Simple Summary
Colorectal cancer is a major global health burden, with surgical resection being the standard treatment aimed at curative tumor removal. Oxidative stress is crucial in colorectal cancer progression and prognosis. This study hypothesizes that the physical removal of colorectal cancer, a primary source of oxidative stress, would reduce blood levels of reactive oxygen metabolite derivatives (d-ROMs), a marker of oxidative stress. This study included 123 patients who underwent radical resection for colorectal cancer. d-ROM levels were measured before and after surgery. The clinicopathological analysis showed a correlation between preoperative d-ROM levels and tumor size. This study confirmed a significant reduction in d-ROM levels following tumor resection. The d-ROM ratio before and after tumor resection was significantly higher in cases with positive lymph node metastasis and larger tumor size. These results suggest that d-ROM levels could serve as a valuable biomarker for monitoring tumor burden in patients with colorectal cancer.
Abstract
Background: Colorectal cancer is a major global health burden, with surgical resection being the standard treatment aimed at curative tumor removal. Oxidative stress plays a crucial role in colorectal cancer progression and prognosis. This study hypothesized that physical removal of colorectal cancer, a primary source of oxidative stress, would reduce blood levels of reactive oxygen metabolite derivatives (d-ROMs), a marker of oxidative stress, and biologic antioxidant potential (BAP) levels, a marker of antioxidant potential. Methods: This study included 123 patients who underwent radical resection for colorectal cancer. d-ROM and BAP levels were measured before and one month after surgery. Results: The clinicopathological analysis showed a correlation between preoperative d-ROM levels and tumor size (p < 0.001). This study confirmed a significant reduction in d-ROM levels following tumor resection, indicating reduced systemic oxidative stress. The reduction was significant in stages II and III, but not in stage I. The d-ROM ratio before and after tumor resection was significantly higher in cases with positive lymph node metastasis and larger tumor size. BAP levels showed no significant changes post-surgery. Conclusions: These results suggest that d-ROMs could serve as a valuable biomarker for monitoring tumor burden and surgical efficacy in patients with colorectal cancer.
1. Introduction
Colorectal cancer (CRC) is a major global health burden, ranking as the third most commonly diagnosed cancer and second leading cause of cancer-related deaths worldwide. In 2020 alone, approximately 1.9 million new cases were diagnosed and 935,000 deaths were reported globally [1]. The standard treatment for CRC typically involves surgical resection aimed at the curative removal of the tumor along with the adjacent lymph nodes [2].
Oxidative stress (OS) is defined as an imbalance between reactive oxygen species (ROS) production and antioxidant defenses. This imbalance can arise from either excessive ROS production or a deficiency in the antioxidant system [3,4,5]. OS plays a role in the initiation, progression, metastasis, and prognosis of CRC, and is integral to cellular signaling processes. However, excessive OS can lead to DNA damage and the oxidation of critical lipids and proteins. These changes disrupt intracellular signaling, cause cellular mutations, impair cellular functions, and promote carcinogenesis [6,7]. Transformation from colorectal adenoma to carcinoma involves the activation of oncogenes, inactivation of tumor suppressor genes, and DNA methylation. These mutations are primarily induced by OS and have significant implications for cancer development [8,9].
The effect of OS on metastasis and tumor growth includes the induction of lipid peroxidation within the mitochondria, leading to the expression of cell cycle-activating proteins, cytokines, growth factors, and adhesion molecules that promote tumor growth and metastasis [10,11,12,13,14,15,16,17]. Reports on OS and CRC prognosis indicate that a high OS in CRC cases is associated with poor outcomes.
OS promotes telomerase activity in tumor cells and increases resistance to chemotherapy and radiotherapy, which may reduce survival rates [18,19,20,21,22,23,24]. Additionally, CRC tissues exhibit higher OS and antioxidant capacity than the surrounding normal tissues [25,26,27,28,29,30,31].
This is attributed to the strong inflammation observed in tumor tissues, where various cytokines are secreted, stimulating neutrophils and monocytes within the tumor and leading to a rapid increase in OS [18,19]. In addition, because of mitochondrial dysfunction, metabolic changes, and frequent genetic mutations, ROS production is significantly increased in cancer cells, resulting in a marked accumulation of oxidized proteins, DNA, and lipids [32,33]. The increase in antioxidant capacity is reported to result from the activation of genes coding for antioxidant enzymes as a cellular adaptation to OS [27]. Despite the observed close relationship between OS and CRC, few studies have examined changes in OS and antioxidant capacity following the surgical resection of CRC.
Recently, methods such as the measurement of reactive oxygen metabolite derivatives (d-ROMs) and biologic antioxidant potential (BAP) have been reported for the quantitative and stable measurement of OS and antioxidant capacity in blood [34]. We previously reported that blood d-ROM levels are associated with advanced tumor size and that high blood d-ROM levels are associated with a poorer prognosis in CRC cases [22,23,24].
In this study, we tested the hypothesis that physical removal of CRC, which is the source of OS, would reduce d-ROM levels. If a decrease is observed, d-ROM levels may reflect tumor burden and could be a valuable tumor marker.
