The Evidence Base for Circulating Tumor DNA-Methylation in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy, Inclusion Criteria, and Data Collection
2.2. Methods for ctDNA Methylation Analysis
2.3. Statistical Methods
3. Results
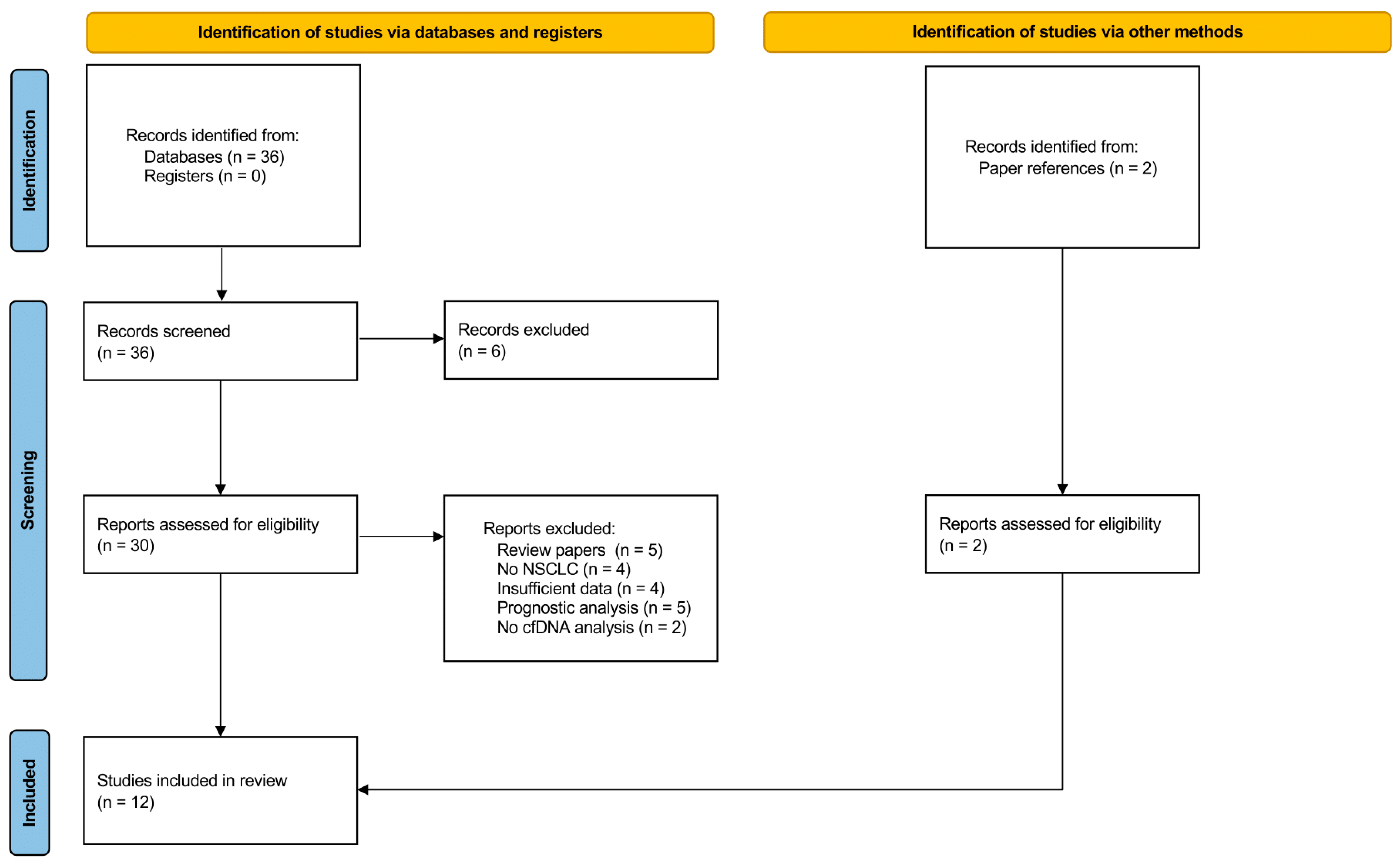
3.1. Study Selection
3.2. Characteristics of Eligible Studies
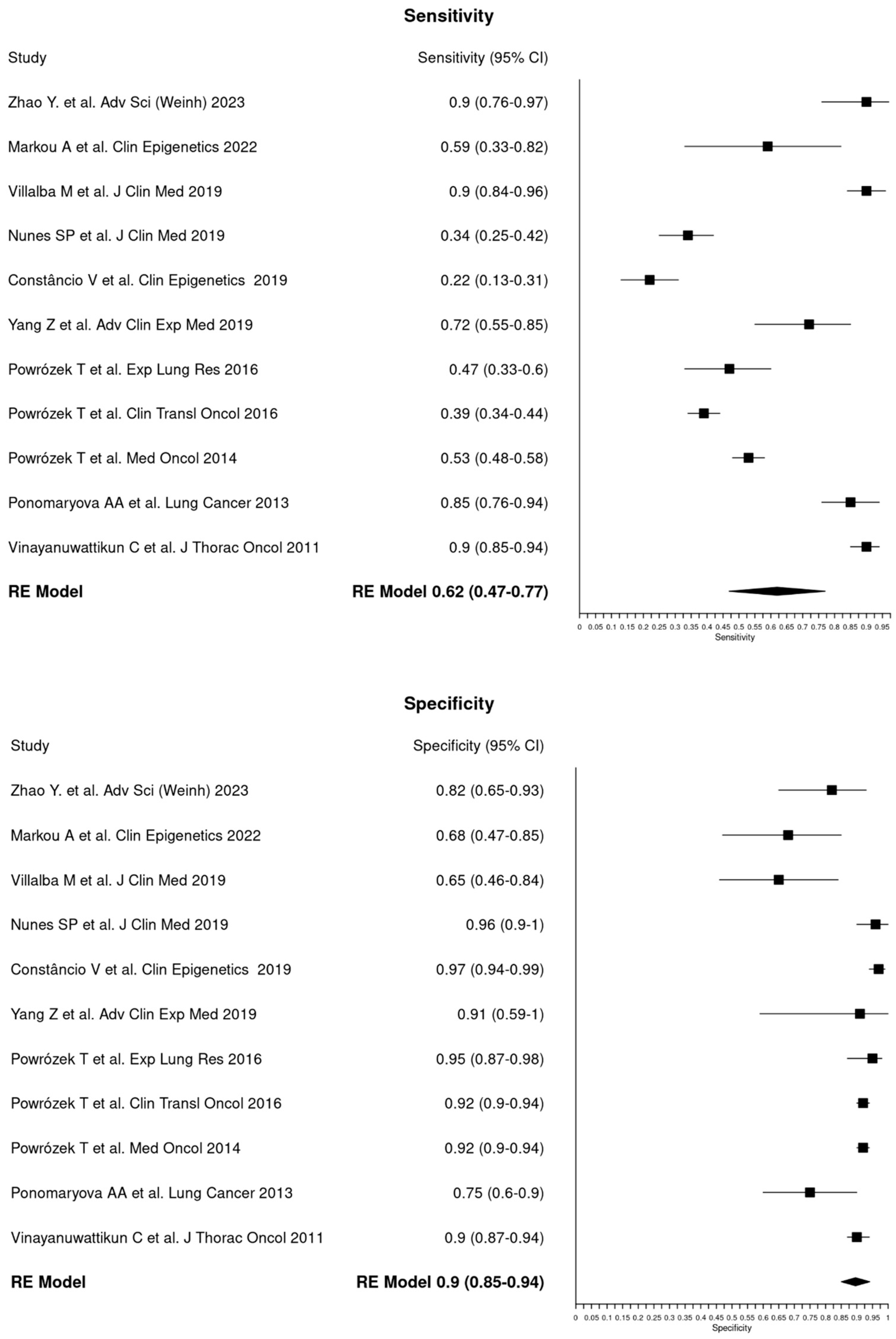
3.3. Diagnostic Accuracy
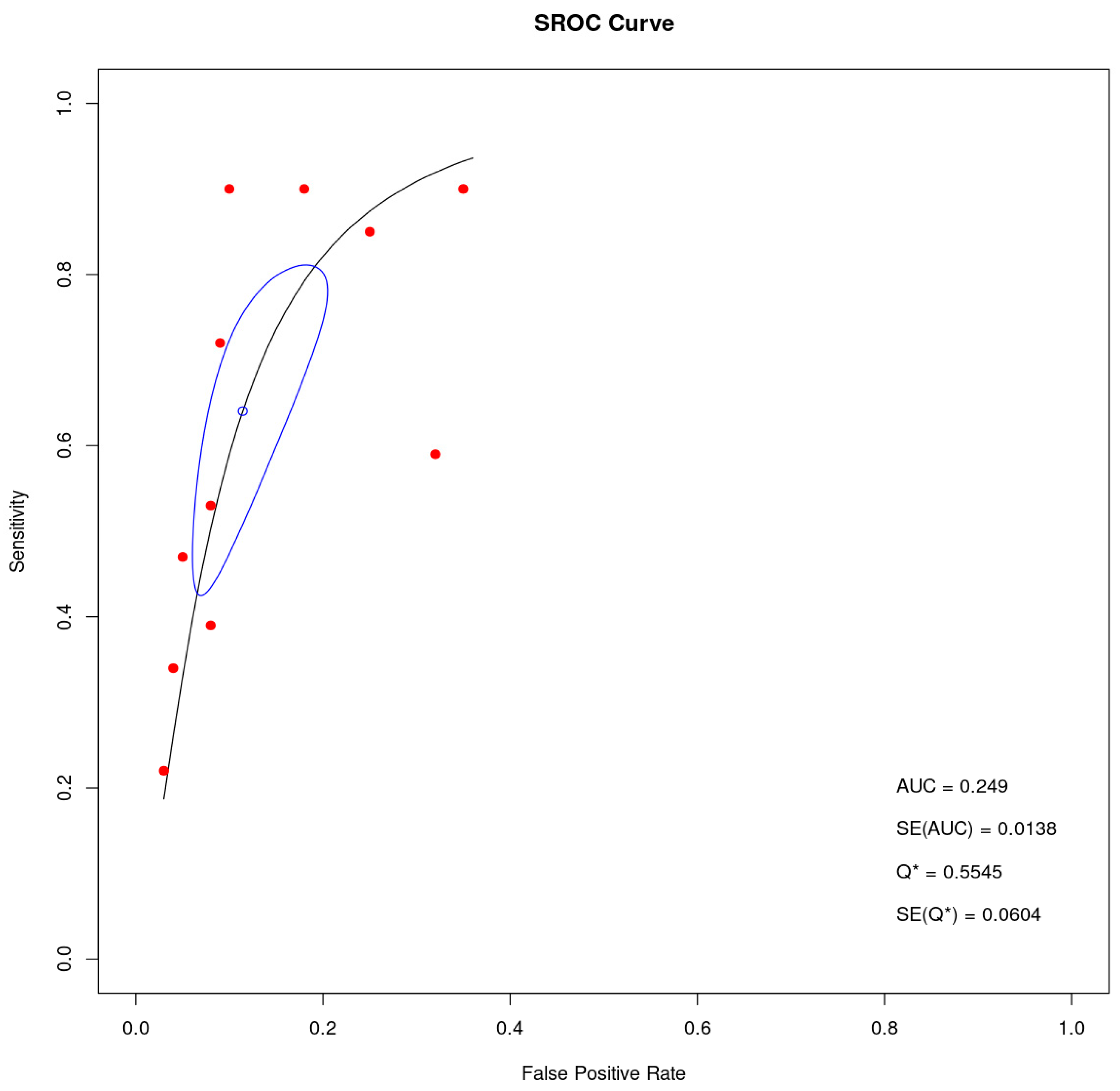
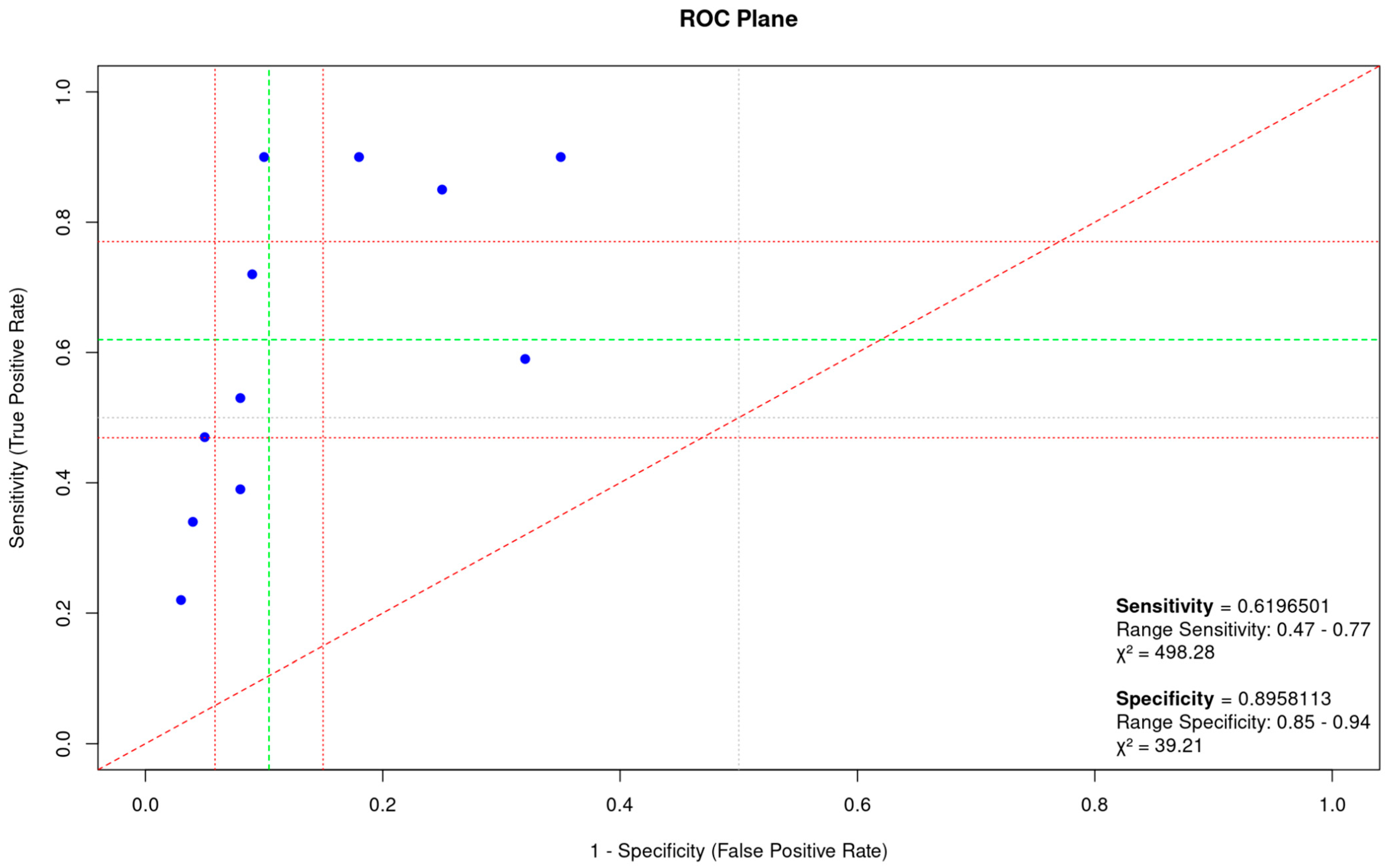
