Receptor for Hyaluronan Mediated Motility (RHAMM)/Hyaluronan Axis in Breast Cancer Chemoresistance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissues
2.2. Immunohistochemistry
2.3. Scoring of RHAMM Immunoreactivity and Other Histochemical Parameters
2.4. Cell Lines and Chemicals
2.5. Establishment of EPI-Resistant Breast Cancer Cell Lines
2.6. Real Time PCR
2.7. Immunoblotting
2.8. Small Interfering RNA (siRNA) Transfection
2.9. Cell Proliferation Assay
2.10. Wound Healing Assay
2.11. Statistical Analysis
3. Results
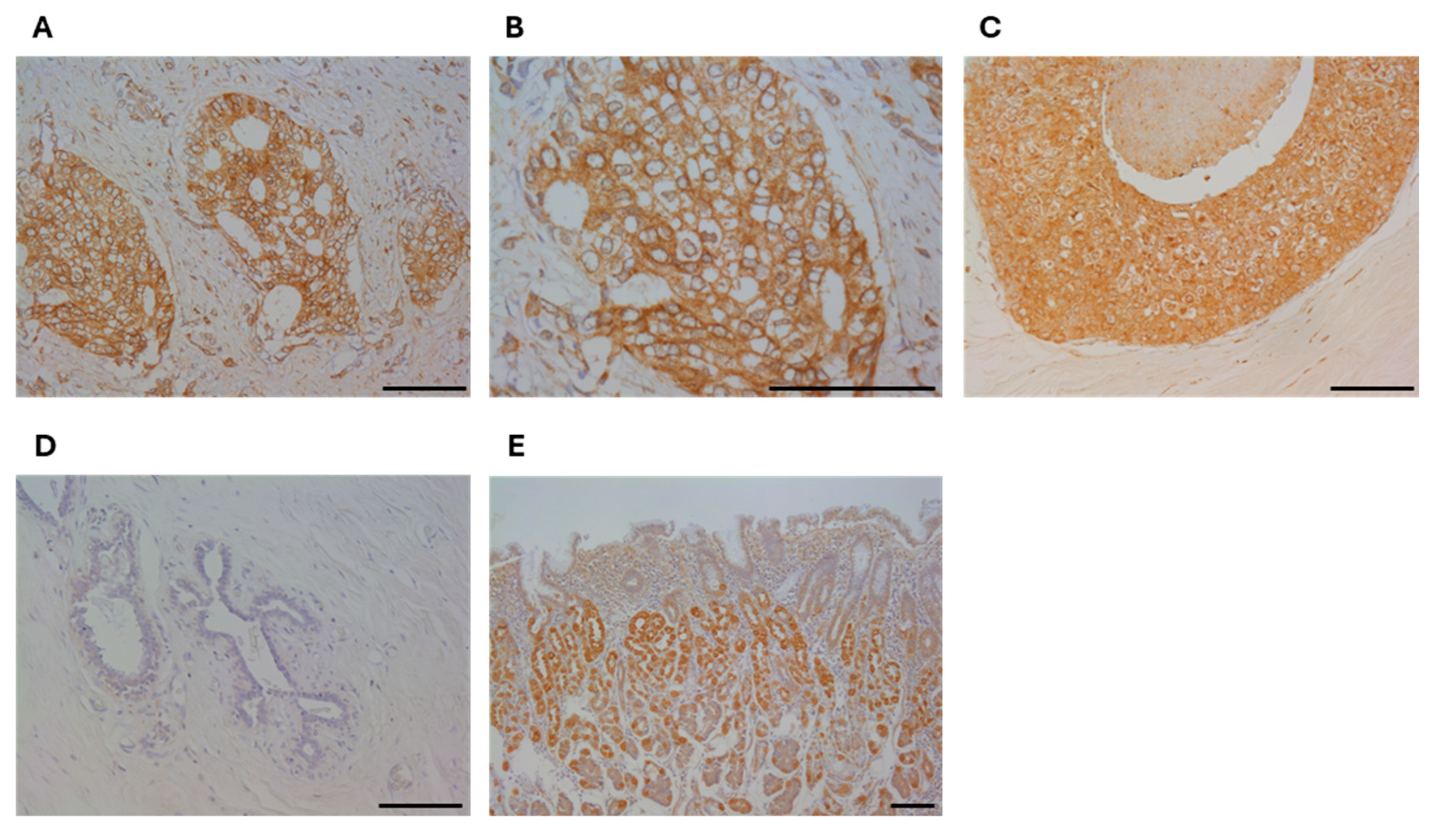
3.1. Immunolocalization of RHAMM in Human Breast Carcinoma Tissues
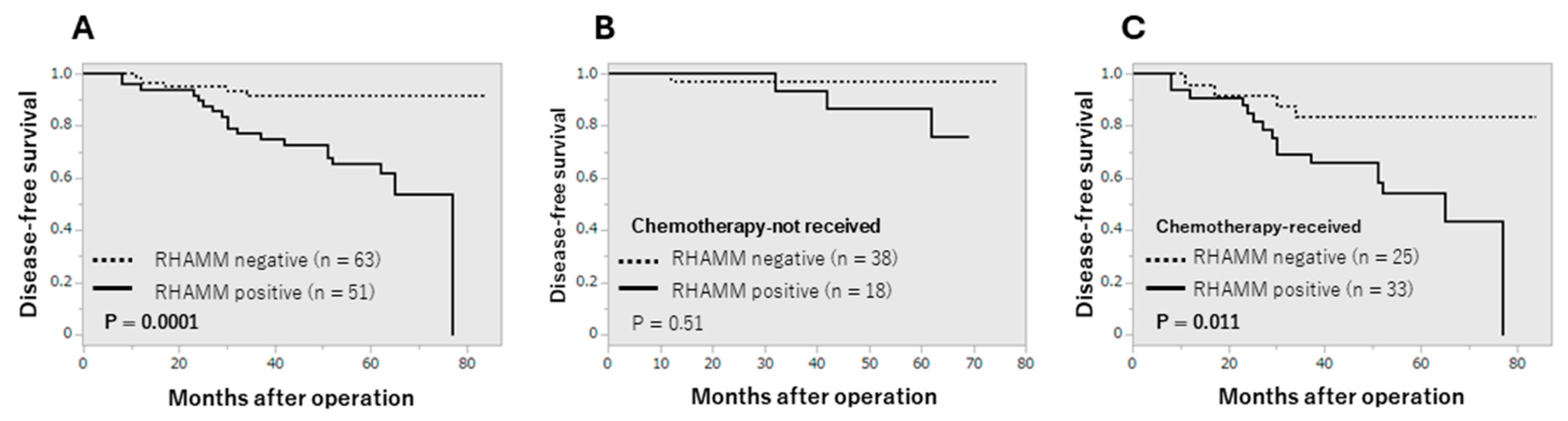
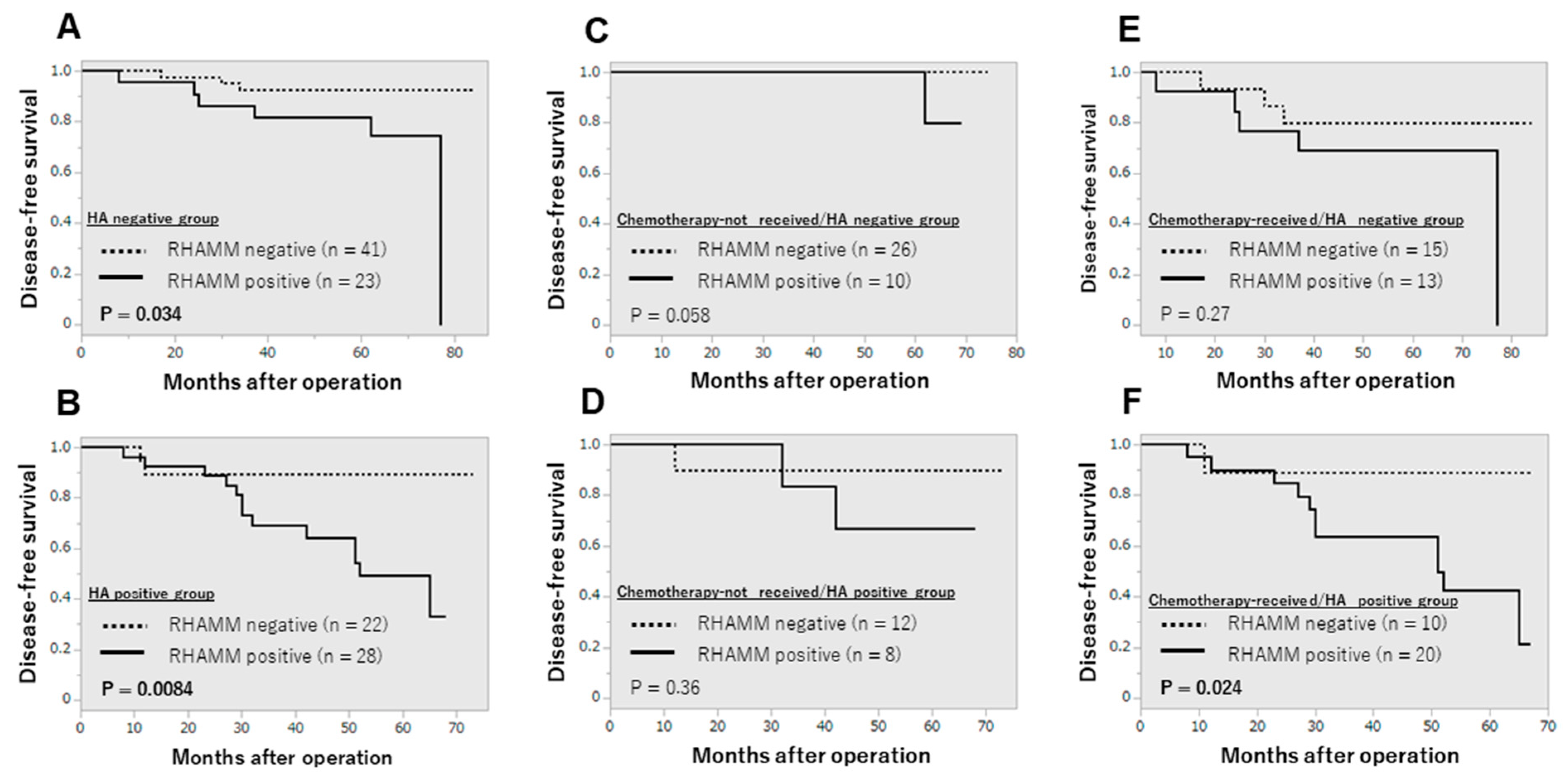
3.2. Clinical Significance of RHAMM
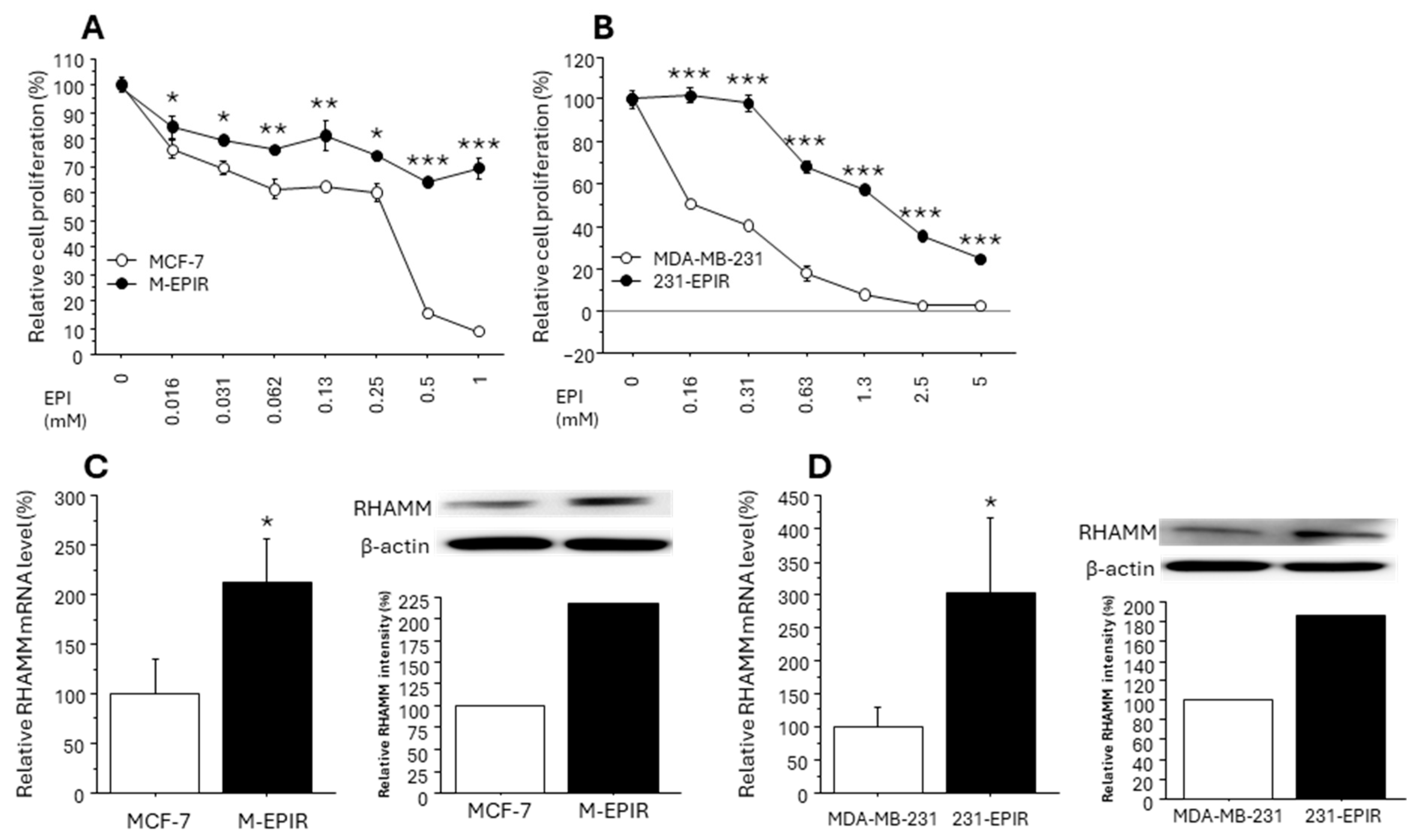
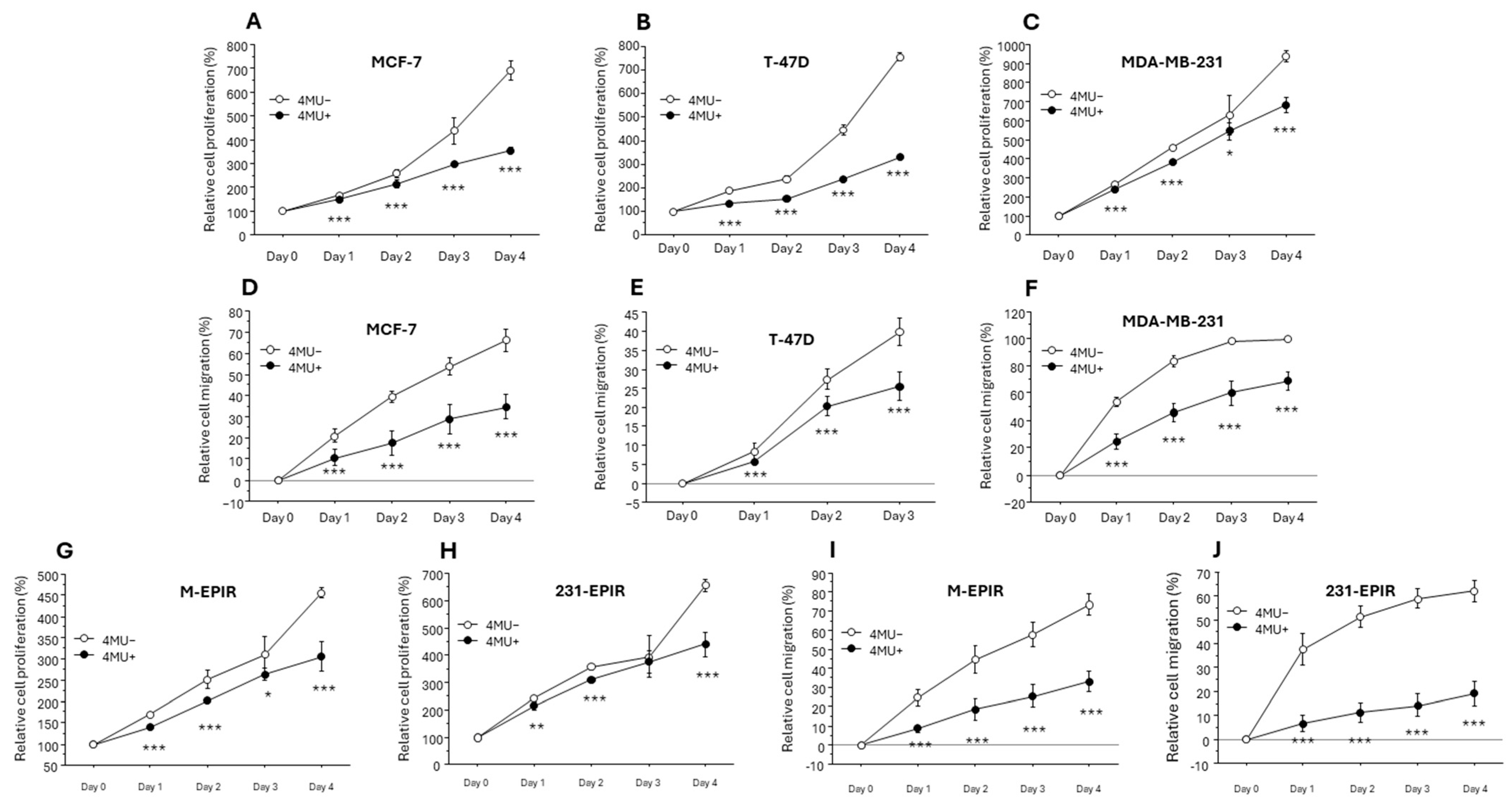
3.3. Increased RHAMM Expression in EPI-Resistant Breast Cancer Cell Lines
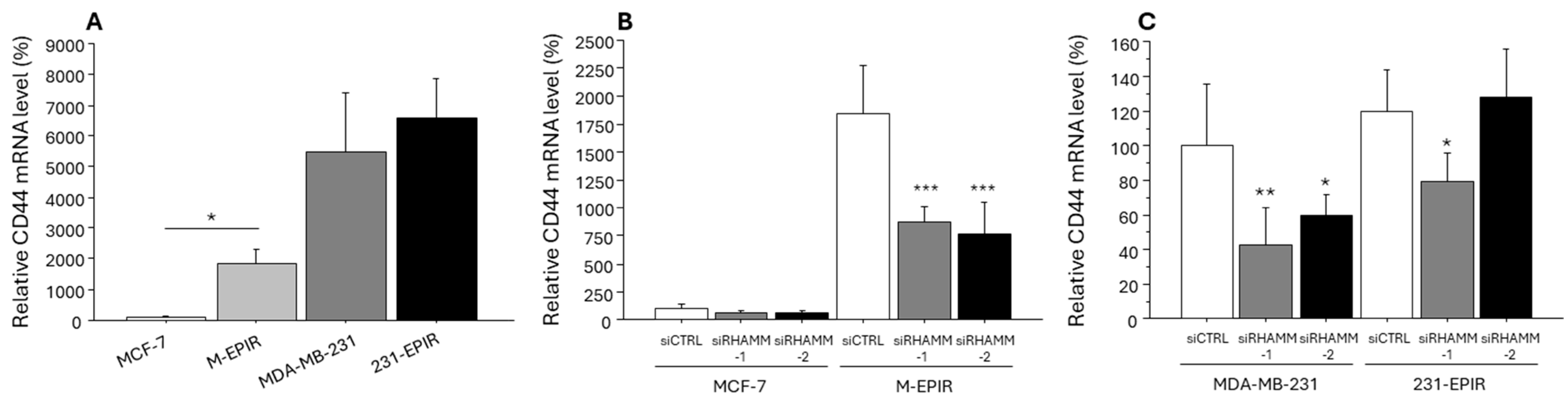
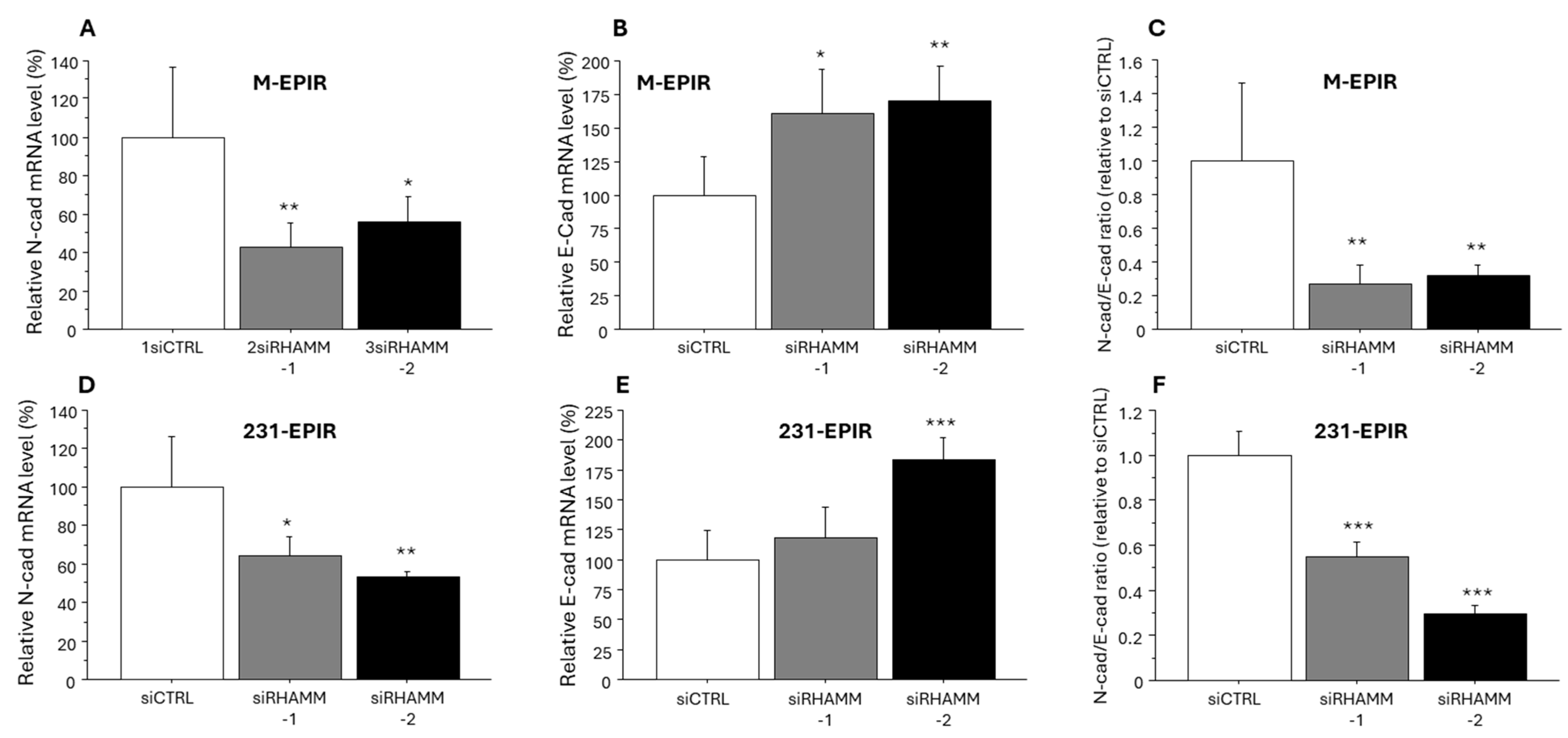
3.4. Possible Regulation of Cancer Stem Cell Marker/Another HA Receptor CD44 and EMT-Related Marker by RHAMM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Iwuarwerk, A.J.; Gray, R.J.; Schneider, B.P.; Smith, M.L.; Wagner, L.I.; Fetting, J.H.; Davidson, N.; Goldstein, L.J.; Miller, K.D.; Sparano, J.A. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: Little evidence of improvement over the past 30 years. Cancer 2013, 119, 1140–1148. [Google Scholar]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular matrix-mediated regulation of cancer stem cells and chemoresistance. Int. J. Biochem. Cell Biol. 2019, 109, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Kosma, V.M.; Sironen, R.; Soini, Y.; Mannermaa, A.; Tumelius, R.; Uljas, E.; Tammi, M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int. J. Cancer 2013, 132, 531–539. [Google Scholar] [CrossRef]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, inflammation, and breast cancer progression. Front. Immunol. 