2. Materials and Methods
2.1. Study Population
This study included 123 patients who underwent a radical resection for CRC at our institution between 2020 and 2023. Patients with synchronous or metachronous cancers, inflammatory bowel disease, immunosuppressive diseases, or severe medical illness, and those who underwent preoperative chemoradiation therapy, emergency surgery, or hemodialysis, were excluded. We collected data on patient age, sex, tumor location, tumor size, depth of tumor invasion, lymph node metastasis, and stage. The histopathological and clinical staging of tumors were assessed based on the TNM classification.
2.2. Measurement of d-ROMs and BAP Levels
We measured the d-ROM and BAP levels before and one month after surgery using blood samples collected during regular examinations.
Serum d-ROM and BAP levels were measured using a Free Radical Elective Evaluator system (FREE Carpe Diem, Wismerll Co., Ltd., Tokyo, Japan) that included a spectrophotometric device. Reader and measurement kits (d-ROMs and BAP test, Wismerll Co., Ltd.) were optimized for the FREE Carpe Diem System according to the manufacturer’s protocol. Briefly, in the d-ROM test, 20 µL of serum sample and 1 mL of buffered solution were carefully mixed in a cuvette, followed by the addition of 20 µL of chromogenic substrate. After thorough mixing, the cuvette was placed in the analyzer’s thermostatic block for 5 min at 37 °C, and absorbance was measured at 505 nm. Results were reported in arbitrary units (U.CARR), with each unit corresponding to 0.8 mg/L of hydrogen peroxide. The reference range was 250–300 U.CARR, and levels ≥ 300 U.CARR indicated serum OS due to excessive free radical production [5,35,36,37].
Briefly, in the BAP test, 10 µL serum samples were added to a 1 mL assay mixture, and the amount of trivalent iron deoxidized over 5 min was measured in µmol/L. When FeCl3 is dissolved in a colorless solution with a chelation acid derivative, it turns red due to Fe3+ ions. This red color is decolorized by the reduction of Fe3+ to Fe2+ ions caused by the antioxidant activity of the plasma. The antioxidant potential of plasma is evaluated by measuring the degree of decolorization with a spectrophotometer. The normal BAP value in healthy subjects is >2200 µmol/L [37].
2.3. Assessment of d-ROM and BAP Levels
The relationship between preoperative d-ROM levels, BAP levels, and clinicopathological factors was examined, as well as the relationship between preoperative and postoperative d-ROM and BAP levels. Additionally, the changes in d-ROM and BAP values before and after tumor resection in each stage were investigated. We also analyzed the d-ROM and BAP ratios (i.e., the ratio of d-ROM and BAP levels before and after treatment) and their relationship with clinicopathological factors.
2.4. Statistical Analysis
Continuous variables were analyzed using a Mann–Whitney U test and are expressed as the median (interquartile range). We conducted a multiple linear regression analysis to identify the factors that might influence preoperative d-ROM levels, preoperative BAP levels, d-ROM ratio, and BAP ratio. The factors examined included age, sex, tumor location, tumor size, serosa invasion, and lymph node metastasis. After establishing the final model, we calculated residuals and created a normal probability plot to evaluate the normality of these residuals. Spearman’s correlation coefficient analysis was employed to explore the relationships between d-ROM and BAP levels. All statistical analyses were conducted using IBM SPSS software version 21.0 (IBM Japan, Ltd., Tokyo, Japan), with significance set at p < 0.05.
3. Results
3.1. Patient Characteristics
The baseline demographic and clinicopathologic data of 123 patients with CRC are summarized in Table 1. In total, 22 cases had tumor depth T1, 20 had T2, 25 had T3, and 56 had T4. Additionally, three cases had stage 0, 38 had stage I, 40 had stage II, and 42 had stage III. Patients underwent curative resection with lymph node dissection at our institution between 2020 and 2023. Patients who had postoperative complications and those who received adjuvant chemotherapy within the first month after surgery were excluded from the analysis.

Table 1.
Comparison of preoperative d-ROM and BAP levels with clinicopathological factors in patients with colorectal cancer using the Mann–Whitney U test.