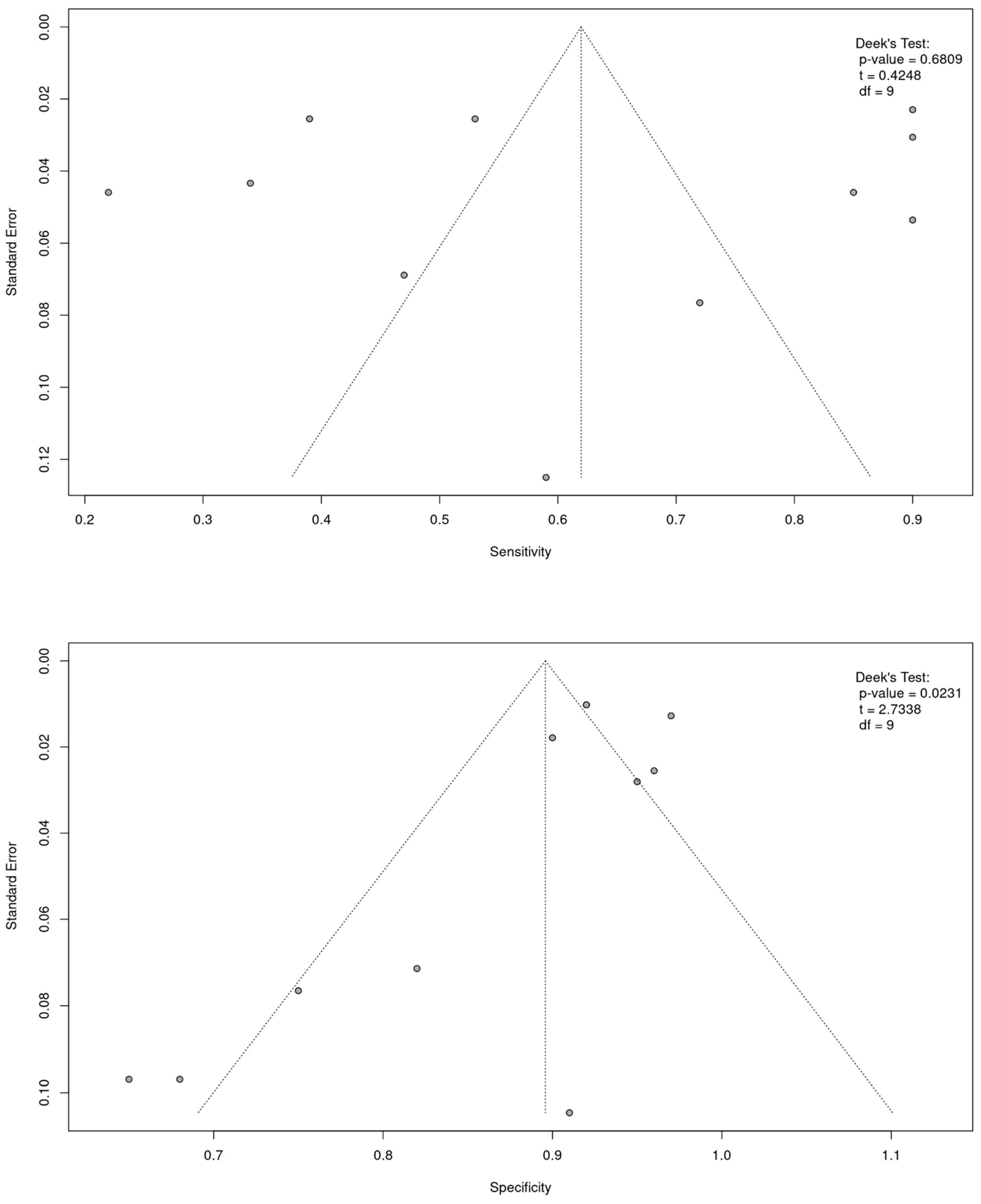
3.4. Heterogeneity and Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2015, 15, 247. [Google Scholar] [CrossRef]
- Wei, Z.; Shah, N.; Deng, C.; Xiao, X.; Zhong, T.; Li, X. Circulating DNA addresses cancer monitoring in non small cell lung cancer patients for detection and capturing the dynamic changes of the disease. SpringerPlus 2016, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Law, E.W.; Settell, M.L.; Kurani, S.S.; Eckert, E.C.; Liu, M.C.; Greenberg-Worisek, A.J. Liquid