2015, 6, 236–248. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Toole, B.P.; Slomiany, M.G. Hyaluronan: A constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008, 18, 244–250. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, P.; Fang, J.; Shao, C.; Shi, Y.; Melino, G.; Peschiaroli, A. Hyaluronic acid metabolism and chemotherapy resistance: Recent advances and therapeutic potential. Mol. Oncol. 2023, 18, 2087–2106. [Google Scholar] [CrossRef]
- Kouvidi, K.; Berdiaki, A.; Nikitovic, D.; Katonis, P.; Afratis, N.; Hascall, V.C.; Karamanos, N.K.; Tzanakakis, G.N. Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J. Biol. Chem. 2011, 286, 38509–38520. [Google Scholar] [CrossRef]
- Schatz-Siemers, N.; Chen, Y.T.; Chen, Z.; Wang, D.; Ellenson, L.H.; Du, Y.N. Expression of the Receptor for Hyaluronic Acid-Mediated Motility (RHAMM) in Endometrial Cancer is Associated with Adverse Histologic Parameters and Tumor Progression. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.W.; Wang, S.; Wu, Z.Z.; Yang, Q.C.; Chen, D.R.; Wan, S.C.; Sun, Z.J. Overexpression of CD168 is related to poor prognosis in oral squamous cell carcinoma. Oral Dis. 2022, 28, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Minato, A.; Kudo, Y.; Noguchi, H.; Kohi, S.; Hasegawa, Y.; Sato, N.; Hirata, K.; Fujimoto, N. Receptor for Hyaluronic Acid-mediated Motility (RHAMM) Is Associated with Prostate Cancer Migration and Poor Prognosis. Cancer Genom. Proteom. 2023, 20, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Thor, A.D.; Moore, D.H.; Zhao, Y.; Kerschmann, R.; Stern, R.; Watson, P.H.; Turley, E.A. The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated protein kinase and is a significant parameter in breast cancer progression. Clin. Cancer Res. 1998, 4, 567–576. [Google Scholar] [PubMed]
- Schütze, A.; Vogeley, C.; Gorges, T.; Twarock, S.; Butschan, J.; Babayan, A.; Klein, D.; Knauer, S.K.; Metzen, E.; Müller, V.; et al. RHAMM splice variants confer radiosensitivity in human breast cancer cell lines. Oncotarget 2016, 7, 21428–21440. [Google Scholar] [CrossRef]