3.2. Comparison of Preoperative d-ROM and BAP Levels with Clinicopathological Factors in Patients with CRC
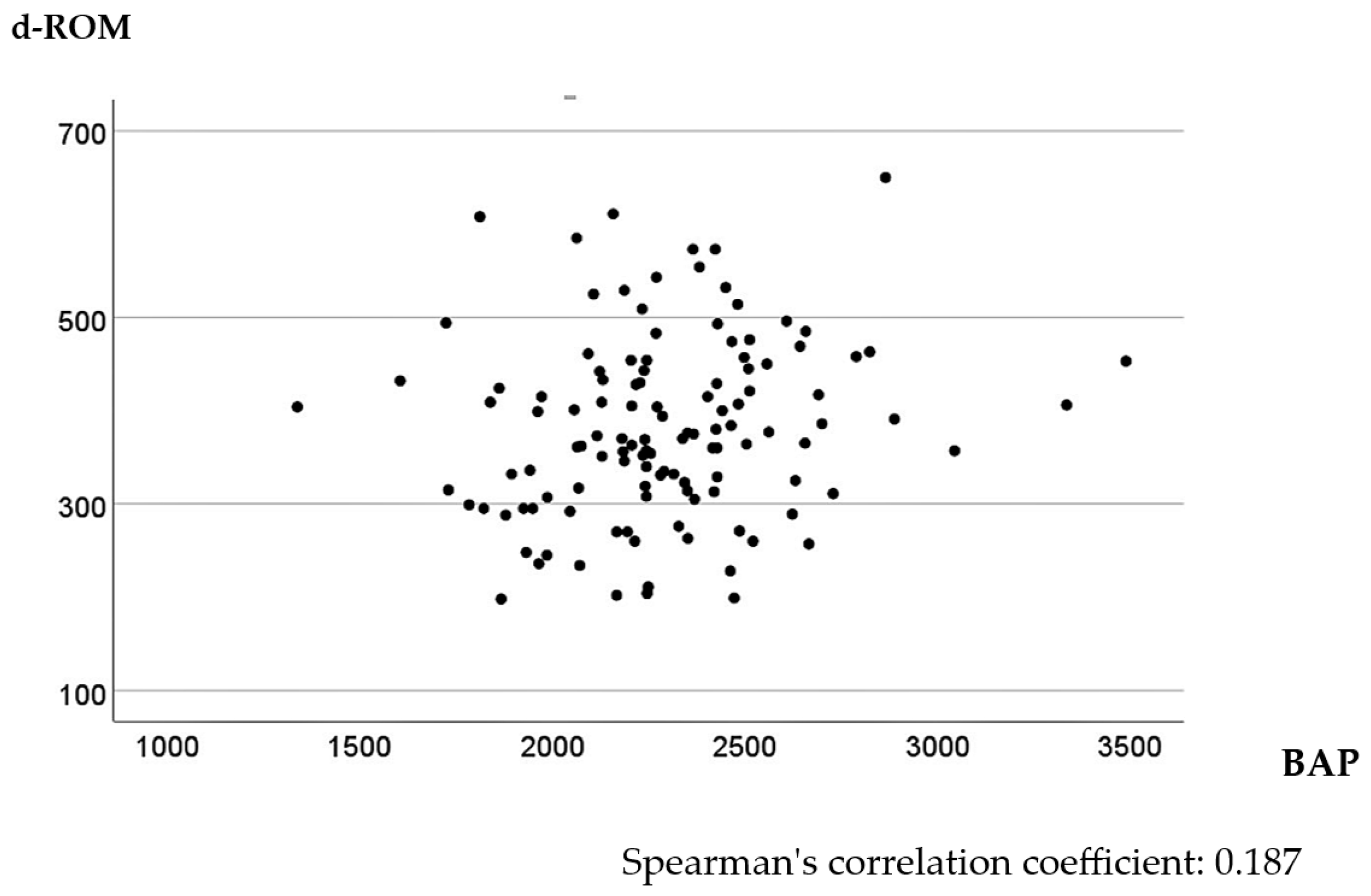
In the univariate analysis, higher d-ROM levels were significantly associated with tumor size > 45 mm, T3 or T4 tumor invasion depth, positive lymph node metastasis, and Stage II or III CRC (p < 0.001, <0.001, =0.004, and =0.005, respectively). The relationship between BAP values and clinicopathological factors was not significant (Table 1). The multivariate linear regression model identified tumor size as a significant explanatory variable for d-ROM level, with larger tumor sizes tending to increase d-ROM levels (Table 2). No factors were associated with BAP levels in the multivariate linear regression model (Table 3). Spearman’s correlation analysis of preoperative d-ROM and BAP values showed little correlation, with a correlation coefficient of 0.187 (p = 0.040) (Figure 1).

Table 2.
Multivariate linear regression analysis results for preoperative d-ROM levels in patients with colorectal cancer.

Table 3.
Multivariate linear regression analysis results for preoperative BAP levels in patients with colorectal cancer.
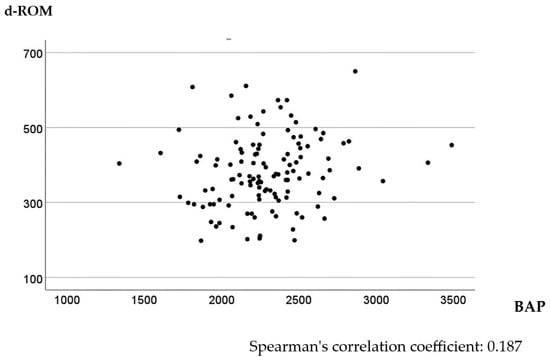
Figure 1.
Scatter plot of d-ROMs and BAP values before surgery.
3.3. Comparison of Postoperative d-ROM and BAP Levels with Clinicopathological Factors in Patients with CRC
No correlation was found between postoperative d-ROM levels and clinicopathological factors in both the univariate and multivariate analyses (Tables S1 and S2). Similarly, no correlation was observed between postoperative BAP values and clinicopathological factors (Tables S1 and S3). The Spearman’s correlation coefficient analysis of postoperative d-ROM and BAP levels showed a correlation coefficient of 0.279 (p < 0.001), indicating a weak correlation (Figure S1).
