; Lang, S.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother. Oncol. 2005, 74, 235–245. [Google Scholar] [CrossRef]
- Dimopoulos, J.C.A.; Petrow, P.; Tanderup, K.; Petric, P.; Berger, D.; Kirisits, C.; Pedersen, E.M.; van Limbergen, E.; Haie-Meder, C.; Pötter, R. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother. Oncol. 2012, 103, 113–122. [Google Scholar] [CrossRef]
- Hellebust, T.P.; Kirisits, C.; Berger, D.; Pérez-Calatayud, J.; de Brabandere, M.; de Leeuw, A.; Dumas, I.; Hudej, R.; Lowe, G.; Wills, R.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: Considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother. Oncol. 2010, 96, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, J.; Jiang, P. High-Dose-Rate Three-Dimensional Image-Guided Adaptive Brachytherapy (3D IGABT) for Locally Advanced Cervical Cancer (LACC): A Narrative Review on Imaging Modality and Clinical Evidence. Curr. Oncol. 2023, 31, 50–65. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Dimopoulos, J.; Kirisits, C.; Berger, D.; Pötter, R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 491–498. [Google Scholar] [CrossRef]
- Rajasooriyar, C.; Hoskin, P. Evolution of Intracavitary Brachytherapy in the Treatment of Cervical Cancer. Acta Sci. Women’s Health 2021, 3, 47–53. [Google Scholar] [CrossRef]
- de Sanctis, V.; Facondo, G.; Vullo, G.; Anzellini, D.; Sanguineti, G.; Nardangeli, A.; Marmiroli, L.; Tortoreto, F.; Gentile, P.; Annessi, I.; et al. Clinical Outcomes and Toxicity of CT-guided High Dose-rate Brachytherapy in Women with Locally-advanced Cervical Cancer. Cancer Diagn. Progn. 2023, 3, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.; Rae, W.I.D.; Alber, M.L. Equivalence of Gyn GEC-ESTRO guidelines for image guided cervical brachytherapy with EUD-based dose prescription. Radiat. Oncol. 2013, 8, 266. [Google Scholar] [CrossRef][Green Version]
- Tanderup, K.; Poetter, R.; Lindegaard, J.; Kirisits, C.; Jurgenliemk-Schulz, I.; de Leeuw, A.; Fortin, I.; Kirchheiner, K.; Georg, D.; Nout, R.; et al. EMBRACE II Study Protocol v.1.0. Available online: https://www.embracestudy.dk/UserUpload/PublicDocuments/EMBRACE%20II%20Protocol.pdf (accessed on 23 September 2024).