- Kahl, I.; Mense, J.; Finke, C.; Boller, A.L.; Lorber, C.; Győrffy, B.; Greve, B.; Götte, M.; Espinoza-Sánchez, N.A. The cell cycle-related genes RHAMM, AURKA, TPX2, PLK1, and PLK4 are associated with the poor prognosis of breast cancer patients. J. Cell Biochem. 2022, 123, 581–600. [Google Scholar] [CrossRef]
- Sasaki, Y.; Miki, Y.; Hirakawa, H.; Onodera, Y.; Takagi, K.; Akahira, J.; Honma, S.; Ishida, T.; Watanabe, M.; Sasano, H.; et al. Immunolocalization of estrogen-producing and metabolizing enzymes in benign breast disease: Comparison with normal breast and breast carcinoma. Cancer Sci. 2010, 101, 2286–2292. [Google Scholar] [CrossRef]
- Takagi, K.; Miki, Y.; Onodera, Y.; Ishida, T.; Watanabe, M.; Sasano, H.; Suzuki, T. ARHGAP15 in Human Breast Carcinoma: A Potent Tumor Suppressor Regulated by Androgens. Int. J. Mol. Sci. 2018, 19, 804. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Huber, B.; Mele, V.; Iezzi, G.; Trippel, M.; Karamitopoulou, E.; Zlobec, I.; Lugli, A. Expression of the hyaluronan-mediated motility receptor RHAMM in tumor budding cells identifies aggressive colorectal cancers. Hum. Pathol. 2015, 46, 1573–1581. [Google Scholar] [CrossRef]
- Sato, A.; Takagi, K.; Yoshimura, A.; Tsukamoto, W.; Yamaguchi-Tanaka, M.; Miki, Y.; Ebata, A.; Miyashita, M.; Suzuki, T. Kallikrein-Related Peptidase 12 (KLK12) in Breast Cancer as a Favorable Prognostic Marker. Int. J. Mol. Sci. 2023, 24, 8419. [Google Scholar] [CrossRef]
- Fujisawa, S.; Takagi, K.; Yamaguchi-Tanaka, M.; Sato, A.; Miki, Y.; Miyashita, M.; Tada, H.; Ishida, T.; Suzuki, T. Clinicopathological significance of hyaluronan and hyaluronidase 2 (HYAL2) in breast cancer. Pathol. Res. Pract. 2024, 260, 155434. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 17, 4287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Shen, W.; Sun, W.; Gao, R.; Jiang, P.; Wang, L.; Liu, Y.; Chen, Y.; Zhou, W.; et al. RHAMM inhibits cell migration via the AKT/GSK3β/Snail axis in luminal A subtype breast cancer. Anat. Rec. 2020, 9, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Milojevic, M.; Qi, F.W.; Pavanel, H.A.; Locke, M.E.O.; Ma, J.; Price, M.; Nelson, A.C.; McCarthy, J.B.; Hill, K.A.; et al. RHAMM regulates MMTV-PyMT-induced lung metastasis by connecting STING-dependent DNA damage sensing to interferon/STAT1 pro-apoptosis signaling. Breast Cancer Res. 2023, 1, 74–91. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Soares da Costa, D.; Paulo, P.M.R.; Reis, R.L.; Pashkuleva, I. Co-localization and crosstalk between CD44 and RHAMM depend on hyaluronan presentation. Acta Biomater. 2021, 119, 114–124. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, L.; Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Gao, F. A novel role of breast cancer-derived hyaluronan on inducement of M2-like tumor-associated macrophages formation. Oncoimmunology 2016, 6, e1172154. [Google Scholar] [CrossRef]
- Brown, K.A.; McInnes, K.J.; Takagi, K.; Ono, K.; Hunger, N.I.; Wang, L.; Sasano, H.; Simpson, E.R. LKB1 expression is inhibited by estradiol-17β in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2011, 127, 439–443. [Google Scholar] [CrossRef]
- Takagi, K.; Miki, Y.; Tanaka, S.; Hashimoto, C.; Watanabe, M.; Sasano, H.; Ito, K.; Suzuki, T. Nucleobindin 2 (NUCB2) in human endometrial carcinoma: A potent prognostic factor associated with cell proliferation and migration. Endocr. J. 2016, 63, 287–299. [Google Scholar] [CrossRef]
- Lompardía, S.L.; Papademetrio, D.L.; Mascaró, M.; Álvarez, E.M.; Hajos, S.E. Human leukemic cell lines synthesize hyaluronan to avoid senescence and resist chemotherapy. Glycobiology 2013, 12, 1463–1476. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Ween, M.P.; Lokman, N.A.; Tan, I.A.; Pyragius, C.E.; Oehler, M.K. Chemotherapy-induced hyaluronan production: A novel chemoresistance mechanism in ovarian cancer. BMC Cancer 2013, 13, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.M.; Horton, K.J.; Coveler, A.L.; Hingorani, S.R.; Harris, W.P. Targeting the Tumor Stroma: The Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20). Curr. Oncol. Rep. 2017, 7, 47–58. [Google Scholar] [CrossRef]