3.4. Changes in d-ROM and BAP Levels Following Resection
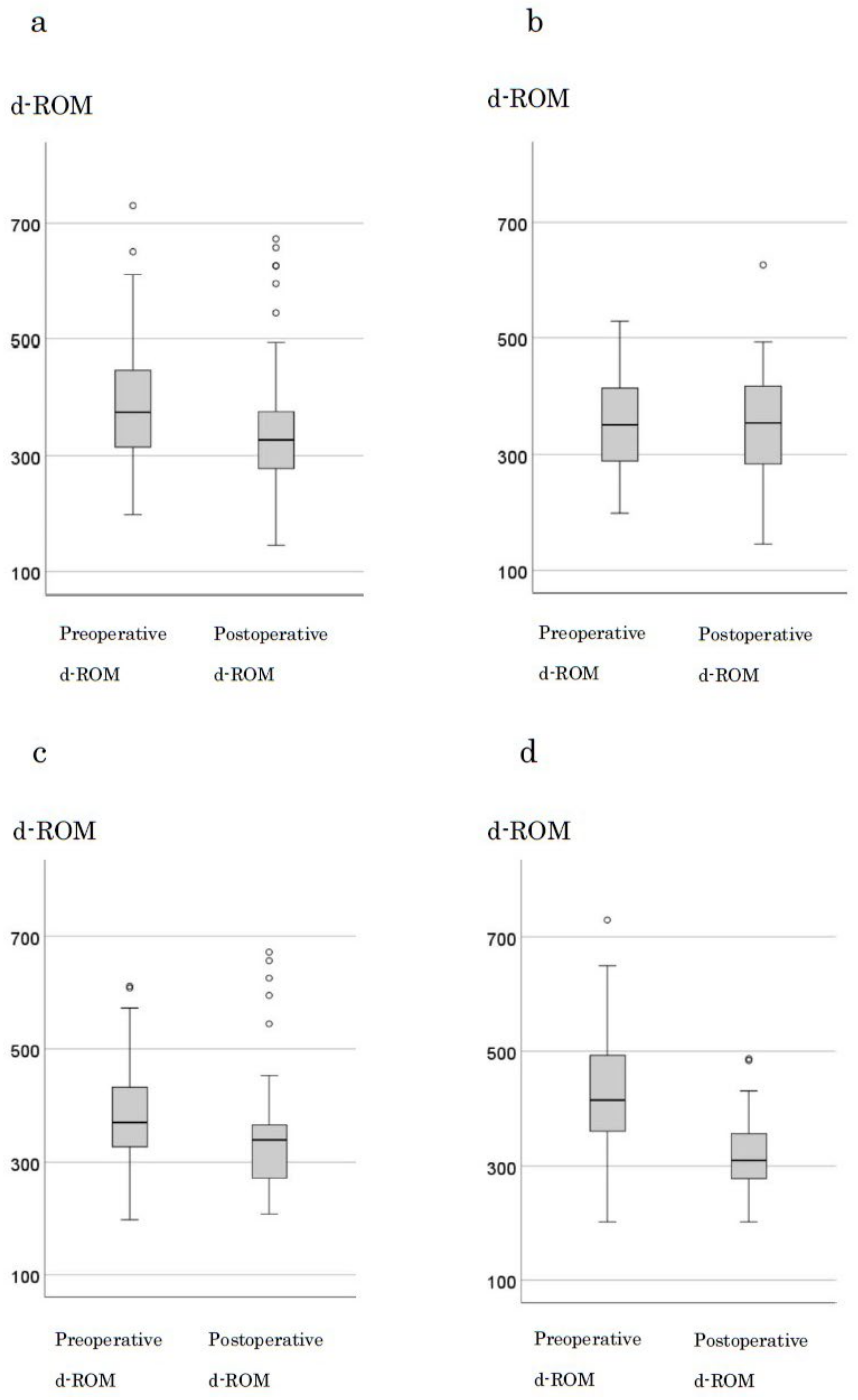
The median preoperative d-ROM and BAP levels in 123 cases were 375 and 327, respectively. A significant decrease in d-ROM levels was observed after tumor resection (p < 0.001) (Figure 2a). When examining changes by stage, in stage0 and I, the median preoperative (351) and postoperative (355) d-ROM levels were not significantly different (p = 0.707; Figure 2b). However, in stage II, the median preoperative and postoperative d-ROM levels were 370 and 339, respectively, with a significant decrease observed (p = 0.03) (Figure 2c). In stage III, the median preoperative and postoperative d-ROM levels were 415 and 310, respectively, with a significant decrease observed following tumor resection (p < 0.001; Figure 2d). BAP levels showed no significant changes following tumor resection with respect to the cases or stages (Figure S2a–d).