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Published: 27 November 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 15 June 2024).
- LENT SOMA tables. Radiother. Oncol. 1995, 35, 17–60. [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 19 August 2024).
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Berger, T.; Seppenwoolde, Y.; Pötter, R.; Assenholt, M.S.; Lindegaard, J.C.; Nout, R.A.; de Leeuw, A.; Jürgenliemk-Schulz, I.; Tan, L.T.; Georg, D.; et al. Importance of Technique, Target Selection, Contouring, Dose Prescription, and Dose-Planning in External Beam Radiation Therapy for Cervical Cancer: Evolution of Practice From EMBRACE-I to II. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 885–894. [Google Scholar] [CrossRef]
- Faye, M.D.; Alfieri, J. Advances in Radiation Oncology for the Treatment of Cervical Cancer. Curr. Oncol. 2022, 29, 928–944. [Google Scholar] [CrossRef]
- Benkhaled, S.; Diakité, K.; Jullian, N.; Poeta, S.; Vandekerkhove, C.; van Houtte, P.; van Gestel, D.; de Caluwé, A. Boost modalities in cervical cancer: Dosimetric comparison between intracavitary BT vs. intracavitary + interstitial BT vs. SBRT. Radiat. Oncol. 2023, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Veeratterapillay, J.; Locks, S.; Morgan, D.; Patil, R.; Chamberlain, H.M. Magnetic Resonance Imaging-Guided Adaptive Brachytherapy for the Treatment of Cervical Cancer and its Impact on Clinical Outcome. Clin. Oncol. 2022, 34, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.A.; Tali, E.T.; Yuh, W.T.; Brown, B.P.; Wen, B.C.; Buller, R.E.; Anderson, B.; Hussey, D.H. Cervical cancer: Application of MR imaging in radiation therapy. Radiology 1993, 189, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.W.L.; Wong, J.S.Y.; Lee, V.W.Y.; Wong, F.C.S.; Tung, S.Y. Throwing the dart blind-folded: Comparison of computed tomography versus magnetic resonance imaging-guided brachytherapy for cervical cancer with regard to dose received by the ‘actual’ targets and organs at risk. J. Contemp. Brachyther. 2017, 9, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Mahantshetty, U.; Poetter, R.; Beriwal, S.; Grover, S.; Lavanya, G.; Rai, B.; Petric, P.; Tanderup, K.; Carvalho, H.; Hegazy, N.; et al. IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother. Oncol. 2021, 160, 273–284. [Google Scholar] [CrossRef]