- Zhao, C.; Thompson, B.J.; Chen, K.; Gao, F.; Blouw, B.; Marella, M.; Zimmerman, S.; Kimbler, T.; Garrovillo, S.; Bahn, J.; et al. The growth of a xenograft breast cancer tumor model with engineered hyaluronan-accumulating stroma is dependent on hyaluronan and independent of CD44. Oncotarget 2019, 61, 6561–6576. [Google Scholar] [CrossRef] [PubMed]
- Morosi, L.; Meroni, M.; Ubezio, P.; Nerini, I.F.; Minoli, L.; Porcu, L.; Panini, N.; Colombo, M.; Blouw, B.; Kang, D.W.; et al. PEGylated recombinant human hyaluronidase (PEGPH20) pre-treatment improves intra-tumour distribution and efficacy of paclitaxel in preclinical models. J. Exp. Clin. Cancer Res. 2021, 40, 286. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Honeth, G.; Bendahl, P.O.; Saal, L.H.; Gruvberger-Saal, S.; Ringnér, M.; Vallon-Christersson, J.; Jönsson, G.; Holm, K.; Lövgren, K.; et al. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer 2011, 11, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, M.; Zhou, F.; Zhang, L.; Meng, X. The Breast Cancer Stem Cells Traits and Drug Resistance. Front. Pharmacol. 2021, 11, 599965. [Google Scholar] [CrossRef]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Spevak, C.C.; Wong, G.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J. Biol. Chem. 2009, 39, 26533–26546. [Google Scholar]
- Chen, L.; Bourguignon, L.Y. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol. Cancer 2014, 8, 52–65. [Google Scholar] [CrossRef]
- Li, J.; Zha, X.M.; Wang, R.; Li, X.D.; Xu, B.; Xu, Y.J.; Yin, Y.M. Regulation of CD44 expression by tumor necrosis factor-α and its potential role in breast cancer cell migration. Biomed. Pharmacother. 2012, 2, 144–150. [Google Scholar] [CrossRef]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT mechanism in breast cancer metastasis and drug resistance: Revisiting molecular interactions and biological functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef] [PubMed]