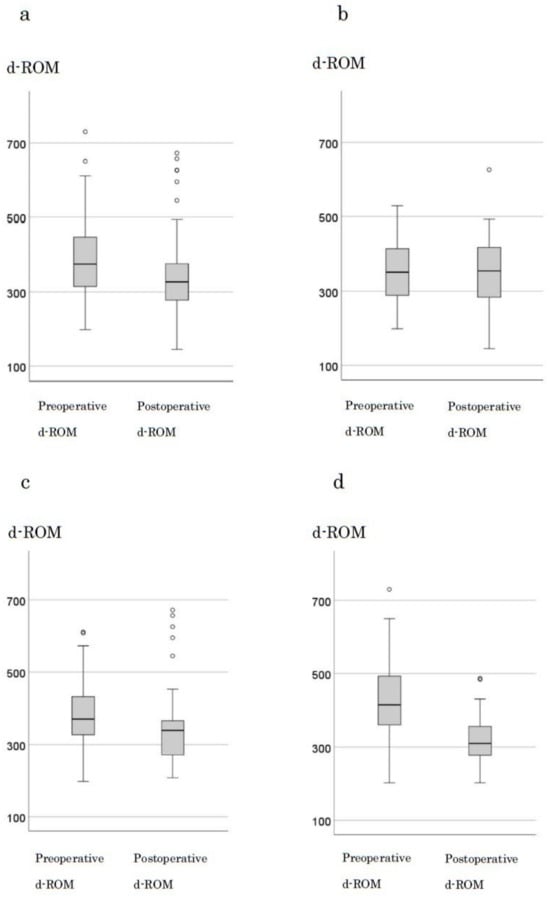
Figure 2.
Changes in d-ROM before and after surgery by stage. (a) all cases (b) stage 0, I cases (c) stage III cases (d) stage IV cases.
3.5. Comparison of Preoperative d-ROM and BAP Ratios with Clinicopathological Factors in Patients with CRC
In the univariate analysis, a higher d-ROM ratio was significantly associated with sex, tumor size > 45 mm, T3 or T4 tumor invasion depth, positive lymph node metastasis, and stage II or III (p = 0.018, <0.001, <0.001, <0.001, and =0.005, respectively). No relationship between the BAP ratio and clinicopathological factors was recognized (Table 4). The multivariate linear regression model identified sex, tumor size, and lymph node metastasis as significant explanatory variables for the d-ROM ratio. A large tumor size and lymph node metastasis tended to increase the d-ROM ratio (Table 5). No factors were associated with the BAP ratio in the multivariate linear regression model (Table S4).

Table 4.
Comparison of clinicopathological factors with d-ROMs and BAP ratio in colorectal cancer patients using Mann–Whitney U test.

Table 5.
Multivariate linear regression analysis results for d-ROM ratio in patients with colorectal cancer.
4. Discussion
Three key findings were obtained from this study. First, a reduction in the OS marker, d-ROM, was observed following tumor resection. Second, d-ROM level was correlated with tumor size. Third, although BAP level, indicating antioxidant capacity, showed a weak correlation with d-ROM levels, no significant change was observed in BAP levels following tumor resection. The increase in OS in colorectal tumor tissue was reported by Chiang et al., who measured advanced oxidation protein products and found higher levels compared to surrounding normal tissue [26], and this observation is supported by other studies [27,28,29,30,31]. The mechanisms behind the increase in OS in tumor tissue are attributed to severe inflammation observed in these tissues, where various cytokines are secreted, stimulating neutrophils and monocytes within the tumor and rapidly increasing OS [18,19]. Furthermore, mitochondrial dysfunction, metabolic changes, and frequent genetic mutations in cancer cells significantly increase ROS production, leading to the accumulation of oxidized proteins, DNA, and lipids [10,38]. Reports indicate that patients with CRC have higher OS levels compared to healthy individuals [17,38,39,40,41,42].
It has been hypothesized that the tumor microenvironment (TME), which is composed of various factors, influences systemic OS, and that removing the TME through surgery reduces systemic OS. Salehi et al. measured changes in OS markers’ MDA, ox-LDL, and AGEs before and after tumor resection in 60 patients with CRC (stages I and II) and found that these levels decreased postoperatively [38]. Similarly, Acevedo-León et al. reported a decrease in OS levels based on the disulfide form of the tripeptide L-glutamylcysteinylglycine (GSSG) in 79 patients with CRC (stages I–IV) following resection [43]. Hristozov et al. found a significant decrease in erythrocyte MDA levels postoperatively compared to preoperatively [44].
We previously reported a reduction in d-ROM levels following tumor reduction by anticancer agents [9]. These results highlight the potential of d-ROMs as a valuable biomarker for tumor burden and provide insights into the role of OS in the progression and prognosis of CRC. In our previous report, we demonstrated a correlation between blood d-ROM levels and tumor size [36]. Inokuma et al. also reported a correlation between blood d-ROM levels and tumor size [45]. In the present study, a multivariate analysis confirmed the correlation between tumor size and d-ROM levels. The preoperative d-ROM level was significantly associated with tumor size, depth of invasion (T3 or T4), lymph node metastasis, and advanced stage (II and III), consistent with previous studies reporting higher OS levels in more aggressive advanced cancers [36].