- Elmali, A.; Biltekin, F.; Sari, S.Y.; Gultekin, M.; Yuce, D.; Yildiz, F. Inter-observer variation of target volume delineation for CT-guided cervical cancer brachytherapy. J. Contemp. Brachyther. 2023, 15, 253–260. [Google Scholar] [CrossRef]
- Xiu, Y.-T.; Meng, F.-X.; Wang, Z.; Zhao, K.-K.; Wang, Y.-L.; Chen, Z.-S.; Sun, B.-S. Prognostic factors for IB2-IIIB cervical cancer patients treated by radiation therapy with high-dose-rate brachytherapy in a single-institution study. J. Contemp. Brachyther. 2022, 14, 332–340. [Google Scholar] [CrossRef]
- Chan, W.-L.; Cheng, M.H.-F.; Wu, J.T.-K.; Choi, C.-W.; Tse, R.P.-Y.; Ho, P.P.-Y.; Cheung, E.E.; Cheung, A.; Test, K.-Y.; Chan, K.K.-L.; et al. Treatment Outcomes of Computer Tomography-Guided Brachytherapy in Cervical Cancer in Hong Kong: A Retrospective Review. Cancers 2022, 14, 3934. [Google Scholar] [CrossRef]
- Gill, B.S.; Kim, H.; Houser, C.J.; Kelley, J.L.; Sukumvanich, P.; Edwards, R.P.; Comerci, J.T.; Olawaiye, A.B.; Huang, M.; Courtney-Brooks, M.; et al. MRI-guided high-dose-rate intracavitary brachytherapy for treatment of cervical cancer: The University of Pittsburgh experience. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 540–547. [Google Scholar] [CrossRef]
- Lalos, O.; Lalos, A. Urinary, climacteric and sexual symptoms one year after treatment of endometrial and cervical cancer. Eur. J. Gynaecol. Oncol. 1996, 17, 128–136. [Google Scholar]
- Glaser, S.M.; Mohindra, P.; Mahantshetty, U.; Beriwal, S. Complications of intracavitary brachytherapy for gynecologic cancers and their management: A comprehensive review. Brachytherapy 2021, 20, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Pan, Y.; Zhang, N.; Han, D.; Guo, X.; Mao, Z.; Cheng, G. Clinical Outcomes of MRI-Guided Adaptive Brachytherapy for Each Fraction in Locally Advanced Cervical Cancer: A Single Institution Experience. Front. Oncol. 2022, 12, 841980. [Google Scholar] [CrossRef] [PubMed]