Biopsy: Emergence of an Alternative Cancer Detection Method. Clin. Transl. Sci. 2020, 13, 845–847. [Google Scholar] [CrossRef]
- Shields, M.D.; Chen, K.; Dutcher, G.; Patel, I.; Pellini, B. Making the Rounds: Exploring the Role of Circulating Tumor DNA (ctDNA) in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 9006. [Google Scholar] [CrossRef]
- Reina, C.; Šabanović, B.; Lazzari, C.; Gregorc, V.; Heeschen, C. Unlocking the future of cancer diagnosis—Promises and challenges of ctDNA-based liquid biopsies in non-small cell lung cancer. Transl. Res. 2024, 272, 41–53. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Risch, A.; Plass, C. Lung cancer epigenetics and genetics. Int. J. Cancer 2008, 123, 1–7. [Google Scholar] [CrossRef]
- Belinsky, S.A.; Nikula, K.J.; Palmisano, W.A.; Michels, R.; Saccomanno, G.; Gabrielson, E.; Baylin, S.B.; Herman, J.G. Aberrant methylation of p16 INK4a is an early event in lung cancer and a potential biomarker for early diagnosis. Proc. Natl. Acad. Sci. USA 1998, 95, 11891–11896. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Plaia, T.W.; Belinsky, S.A.; Baylin, S.B.; Herman, J.G. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 12754–12759. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. PRISMA 2020 Statement. Available online: https://www.prisma-statement.org/prisma-2020-statement (accessed on 26 June 2024).