Tumor size was a significant explanatory variable for d-ROM levels in the multivariate analysis, indicating that larger tumors produce more ROS and increase systemic OS. The significant reduction in d-ROM levels following CRC resection suggests that removing the tumor, which is the main source of ROS, notably reduces systemic OS. This decrease was particularly pronounced in patients with stage II and III CRC, suggesting that tumor burden plays a crucial role in systemic OS levels. Salehi et al. reported a reduction in OS following CRC resection, but did not confirm the association between preoperative OS levels and clinicopathological factors, possibly due to the limited sample size of 60 stage I and II cases [38].
The findings on antioxidant markers following tumor resection are inconsistent, with some studies reporting a decrease and others showing an increase. For instance, Salehi et al. reported an increase in antioxidant factors after the resection of high oxidative stress (OS) tumor tissue [38], whereas Acevedo-León et al. documented a decrease in antioxidant factors following similar procedures [43]. In our study, we observed a weak correlation between d-ROM and BAP levels, suggesting that the decrease in d-ROM levels post-resection might lead to a decrease in BAP levels. However, no significant change in BAP levels was detected after surgery, despite the reduction in ROS production indicated by lower d-ROM levels. This suggests that while tumor removal reduces ROS production, the overall systemic antioxidant capacity, as measured by BAP, remains largely unaffected.
Additionally, no correlation was found between BAP levels and clinicopathological factors, in contrast to the clear correlation between d-ROM levels and tumor progression. It is plausible that BAP, as a marker of antioxidant potential, is less influenced by the tumor itself and more dependent on other physiological mechanisms, explaining the lack of change in BAP levels post-resection. Previous studies have reported that antioxidant capacity can increase when cells are stimulated by heightened OS [46], but our results suggest that strategies focusing on reducing ROS production, rather than enhancing antioxidant defenses, may be more effective in managing OS in patients with CRC.
Despite the strengths of this study, some limitations are acknowledged. First, the measurement of d-ROMs as a marker of oxidative stress may lack sufficient sensitivity in early-stage colorectal cancer, where its clinical significance appears limited. However, d-ROMs are considered to be effective in advanced colorectal cancer, and their utility in assessing treatment efficacy has also been demonstrated in stage IV cases, as previously reported in [36]. Although this study included a larger sample size compared to other reports examining changes in oxidative stress (OS) following colorectal cancer (CRC) resection, and allowed for multivariate analysis, the cohort was limited to patients from a single institution, which may restrict the generalizability of the findings. Furthermore, long-term follow-up is required to assess the sustained impact of tumor resection on OS and antioxidant capacity. Additionally, while a significant reduction in d-ROM levels was observed post-resection, the precise mechanisms driving this reduction remain unclear. Future research should focus on elucidating the molecular pathways responsible for the reduction in OS following CRC resection and explore the potential benefits of combining surgical resection with other therapeutic strategies to further mitigate OS and improve patient outcomes.
5. Conclusions
The physical removal of CRC, a primary source of OS, reduces blood levels of d-ROMs, a marker of OS. These results suggest that d-ROMs could serve as a valuable biomarker for monitoring tumor burden and the efficacy of surgery in patients with CRC.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers16203550/s1, Figure S1: Scatter plot of d-ROMs and BAP values after surgery; Figure S2: Changes in d-ROM Before and After Surgery by Stage; Table S1: Comparison of postoperative d-ROM and BAP values with clinicopathological factors in colorectal cancer patients using the Mann-Whitney U test; Table S2: Multivariate linear regression analysis results for postoperative d-ROM levels in patients with colorectal cancer; Table S3: Multivariate linear regression analysis results for postoperative d-ROM levels in patients with colorectal cancer; Table S4: Multivariate linear regression analysis results for BAP ratio in patients with colorectal cancer.
Author Contributions
K.S. and T.G. were involved in the study design and data interpretation. Y.K. and K.K. were involved in the data analysis. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no external funding was received for this study.
Institutional Review Board Statement
The protocol for this research project was approved by a suitable Institutional Ethics Committee and conformed to the provisions of the Declaration of Helsinki. The Research Ethics Committee of the University of Fukui approved the study on 1 April 2017 (Approval No. 20200058, 1 April 2017).
Informed Consent Statement
Written informed consent was obtained from participants prior to the study.
Data Availability Statement
All data included in this study are available from the corresponding author upon request.