- Horeweg, N.; Creutzberg, C.L.; Rijkmans, E.C.; Laman, M.S.; Velema, L.A.; Coen, V.L.; Stam, T.C.; Kerkhof, E.M.; Kroep, J.R.; de Kroon, C.D.; et al. Efficacy and toxicity of chemoradiation with image-guided adaptive brachytherapy for locally advanced cervical cancer. Int. J. Gynecol. Cancer 2019, 29, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Voigtländer, S.; Hakimhashemi, A.; Grundmann, N.; Radespiel-Tröger, M.; Inwald, E.C.; Ortmann, O.; Gerken, M.; Klug, S.J.; Klinkhammer-Schalke, M.; Meyer, M.; et al. Impact of the COVID-19 pandemic on reported cancer diagnoses in Bavaria, Germany. J. Cancer Res. Clin. Oncol. 2023, 149, 7493–7503. [Google Scholar] [CrossRef]
- Elemes, S.; Stachteas, P.; Haidich, A.-B.; Mamopoulos, A.; Smyrnakis, E. The Impact of the COVID-19 Pandemic on Breast and Cervical Cancer Screening: A Systematic Review. In Vivo 2023, 37, 1455–1476. [Google Scholar] [CrossRef]


| Variable | CR 38 (79%) | No CR 10 (21%) | Odds Ratio (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Age at start of therapy | x + 1 vs. x years | 49.5 (39.3–64.0) | 61.0 (46.5–68.3) | 0.96 (0.91, 1.01) | 0.134 |
| FIGO stage | I | 7 (15%) | 0 | NE | NE |
| II | 10 (21%) | 3 (6%) | 1.11 (0.24, 5.23) | 0.894 | |
| III | 21 (44%) | 7 (15%) | Reference | ||
| Lymph node involvement | No lymph node involvement | 21 (44%) | 3 (6%) | Reference | |
| Pelvic lymph node metastasis | 8 (17%) | 3 (6%) | 0.38 (0.06, 2.29) | 0.292 | |
| Para-aortic lymph node metastasis | 9 (19%) | 4 (8%) | 0.32 (0.06, 1.74) | 0.188 | |
| Complete therapy | Yes | 27 (56%) | 6 (13%) | Reference | |
| No | 11 (23%) | 4 (8%) | 0.61 (0.14, 2.60) | 0.505 | |
| EBRT boost received | No | 10 (21%) | 4 (8%) | Reference | |
| Yes | 28 (58%) | 6 (13%) | 1.87 (0.44, 8.01) | 0.401 | |
| MRI pre EBRT or for BT | No MRI | 12 (25%) | 2 (4%) | Reference | |
| At least one MRI | 26 (54%) | 8 (17%) | 0.54 (0.1, 2.95) | 0.478 | |
| MRI for BT | No MRI | 25 (52%) | 7 (15%) | Reference | |
| At least one MRI | 13 (27%) | 3 (6%) | 1.21 (0.27, 5.49) | 0.802 | |
| GTVres D98 [Gy] | x + 1 vs. x Gy | 55.9 (51.3–61.3) | 52.0 (48.3–56.8) | 1.07 (0.96, 1.20) | 0.205 |
| IR-CTV D98 [Gy] | x + 1 vs. x Gy | 44.1 (39.7–46.4) | 42.0 (36.0–45.2) | 1.04 (0.93, 1.17) | 0.474 |
| HR-CTV D90 [Gy] | x + 1 vs. x Gy | 55.1 (52.8–58.9) | 53.8 (49.0–57.9) | 1.08 (0.94, 1.23) | 0.275 |