- Gooding, A.J.; Schiemann, W.P. Epithelial-Mesenchymal Transition Programs and Cancer Stem Cell Phenotypes: Mediators of Breast Cancer Therapy Resistance. Mol. Cancer Res. 2020, 18, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, X.Q.; Zheng, J.; Xu, T.T.; Wu, K.; Ru, C.H. RHAMM regulates the growth and migration of lung adenocarcinoma A549 cell line by regulating Cdc2/CyclinB1 and MMP9 genes Running title: RHAMM regulates the growth of lung adenocarcinoma cells. Math Biosci. Eng. 2020, 17, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, L.; Ding, Y.; Li, F.; Chen, X.; Ouyang, Y.; Zhang, Y.; Zhang, D. Hyaluronan-mediated motility receptor confers resistance to chemotherapy via TGFβ/Smad2-induced epithelial-mesenchymal transition in gastric cancer. FASEB J. 2019, 33, 6365–6377. [Google Scholar] [CrossRef]
- Martínez-Ramos, C.; Lebourg, M. Three-dimensional constructs using hyaluronan cell carrier as a tool for the study of cancer stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1249–1257. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 23, 9–16. [Google Scholar] [CrossRef]

| All Cases | HA Negative | HA Positive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RHAMM | RHAMM | RHAMM | ||||||||
| Negative (n = 63) | Positive (n = 51) | p | Negative (n = 41) | Positive (n = 23) | p | Negative (n = 22) | Positive (n = 28) | p | ||
| Age * | 57 (27–87) | 57 (29–82) | 0.93 | 56 (33–87) | 56 (29–82) | 0.93 | 58 (27–77) | 57 (40–82) | 0.77 | |
| Menopause | ||||||||||
| Pre | 24 | 17 | 15 | 10 | 0.59 | 9 | 7 | 0.23 | ||
| Post | 39 | 34 | 0.60 | 26 | 13 | 13 | 21 | |||
| pT | ||||||||||
| pT1 | 47 | 29 | 0.046 | 30 | 18 | 0.65 | 17 | 11 | 0.0072 | |
| pT2–4 | 16 | 22 | 11 | 5 | 5 | 17 | ||||
| Lymph node metastasis | ||||||||||