Acknowledgments
The authors thank Masae Saitoh for the assistance in the patient data collection and administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ros) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Carini, F.; Mazzola, M.; Rappa, F.; Jurjus, A.; Geagea, A.G.; Al Kattar, S.; Bou-Assi, T.; Jurjus, R.; Damiani, P.; Leone, A.; et al. Colorectal carcinogenesis: Role of oxidative stress and antioxidants. Anticancer Res. 2017, 37, 4759–4766. [Google Scholar]
- Bardelcíková, A.; Šoltys, J.; Mojžiš, J. Oxidative stress, inflammation and colorectal cancer: An overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Perše, M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? BioMed Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef]
- Kang, M.; Jeong, S.; Park, S.; Nam, S.; Chung, J.W.; Kim, K.O.; An, J.; Kim, J.H. Significance of 8-OHdG Expression as a Predictor of Survival in Colorectal Cancer. Cancers 2023, 15, 4613. [Google Scholar] [CrossRef]
- Sawai, K.; Goi, T.; Kimura, Y.; Koneri, K. Monitoring metastatic colorectal cancer progression according to reactive oxygen metabolite derivative levels. Cancers 2023, 15, 5517. [Google Scholar] [CrossRef]
- Delimaris, I.; Faviou, E.; Antonakos, G.; Stathopoulou, E.; Zachari, A.; Dionyssiou-Asteriou, A. Oxidized ldl, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin. Biochem. 2007, 40, 1129–1134. [Google Scholar] [CrossRef]
- Zabirnyk, O.; Liu, W.; Khalil, S.; Sharma, A.; Phang, J.M. Oxidized low-density lipoproteins upregulate proline oxidase to initiate ros-dependent autophagy. Carcinogenesis 2010, 31, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Sanjuán, J.; Calvo-Nieves, M.D.; Aguirre-Gervás, B.; Herreros-Rodríguez, J.; Velayos-Jiménez, B.; Castro-Alija, M.J.; Muñoz-Moreno, M.F.; Sánchez, D.; Zamora-González, N.; Bajo-Grañeras, R.; et al. Early detection of high oxidative activity in patients with adenomatous intestinal polyps and colorectal adenocarcinoma: Myeloperoxidase and oxidized low-density lipoprotein in serum as new markers of oxidative stress in colorectal cancer. Lab. Med. 2015, 46, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zettler, M.E.; Prociuk, M.A.; Austria, J.A.; Massaeli, H.; Zhong, G.M.; Pierce, G.N. Oxldl stimulates cell proliferation through a general induction of cell cycle proteins. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H644–H653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bitorina, A.V.; Oligschlaeger, Y.; Shiri-Sverdlov, R.; Theys, J. Low profile high value target: The role of oxLDL in cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158518. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, T.; Haraguchi, M.; Fujita, F.; Tajima, Y.; Kanematsu, T. Oxidative stress and tumor progression in colorectal cancer. Hepato-Gastroenterology 2009, 56, 343–347. [Google Scholar]
- Inokuma, T.; Haraguchi, M.; Fujita, F.; Torashima, Y.; Eguchi, S.; Kanematsu, T. Suppression of reactive oxygen species develops lymph node metastasis in colorectal cancer. Hepato-Gastroenterology 2012, 59, 2480–2483. [Google Scholar]
- Wu, R.; Feng, J.F.; Yang, Y.W.; Dai, C.M.; Lu, A.Y.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.M.; Wang, D.; et al. Significance of serum total oxidant/antioxidant status in patients with colorectal cancer. PLoS ONE 2017, 12, e0170003. [Google Scholar] [CrossRef]
- Afanas’ev, I. Reactive oxygen species signaling in cancer: Comparison with aging. Aging Dis. 2011, 2, 219–230. [Google Scholar]
- Rodic, S.; Vincent, M.D. Reactive oxygen species (ros) are a key determinant of cancer’s metabolic phenotype. Int. J. Cancer 2018, 142, 440–448. [Google Scholar] [CrossRef]
- Rahman, S.H.; Ammori, B.J.; Holmfield, J.; Larvin, M.; McMahon, M.J. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J. Gastrointest. Surg. 2003, 7, 26–36. [Google Scholar] [CrossRef]
- Li, P.Y.; Wu, M.L.; Wang, J.; Sui, Y.L.; Liu, S.L.; Shi, D.Y. Nac selectively inhibit cancer telomerase activity: A higher redox homeostasis threshold exists in cancer cells. Redox Biol. 2016, 8, 91–97. [Google Scholar] [CrossRef]
- Floyd, R.A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis 1990, 11, 1447–1450. [Google Scholar] [CrossRef]
- Hussain, S.P.; Aguilar, F.; Amstad, P.; Cerutti, P. Oxy-radical induced mutagenesis of hotspot codons 248 and 249 of the human p53 gene. Oncogene 1994, 9, 2277–2281. [Google Scholar]