| HR-CTV D98 [Gy] | x + 1 vs. x Gy | 49.9 (46.5–53.7) | 47.5 (45.3–51.8) | 1.05 (0.93, 1.19) | 0.418 |
| GTVres D98 deviation from GGE | x + 1 vs. x Gy | 34.1 (28.7–38.7) | 38.0 (33.3–41.7) | 0.93 (0.84, 1.04) | 0.205 |
| IR-CTV D98 deviation from GGE | x + 1 vs. x Gy | 15.9 (13.6–20.3) | 18.0 (14.8–24.0) | 0.96 (0.86, 1.08) | 0.474 |
| HR-CTV D90 deviation from GGE | x + 1 vs. x Gy | 29.9 (26.1–32.2) | 31.2 (27.1–36.0) | 0.93 (0.81, 1.06) | 0.275 |
| HR-CTV D98 deviation from GGE | x + 1 vs. x Gy | 25.1 (21.3–28.5) | 27.5 (23.2–29.7) | 0.95 (0.84, 1.07) | 0.418 |
| IR-CTV V150 [%] | x + 1 vs. x % | 9.3 (7.5–11.2) | 8.0 (4.4–9.8) | 1.30 (0.98, 1.73) | 0.073 |
| IR-CTV V200 [%] | x + 1 vs. x % | 4.3 (3.5–5.8) | 3.6 (2.1–4.6) | 1.77 (1.01, 3.09) | 0.046 |
| HR-CTV V150 [%] | x + 1 vs. x % | 20.7 (15.9–24.8) | 16.5 (9.8–19.6) | 1.12 (0.99, 1.27) | 0.063 |
| HR-CTV V200 [%] | x + 1 vs. x % | 10.2 (7.4–12.0) | 7.8 (4.5–9.4) | 1.22 (0.98, 1.50) | 0.073 |
| Point A GGE | Recommendations not met | 16 (33%) | 5 (10%) | Reference | |
| Recommendations met | 22 (46%) | 5 (10%) | 1.38 (0.34, 5.56) | 0.655 |
| Variable | Events/Total 13/43 | Hazard Ratio (95% CI) | p-Value | |
|---|---|---|---|---|
| Age at start of therapy | x + 1 vs. x years | - | 1.01 (0.97, 1.06) | 0.551 ** |
| FIGO stage | I | 1/7 | Reference | |
| II | 3/12 | 1.62 (0.17, 15.57) | 0.511 * | |
| III | 9/24 | 2.69 (0.34, 21.29) | ||
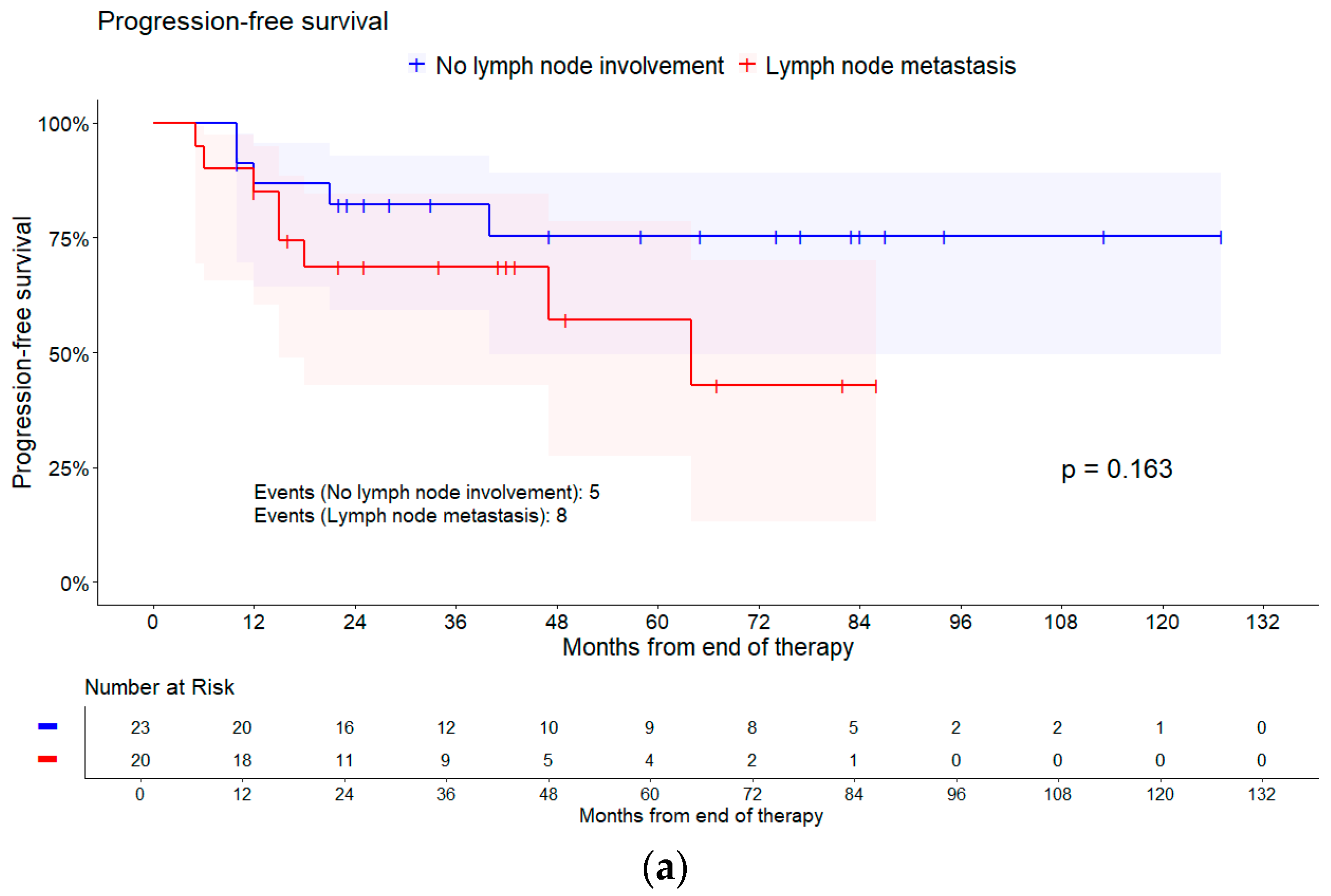
| Lymph node involvement | No lymph node involvement | 5/23 | Reference | |
| Pelvic lymph node metastasis | 3/9 | 1.50 (0.36, 6.27) | 0.192 * | |
| Para-aortic lymph node metastasis | 5/11 | 3.04 (0.86, 10.76) | ||
| Complete therapy | Yes | 10/32 | Reference | |
| No | 3/11 | 1.07 (0.29, 3.91) | 0.921 * | |
| EBRT boost received | No | 3/12 | Reference | |
| Yes | 10/31 | 1.43 (0.39, 5.20) | 0.586 * | |
| MRI pre EBRT or for BT | No MRI | 6/13 | Reference | |
| At least one MRI | 7/30 | 0.36 (0.12, 1.09) | 0.058 * | |
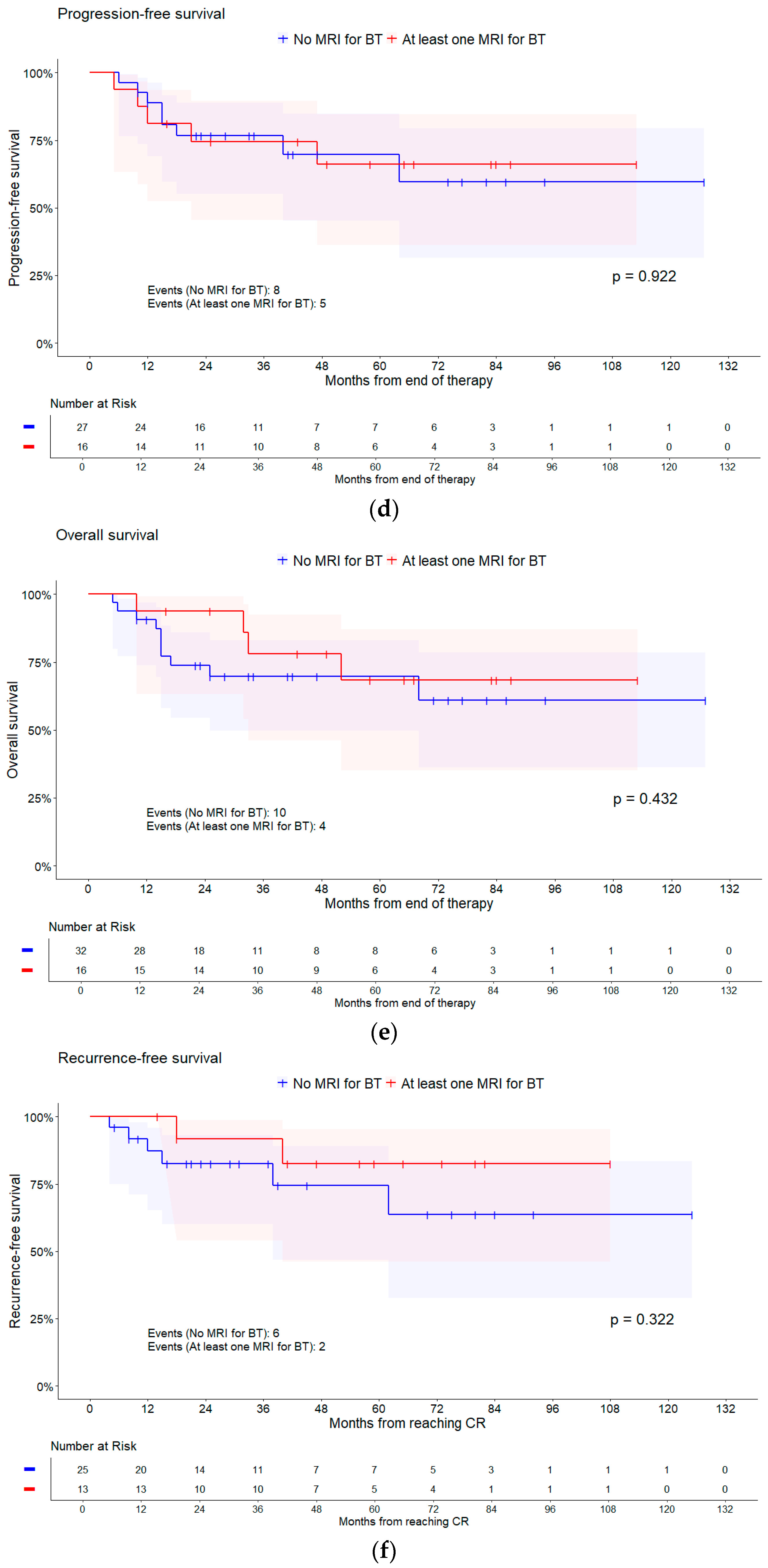
| MRI for BT | No MRI | 8/27 | Reference | |
| At least one MRI | 5/16 | 0.95 (0.31, 2.91) | 0.922 * | |
| GTVres D98 [Gy] | x + 1 vs. x Gy | - | 0.99 (0.93, 1.06) | 0.839 ** |