- Nunes, S.P.; Diniz, F.; Moreira-Barbosa, C.; Constâncio, V.; Silva, A.V.; Oliveira, J.; Soares, M.; Paulino, S.; Cunha, A.L.; Rodrigues, J.; et al. Subtyping Lung Cancer Using DNA Methylation in Liquid Biopsies. J. Clin. Med. 2019, 8, 1500. [Google Scholar] [CrossRef] [PubMed]
- Constâncio, V.; Nunes, S.P.; Moreira-Barbosa, C.; Freitas, R.; Oliveira, J.; Pousa, I.; Oliveira, J.; Soares, M.; Dias, C.G.; Dias, T.; et al. Early detection of the major male cancer types in blood-based liquid biopsies using a DNA methylation panel. Clin. Epigenetics 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Krawczyk, P.; Kuźnar-Kamińska, B.; Batura-Gabryel, H.; Milanowski, J. Analysis of RTEL1 and PCDHGB6 promoter methylation in circulating-free DNA of lung cancer patients using liquid biopsy: A pilot study. Exp. Lung Res. 2016, 42, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Krawczyk, P.; Nicoś, M.; Kuźnar-Kamińska, B.; Batura-Gabryel, H.; Milanowski, J. Methylation of the DCLK1 promoter region in circulating free DNA and its prognostic value in lung cancer patients. Clin Transl Oncol. 2016, 18, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Krawczyk, P.; Kucharczyk, T.; Milanowski, J. Septin 9 promoter region methylation in free circulating DNA—potential role in noninvasive diagnosis of lung cancer: Preliminary report. Med. Oncol. 2014, 31, 917. [Google Scholar] [CrossRef]
- Ponomaryova, A.A.; Rykova, E.Y.; Cherdyntseva, N.V.; Skvortsova, T.E.; Dobrodeev, A.Y.; Zav’yalov, A.A.; Bryzgalov, L.O.; Tuzikov, S.A.; Vlassov, V.V.; Laktionov, P.P. Potentialities of aberrantly methylated circulating DNA for diagnostics and post-treatment follow-up of lung cancer patients. Lung Cancer 2013, 81, 397–403. [Google Scholar] [CrossRef]
- Ponomaryova, A.A.; Rykova, E.Y.; Cherdyntseva, N.V.; Skvortsova, T.E.; Dobrodeev, A.Y.; Zav’yalov, A.A.; Tuzikov, S.A.; Vlassov, V.V.; Laktionov, P.P. RARβ2 gene methylation level in the circulating DNA from blood of patients with lung cancer. Eur. J. Cancer Prev. 2011, 20, 453–455. [Google Scholar] [CrossRef]
- Zhao, Y.; O’Keefe, C.M.; Hsieh, K.; Cope, L.; Joyce, S.C.; Pisanic, T.R.; Herman, J.G.; Wang, T. Multiplex Digital Methylation-Specific PCR for Noninvasive Screening of Lung Cancer. Adv. Sci. 2023, 10, e2206518. [Google Scholar] [CrossRef]
- Markou, A.; Londra, D.; Tserpeli, V.; Kollias, I.; Tsaroucha, E.; Vamvakaris, I.; Potaris, K.; Pateras, I.; Kotsakis, A.; Georgoulias, V.; et al. DNA methylation analysis of tumor suppressor genes in liquid biopsy components of early stage NSCLC: A promising tool for early detection. Clin. Epigenetics 2022, 14, 61. [Google Scholar] [CrossRef]
- Villalba, M.; Exposito, F.; Pajares, M.J.; Sainz, C.; Redrado, M.; Remirez, A.; Wistuba, I.; Behrens, C.; Jantus-Lewintre, E.; Camps, C.; et al. TMPRSS4: A Novel Tumor Prognostic Indicator for the Stratification of Stage IA Tumors and a Liquid Biopsy Biomarker for NSCLC Patients. J. Clin. Med. 2019, 8, 2134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qi, W.; Sun, L.; Zhou, H.; Zhou, B.; Hu, Y. DNA methylation analysis of selected genes for the detection of early-stage lung cancer using circulating cell-free DNA. Adv. Clin. Exp. Med. 2018, 28, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Vinayanuwattikun, C.; Sriuranpong, V.; Tanasanvimon, S.; Chantranuwat, P.; Mutirangura, A. Epithelial-specific methylation marker: A potential plasma biomarker in advanced non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Jin, Y.; Zhao, H.; Wu, M.; Zhang, K.; Wei, Z.; Wang, X.; Wang, Z.; Li, Y.; Yang, F.; et al. Potential clinical utility of liquid biopsy in early-stage non-small cell lung cancer. BMC Med. 2022, 20, 480. [Google Scholar] [CrossRef] [PubMed]