| negative | 50 | 28 | 0.0052 | 34 | 16 | 0.21 | 16 | 12 | 0.035 | |
| positive | 13 | 23 | 7 | 7 | 6 | 16 | ||||
| Stage | ||||||||||
| 1 | 44 | 22 | 0.011 | 29 | 14 | 0.11 | 15 | 8 | 0.020 | |
| 2 | 10 | 19 | 6 | 8 | 4 | 11 | ||||
| 3 | 9 | 10 | 6 | 1 | 3 | 9 | ||||
| Histological grade | ||||||||||
| 1 | 30 | 15 | 0.064 | 21 | 7 | 0.25 | 9 | 8 | 0.37 | |
| 2 | 24 | 21 | 15 | 11 | 9 | 10 | ||||
| 3 | 9 | 15 | 5 | 5 | 4 | 10 | ||||
| ER | ||||||||||
| negative | 9 | 13 | 0.13 | 6 | 7 | 0.13 | 3 | 6 | 0.48 | |
| positive | 54 | 38 | 35 | 16 | 19 | 22 | ||||
| PR | ||||||||||
| negative | 19 | 18 | 0.56 | 13 | 9 | 0.55 | 6 | 9 | 0.71 | |
| positive | 44 | 33 | 28 | 14 | 16 | 19 | ||||
| HER2 | ||||||||||
| negative | 54 | 43 | 0.83 | 34 | 10 | 0.67 | 20 | 23 | 0.38 | |
| positive | 9 | 8 | 7 | 3 | 2 | 5 | ||||
| Ki67 LI (%) * | 9 (1–60) | 18 (1–53) | 0.012 | 8 (1–60) | 7 (1–49) | 0.37 | 10.5 (1–41) | 18 (1–53) | 0.019 | |
| Subtype | ||||||||||
| Luminal A | 34 | 19 | 0.18 | 23 | 11 | 0.095 | 11 | 8 | 0.31 | |
| Luminal B | 20 | 19 | 12 | 5 | 8 | 14 | ||||
| HER2 | 4 | 3 | 4 | 1 | 0 | 2 | ||||
| TNBC | 5 | 10 | 2 | 6 | 3 | 4 | ||||
| HA | ||||||||||
| negative | 41 | 23 | ||||||||
| positive | 22 | 28 | 0.033 | |||||||
| Univariate * | Multivariate | ||||
|---|---|---|---|---|---|
| p | Relative Risk (95% CI) | p | Relative Risk (95% CI) | ||
| pT | pT2–4/pT1 | 0.0002 | 5.0 (2.1–12) | 0.056 | 3.3 (0.97–11) |
| Lymph node metastasis | positive/negative | 0.0024 | 3.5 (1.6–8.0) | 0.98 | 0.99 (0.32–3.1) |
| Histological grade | 3/1 + 2 | 0.0037 | 3.4 (1.5–7.6) | 0.18 | 0.46 (0.15–1.4) |
| ER | negative/positive | 0.0062 | 3.2 (1.4–7.3) | 0.61 | 0.73 (0.21–2.5) |
| PR | negative/positive | 0.0007 | 4.4 (1.9–10) | 0.007 | 4.6 (1.5–14) |
| HER2 | positive/negative | 0.22 | 0.41 (0.096–1.7) | ||
| Ki67 LI | ≥20%/<20% | 0.0002 | 4.8 (2.1–11) | 0.097 | 2.7 (0.83–9.0) |
| HA | positive/negative | 0.0097 | 3.1 (1.3–7.4) | 0.22 | 1.9 (0.67–5.4) |
| RHAMM | positive/negative | 0.0006 | 5.6 (2.1–15) | 0.005 | 4.8 (1.6–15) |