| IR-CTV D98 [Gy] | x + 1 vs. x Gy | - | 1.00 (0.91, 1.09) | 0.907 ** |
| HR-CTV D90 [Gy] | x + 1 vs. x Gy | - | 1.00 (0.93, 1.08) | 0.943 ** |
| HR-CTV D98 [Gy] | x + 1 vs. x Gy | - | 1.01 (0.93, 1.09) | 0.880 ** |
| GTVres D98 deviation from GGE | x + 1 vs. x Gy | - | 1.01 (0.94, 1.08) | 0.839 ** |
| IR-CTV D98 deviation from GGE | x + 1 vs. x Gy | - | 1.01 (0.92, 1.10) | 0.907 ** |
| HR-CTV D90 deviation from GGE | x + 1 vs. x Gy | - | 1.00 (0.93, 1.07) | 0.943 ** |
| HR-CTV D98 deviation from GGE | x + 1 vs. x Gy | - | 0.99 (0.92, 1.07) | 0.880 ** |
| Point A GGE | Recommendations not met | 7/19 | Reference | |
| Recommendations met | 6/24 | 0.62 (0.21, 1.86) | 0.385 * |
| Variable | Events/Total 14/48 | Hazard Ratio (95% CI) | p-Value | |
|---|---|---|---|---|
| Age at start of therapy | x + 1 vs. x years | - | 1.05 (1.01, 1.09) | 0.027 ** |
| FIGO stage | I | 1/7 | Reference | |
| II | 4/13 | 1.94 (0.22, 17.47) | 0.691 * | |
| III | 9/28 | 2.36 (0.30, 18.73) | ||
| Lymph node involvement | No lymph node involvement | 6/24 | Reference | |
| Pelvic lymph node metastasis | 4/11 | 1.3 (0.46, 5.81) | 0.592 * | |
| Para-aortic lymph node metastasis | 4/13 | 1.82 (0.50, 6.61) | ||
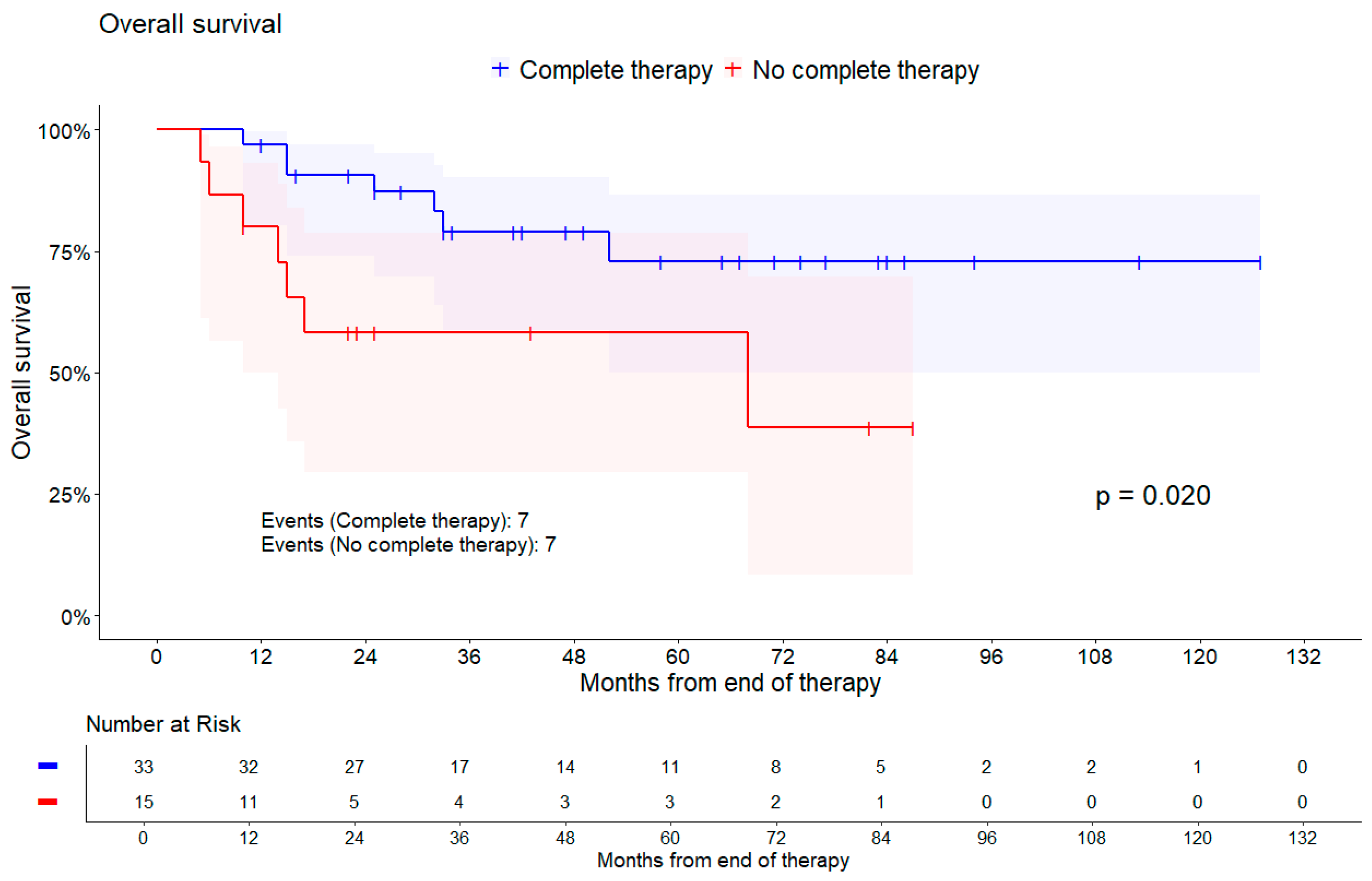
| Complete therapy | Yes | 7/33 | Reference | |
| No | 7/15 | 3.27 (1.13, 9.42) | 0.020 * | |
| EBRT boost received | No | 4/14 | Reference | |
| Yes | 10/34 | 0.97 (0.30, 3.08) | 0.954 * | |
| MRI pre EBRT or for BT | No MRI | 5/14 | Reference | |
| At least one MRI | 9/34 | 0.54 (0.18, 1.62) | 0.257 * | |
| MRI for BT | No MRI | 10/32 | Reference | |
| At least one MRI | 4/16 | 0.63 (0.20, 2.03) | 0.432 * | |
| GTVres D98 [Gy] | x + 1 vs. x Gy | - | 1.01 (0.95, 1.07) | 0.833 ** |
| IR-CTV D98 [Gy] | x + 1 vs. x Gy | - | 1.04 (0.95, 1.14) | 0.400 ** |
| HR-CTV D90 [Gy] | x + 1 vs. x Gy | - | 1.01 (0.95, 1.08) | 0.678 ** |
| HR-CTV D98 [Gy] | x + 1 vs. x Gy | - | 1.02 (0.96, 1.10) | 0.515 ** |
| GTVres D98 deviation from GGE | x + 1 vs. x Gy | - | 0.99 (0.93, 1.06) | 0.833 ** |
| IR-CTV D98 deviation from GGE | x + 1 vs. x Gy | - | 0.96 (0.88, 1.05) | 0.400 ** |
| HR-CTV D90 deviation from GGE | x + 1 vs. x Gy | - | 0.99 (0.93, 1.05) | 0.678 ** |