- Kerachian, M.A.; Azghandi, M.; Mozaffari-Jovin, S.; Thierry, A.R. Guidelines for pre-analytical conditions for assessing the methylation of circulating cell-free DNA. Clin. Epigenetics 2021, 13, 193. [Google Scholar] [CrossRef]
- Cree, I.A.; Uttley, L.; Woods, H.B.; Kikuchi, H.; Reiman, A.; Harnan, S.; Whiteman, B.L.; Philips, S.T.; Messenger, M.; Cox, A.; et al. The evidence base for circulating tumour DNA blood-based biomarkers for the early detection of cancer: A systematic mapping review. BMC Cancer 2017, 17, 697. [Google Scholar] [CrossRef]
- Nie, K.; Jia, Y.; Zhang, X. Cell-free circulating tumor DNA in plasma/serum of non-small cell lung cancer. Tumor Biol. 2014, 36, 7–19. [Google Scholar] [CrossRef]
- Heeke, S.; Gay, C.M.; Estecio, M.R.; Tran, H.; Morris, B.B.; Zhang, B.; Tang, X.; Raso, M.G.; Rocha, P.; Lai, S.; et al. Tumor- and circulating-free DNA methylation identifies clinically relevant small cell lung cancer subtypes. Cancer Cell 2024, 42, 225–237.e5. [Google Scholar] [CrossRef]
- Haq, S.U.; Schmid, S.; Aparnathi, M.K.; Hueniken, K.; Zhan, L.J.; Sacdalan, D.; Li, J.J.; Meti, N.; Patel, D.; Cheng, D.; et al. Cell-free DNA methylation-defined prognostic subgroups in small-cell lung cancer identified by leukocyte methylation subtraction. iScience 2022, 25, 105487. [Google Scholar] [CrossRef]
- Chemi, F.; Pearce, S.P.; Clipson, A.; Hill, S.M.; Conway, A.-M.; Richardson, S.A.; Kamieniecka, K.; Caeser, R.; White, D.J.; Mohan, S.; et al. cfDNA methylome profiling for detection and subtyping of small cell lung cancers. Nat. Cancer 2022, 3, 1260–1270. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Waqar, S.N.; Krug, L.M.; Dowlati, A.; Hann, C.L.; Chiappori, A.; Owonikoko, T.K.; Woo, K.M.; Cardnell, R.J.; Fujimoto, J.; et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination with Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Metzenmacher, M.; Hegedüs, B.; Forster, J.; Schramm, A.; Horn, P.A.; Klein, C.A.; Bielefeld, N.; Ploenes, T.; Aigner, C.; Theegarten, D.; et al. Combined multimodal ctDNA analysis and radiological imaging for tumor surveillance in Non-small cell lung cancer. Transl. Oncol. 2021, 15, 101279. [Google Scholar] [CrossRef] [PubMed]
- Mastoraki, S.; Balgkouranidou, I.; Tsaroucha, E.; Klinakis, A.; Georgoulias, V.; Lianidou, E. KMT2C promoter methylation in plasma-circulating tumor DNA is a prognostic biomarker in non-small cell lung cancer. Mol. Oncol. 2020, 15, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, X.; Gao, C.; Xing, Y.; Qi, Z.; Liu, R.; Wang, Y.; Zhang, X.; Yang, Y.-G.; Li, X.; et al. 5-Hydroxymethylome in Circulating Cell-Free DNA as A Potential Biomarker for Non-Small-Cell Lung Cancer. Genom. Proteom. Bioinform. 2018, 16, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, C.; Bianchi, F.; Raviele, P.R.; Vacirca, D.; Bertalot, G.; Rampinelli, C.; Lazzeroni, M.; Bonnani, B.; Veronesi, G.; Fusco, N.; et al. Circulating and tissue biomarkers in early-stage non-small. Ecancermedicalscience 2017, 11, 717. [Google Scholar] [CrossRef]
- Bossé, Y.; Dasgupta, A.; Abadier, M.; Guthrie, V.; Song, F.; Armero, V.S.; Gaudreault, N.; Orain, M.; Lamaze, F.C.; Melton, C.; et al. Prognostic implication of methylation-based circulating tumor DNA detection prior to surgery in stage I non-small cell lung cancer. Cancer Lett. 2024, 594, 216984. [Google Scholar] [CrossRef]
- Chen, K.; Kang, G.; Zhang, Z.; Lizaso, A.; Beck, S.; Lyskjær, I.; Chervova, O.; Li, B.; Shen, H.; Wang, C.; et al. Individualized dynamic methylation-based analysis of cell-free DNA in postoperative monitoring of lung cancer. BMC Med. 2023, 21, 255. [Google Scholar] [CrossRef]