- Collado, R.; Oliver, I.; Tormos, C.; Egea, M.; Miguel, A.; Cerdá, C.; Ivars, D.; Borrego, S.; Carbonell, F.; Sáez, G.T. Early ros-mediated DNA damage and oxidative stress biomarkers in monoclonal B lymphocytosis. Cancer Lett. 2012, 317, 144–149. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. Ros stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Chiang, F.F.; Chao, T.H.; Huang, S.C.; Cheng, C.H.; Tseng, Y.Y.; Huang, Y.C. Cysteine regulates oxidative stress and glutathione-related antioxidative capacity before and after colorectal tumor resection. Int. J. Mol. Sci. 2022, 23, 9581. [Google Scholar] [CrossRef]
- Strzelczyk, J.K.; Wielkoszyński, T.; Krakowczyk, Ł.; Adamek, B.; Zalewska-Ziob, M.; Gawron, K.; Kasperczyk, J.; Wiczkowski, A. The activity of antioxidant enzymes in colorectal adenocarcinoma and corresponding normal mucosa. Acta Biochim. Pol. 2012, 59, 549–556. [Google Scholar] [CrossRef]
- Ozdemirler, G.; Pabuççuoglu, H.; Bulut, T.; Bugra, D.; Uysal, M.; Toker, G. Increased lipoperoxide levels and antioxidant system in colorectal cancer. J. Cancer Res. Clin. Oncol. 1998, 124, 555–559. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Sulkowski, S.; Koda, M.; Zalewski, B.; Kanczuga-Koda, L.; Sulkowska, M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403–406. [Google Scholar] [CrossRef]
- Rainis, T.; Maor, I.; Lanir, A.; Shnizer, S.; Lavy, A. Enhanced oxidative stress and leucocyte activation in neoplastic tissues of the colon. Dig. Dis. Sci. 2007, 52, 526–530. [Google Scholar] [CrossRef]
- Kekec, Y.; Paydas, S.; Tuli, A.; Zorludemir, S.; Sakman, G.; Seydaoglu, G. Antioxidant enzyme levels in cases with gastrointesinal cancer. Eur. J. Intern. Med. 2009, 20, 403–406. [Google Scholar] [CrossRef]
- Demple, B.; Harrison, L. Repair of oxidative damage to DNA: Enzymology and biology. Annu. Rev. Biochem. 1994, 63, 915–948. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Sugimoto, K.; Sakamoto, K.; Kawai, M.; Kawano, S.; Munakata, S.; Ishiyama, S.; Takahashi, M.; Kojima, Y.; Tomiki, Y. Serum oxidative stress is an independent prognostic marker in colorectal cancer. Transl. Cancer Res. 2019, 8, 1699–1708. [Google Scholar] [CrossRef]
- Kilk, K.; Meitern, R.; Härmson, O.; Soomets, U.; Hõrak, P. Assessment of oxidative stress in serum by d-roms test. Free Radic. Res. 2014, 48, 883–889. [Google Scholar] [CrossRef]
- Sawai, K.; Goi, T.; Sakamoto, S.; Matsunaka, T.; Maegawa, N.; Koneri, K. Oxidative stress as a biomarker for predicting the prognosis of patients with colorectal cancer. Oncology 2022, 100, 612–619. [Google Scholar] [CrossRef]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002, 44, 37–40. [Google Scholar]
- Salehi, S.S.; Mirmiranpour, H.; Rabizadeh, S.; Esteghamati, A.; Tomasello, G.; Alibakhshi, A.; Najafi, N.; Rajab, A.; Nakhjavani, M. Improvement in redox homeostasis after cytoreductive surgery in colorectal adenocarcinoma. Oxid. Med. Cell. Longev. 2021, 2021, 8864905. [Google Scholar] [CrossRef]
- Acevedo-León, D.; Gómez-Abril, S.Á.; Sanz-García, P.; Estañ-Capell, N.; Bañuls, C.; Sáez, G. The role of oxidative stress, tumor and inflammatory markers in colorectal cancer patients: A one-year follow-up study. Redox Biol. 2023, 62, 102662. [Google Scholar] [CrossRef]
- Yücel, A.F.; Kemik, O.; Kemik, A.S.; Purisa, S.; Tüzün, I.S. Relationship between the levels of oxidative stress in mesenteric and peripheral serum and clinicopathological variables in colorectal cancer. Balk. Med. J. 2012, 29, 144–147. [Google Scholar] [CrossRef]
- Hendrickse, C.W.; Kelly, R.W.; Radley, S.; Donovan, I.A.; Keighley, M.R.; Neoptolemos, J.P. Lipid peroxidation and prostaglandins in colorectal cancer. Br. J. Surg. 1994, 81, 1219–1223. [Google Scholar] [CrossRef]
- Skrzydewska, E.; Stankiewicz, A.; Michalak, K.; Sulkowska, M.; Zalewski, B.; Piotrowski, Z. Antioxidant status and proteolytic-antiproteolytic balance in colorectal cancer. Folia Histochem. Cytobiol. 2001, 39 (Suppl. S2), 98–99. [Google Scholar]
- Acevedo-León, D.; Monzó-Beltrán, L.; Gómez-Abril, S.Á.; Estañ-Capell, N.; Camarasa-Lillo, N.; Pérez-Ebri, M.L.; Escandón-Álvarez, J.; Alonso-Iglesias, E.; Santaolaria-Ayora, M.L.; Carbonell-Moncho, A.; et al. The effectiveness of glutathione redox status as a possible tumor marker in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6183. [Google Scholar] [CrossRef]
- Hristozov, D.; Gadjeva, V.; Vlaykova, T.; Dimitrov, G. Evaluation of oxidative stress in patients with cancer. Arch. Physiol. Biochem. 2001, 109, 331–336. [Google Scholar] [CrossRef]
- Okur, H.K.; Yuksel, M.; Lacin, T.; Baysungur, V.; Okur, E. Detection of reactive oxygen metabolites in malignant and adjacent normal tissues of patients with lung cancer. World J. Surg. Oncol. 2013, 11, 9. [Google Scholar] [CrossRef]
- Colon Cancer Laparoscopic or Open Resection Study Group; Buunen, M.; Veldkamp, R.; Hop, W.C.J.; Kuhry, E.; Jeekel, J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).