| HR-CTV D98 deviation from GGE | x + 1 vs. x Gy | - | 0.98 (0.91, 1.05) | 0.515 ** |
| Point A GGE | Recommendations not met | 7/21 | Reference | |
| Recommendations met | 7/27 | 0.88 (0.31, 2.51) | 0.806 * |
| Variable | Events/Total 8/38 | Hazard Ratio (95% CI) | p-Value | |
|---|---|---|---|---|
| Age at start of therapy | x + 1 vs. x years | - | 1.02 (0.97, 1.08) | 0.428 ** |
| FIGO stage | I | 1/7 | Reference | |
| II | 1/10 | 0.59 (0.04, 9.40) | 0.427 * | |
| III | 6/21 | 2.01 (0.24, 16.73) | ||
| Lymph node involvement | No lymph node involvement | 3/21 | Reference | |
| Pelvic lymph node metastasis | 2/8 | 1.60 (0.27, 9.61) | 0.162 * | |
| Para-aortic lymph node metastasis | 3/9 | 4.38 (0.85, 22.61) | ||
| Complete therapy | Yes | 5/27 | Reference | |
| No | 3/11 | 2.16 (0.51, 9.11) | 0.284 * | |
| EBRT boost received | No | 1/10 | Reference | |
| Yes | 7/28 | 3.07 (0.38, 24.99) | 0.270 * | |
| MRI pre EBRT or for BT | No MRI | 5/12 | Reference | |
| At least one MRI | 3/26 | 0.14 (0.03, 0.61) | 0.003 * | |
| MRI for BT | No MRI | 6/25 | Reference | |
| At least one MRI | 2/13 | 0.45 (0.09, 2.27) | 0.322 * | |
| GTVres D98 [Gy] | x + 1 vs. x Gy | - | 1.03 (0.95, 1.10) | 0.492 ** |
| IR-CTV D98 [Gy] | x + 1 vs. x Gy | - | 1.02 (0.91, 1.14) | 0.723 ** |
| HR-CTV D90 [Gy] | x + 1 vs. x Gy | - | 1.03 (0.96,1.11) | 0.409 ** |
| HR-CTV D98 [Gy] | x + 1 vs. x Gy | - | 1.03 (0.95, 1.12) | 0.436 ** |
| GTVres D98 deviation from GGE | x + 1 vs. x Gy | - | 0.98 (0.91, 1.05) | 0.492 ** |
| IR-CTV D98 deviation from GGE | x + 1 vs. x Gy | - | 0.98 (0.87, 1.10) | 0.723 ** |
| HR-CTV D90 deviation from GGE | x + 1 vs. x Gy | - | 0.97 (0.90, 1.04) | 0.409 ** |
| HR-CTV D98 deviation from GGE | x + 1 vs. x Gy | - | 0.97 (0.89, 1.05) | 0.436 ** |
| Point A GGE | Recommendations not met | 4/16 | Reference | |
| Recommendations met | 4/22 | 0.72 (0.18, 2.86) | 0.631 * |
| Dose–Volume Parameter | GTVres | HR-CTV | IR-CTV | |
|---|---|---|---|---|
| D98 [Gy] EQD2(α/β=10) | Median | 55.3 | 49.6 | 43.4 |
| IQR | 50.2–60.8 | 46.5–53.3 | 39.5–46.2 | |
| Min–Max | 40.2–82.1 | 36.7–73.5 | 27.9–56.9 | |
| D90 [Gy] EQD2(α/β=10) | Median | 55.0 | ||
| IQR | 51.8–58.6 | |||
| Min–Max | 45.4–83.6 | |||