| Univariate * | Multivariate | ||||
|---|---|---|---|---|---|
| p | Relative Risk (95% CI) | p | Relative Risk (95% CI) | ||
| pT | pT2–4/pT1 | 0.066 | 2.6 (0.94–7.2) | 0.018 | 3.9 (1.3–12) |
| Lymph node metastasis | positive/negative | 0.24 | 1.8 (0.68–4.7) | ||
| Histological grade | 3/1 + 2 | 0.21 | 1.8 (0.72–4.3) | ||
| ER | negative/positive | 0.23 | 0.58 (024–1.4) | ||
| PR | negative/positive | 0.056 | 2.7 (0.98–7.5) | 0.009 | 4.8 (1.5–15) |
| HER2 | positive/negative | 0.077 | 0.27 (0.062–1.2) | 0.017 | 0.16 (0.035–0.72) |
| Ki67 LI | ≥20%/<20% | 0.044 | 2.6 (1.0–6.6) | 0.63 | 1.3 (0.44–3.9) |
| HA | positive/negative | 0.20 | 1.8 (0.72–4.7) | ||
| RHAMM | positive/negative | 0.018 | 3.8 (1.3–11) | 0.01 | 4.4 (1.4–14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, S.; Takagi, K.; Yamaguchi-Tanaka, M.; Sato, A.; Miki, Y.; Miyashita, M.; Tada, H.; Ishida, T.; Suzuki, T. Receptor for Hyaluronan Mediated Motility (RHAMM)/Hyaluronan Axis in Breast Cancer Chemoresistance. Cancers 2024, 16, 3600. https://doi.org/10.3390/cancers16213600
Fujisawa S, Takagi K, Yamaguchi-Tanaka M, Sato A, Miki Y, Miyashita M, Tada H, Ishida T, Suzuki T. Receptor for Hyaluronan Mediated Motility (RHAMM)/Hyaluronan Axis in Breast Cancer Chemoresistance. Cancers. 2024; 16(21):3600. https://doi.org/10.3390/cancers16213600
Chicago/Turabian StyleFujisawa, Shiori, Kiyoshi Takagi, Mio Yamaguchi-Tanaka, Ai Sato, Yasuhiro Miki, Minoru Miyashita, Hiroshi Tada, Takanori Ishida, and Takashi Suzuki. 2024. "Receptor for Hyaluronan Mediated Motility (RHAMM)/Hyaluronan Axis in Breast Cancer Chemoresistance" Cancers 16, no. 21: 3600. https://doi.org/10.3390/cancers16213600
APA StyleFujisawa, S., Takagi, K., Yamaguchi-Tanaka, M., Sato, A., Miki, Y., Miyashita, M., Tada, H., Ishida, T., & Suzuki, T. (2024). Receptor for Hyaluronan Mediated Motility (RHAMM)/Hyaluronan Axis in Breast Cancer Chemoresistance. Cancers, 16(21), 3600. https://doi.org/10.3390/cancers16213600