| V150 [%] | Median | 19.2 | 8.5 | |
| IQR | 15.0–24.4 | 7.1–11.1 | ||
| Min–Max | 6.0–71.4 | 4.0–27.2 | ||
| V200 [%] | Median | 9.8 | 4.3 | |
| IQR | 6.9–11.8 | 3.1–5.4 | ||
| Min–Max | 0.4–44.7 | 0.8–14.7 |
| GTVres D98 [Gy] | IR-CTV D98 [Gy] | HR-CTV D90 [Gy] | HR-CTV D98 [Gy] | |
|---|---|---|---|---|
| Median | 34.7 | 16.6 | 30.0 | 25.4 |
| IQR | 29.2–39.8 | 13.8–20.5 | 26.4–33.2 | 21.7–28.5 |
| Min–Max | 7.9–49.8 | 3.1–32.1 | 1.4–39.6 | 1.5–38.3 |
| Acute Complications 1 | Long-Term Complications 2 | |||
|---|---|---|---|---|
| Bladder | Rectum | Bladder | Rectum | |
| No complications | 29 (60%) | 17 (35%) | 20 (42%) | 18 (38%) |
| Grade I | 11 (30%) | 20 (42%) | 5 (10%) | 8 (17%) |
| Grade II | 1 (2%) | 8 (17%) | 7 (15%) | 11 (23%) |
| Grade III | 7 (15%) | 3 (6%) | 8 (17%) | 1 (2%) |
| Grade IV | 0 | 0 | 3 (6%) | 5 (10%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönicke, T.; Koch, R.; Vogt, I.; Falke, I.; Eich, H.T.; Reinartz, G. Retrospective Evaluation of GEC-ESTRO Constraints for Definitive Radiochemotherapy with Brachytherapy and Correlation with Oncologic Outcome in Cervical Cancer: A Monocenter Study. Cancers 2024, 16, 3495. https://doi.org/10.3390/cancers16203495
Schönicke T, Koch R, Vogt I, Falke I, Eich HT, Reinartz G. Retrospective Evaluation of GEC-ESTRO Constraints for Definitive Radiochemotherapy with Brachytherapy and Correlation with Oncologic Outcome in Cervical Cancer: A Monocenter Study. Cancers. 2024; 16(20):3495. https://doi.org/10.3390/cancers16203495
Chicago/Turabian StyleSchönicke, Tom, Raphael Koch, Isabel Vogt, Isabel Falke, Hans Theodor Eich, and Gabriele Reinartz. 2024. "Retrospective Evaluation of GEC-ESTRO Constraints for Definitive Radiochemotherapy with Brachytherapy and Correlation with Oncologic Outcome in Cervical Cancer: A Monocenter Study" Cancers 16, no. 20: 3495. https://doi.org/10.3390/cancers16203495
APA StyleSchönicke, T., Koch, R., Vogt, I., Falke, I., Eich, H. T., & Reinartz, G. (2024). Retrospective Evaluation of GEC-ESTRO Constraints for Definitive Radiochemotherapy with Brachytherapy and Correlation with Oncologic Outcome in Cervical Cancer: A Monocenter Study. Cancers, 16(20), 3495. https://doi.org/10.3390/cancers16203495