- Wen, S.W.C.; Nederby, L.; Andersen, R.F.; Nyhus, C.H.; Hilberg, O.; Jakobsen, A.; Hansen, T.F. NK cell activity and methylated HOXA9 ctDNA as prognostic biomarkers in patients with non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. Br. J. Cancer 2023, 129, 135–142. [Google Scholar] [CrossRef]
- Guo, D.; Yang, L.; Yang, J.; Shi, K. Plasma cell-free DNA methylation combined with tumor mutation detection in prognostic prediction of patients with non-small cell lung cancer (NSCLC). Medicine 2020, 99, e20431. [Google Scholar] [CrossRef]
- Balgkouranidou, I.; Chimonidou, M.; Milaki, G.; Tsarouxa, E.G.; Kakolyris, S.; Welch, D.R.; Georgoulias, V.; Lianidou, E.S. Breast cancer metastasis suppressor-1 promoter methylation in cell-free DNA provides prognostic information in non-small cell lung cancer. Br. J. Cancer 2014, 110, 2054–2062. [Google Scholar] [CrossRef]
- Coyne, G.O.; Wang, L.; Zlott, J.; Juwara, L.; Covey, J.M.; Beumer, J.H.; Cristea, M.C.; Newman, E.M.; Koehler, S.; Nieva, J.J.; et al. Intravenous 5-fluoro-2′-deoxycytidine administered with tetrahydrouridine increases the proportion of p16-expressing circulating tumor cells in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2020, 85, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Y.; Li, J.; Li, X.; Cheng, N.; Wang, Q.; Cai, W.; Zhao, C.; He, Y.; Chang, J.; et al. Histone deacetylation, as opposed to promoter methylation, results in epigenetic BIM silencing and resistance to EGFR TKI in NSCLC. Oncol. Lett. 2017, 15, 1089–1096. [Google Scholar] [CrossRef] [PubMed]


| Reference | No. of NSCLC Cases | Country | Males/Females | Median Age (Range) | % Smoker (Former or Current) | NSCLC Stage (I–IV) | Specimen Type for ctDNA | Detection Assay in ctDNA |
|---|---|---|---|---|---|---|---|---|
| Zhao Y. et al. Adv. Sci. (Weinh) 2023 [20] | 39 | United States | 18/21 | 68 (30–85) | 97.4 | I, II, III, IV | Plasma | mdMSP |
| Markou A et al. Clin Epigenetics 2022 [21] | 42 | Greece | 32/10 | 69 (39–89) | 78.6 | IA–IIIA | Plasma | QMSP |
| Villalba M et al. J Clin Med 2019 [22] | 89 | Spain | 66/23 | 61.5 (30–86) | 83.1 | I, II, III, IV | Plasma | ddPCR |
| Nunes SP et al. J Clin Med 2019, Constâncio V et al. Clin Epigenetics 2019 [13,14] | 110 | Portugal | 75/35 | 66.5 (38–89) | 75.5 | I, II, III, IV | Plasma | QMSP, multiplex QMSP |
| Yang Z et al. Adv Clin Exp Med 2019 [23] | 39 | China | 24/15 | 51 (NA) | 74.4 | I | Plasma | QMSP |
| Powrózek T et al. Exp Lung Res 2016, Powrózek T et al. Clin Transl Oncol 2016, Powrózek T et al. Med Oncol 2014 [15,16,17] | 55 | Poland | 40/15 | 62 (NA) | 88.6 | I, II, IIIA, IIIB, IV | Plasma | QMSP, real-time PCR |
| Ponomaryova AA et al. Lung Cancer 2013, Ponomaryova AA et al. Eur J Cancer Prev 2011 [18,19] | 60 | Russia | 52/8 | NA | 83.0 | I, II, III | Plasma | QMSP |
| Vinayanuwattikun C et al. J. Thorac. Oncol. 2011 [24] | 38 | Thailand | 20/18 | NA | 50.0 | III, IV | Plasma | AQAMA-PCR |
| Total of cases | 472 |
| Reference | Gene | N° NSCLC Patients | N° Controls | Sensitivity | 95% CI Sensitivity | Specificity | 95% CI Specificity |
|---|---|---|---|---|---|---|---|
| Zhao Y. et al. Adv. Sci. (Weinh) 2023 [20] | SOX17, CDO1, TAC1, HOXA7 | 39 | 33 | 0.90 | 0.76–0.97 | 0.82 | 0.65–0.93 |
| Markou A et al. Clin Epigenetics 2022 [21] | APC, RASSF1A, FOXA1, SLFN11, SHOX2 | 42 | 12 | 0.59 | 0.33–0.82 | 0.68 | 0.47–0.85 |
| Villalba M et al. J Clin Med 2019 [22] | TMPRSS4 | 89 | 25 | 0.90 | 0.84–0.96 | 0.65 | 0.46–0.84 |
| Nunes SP et al. J Clin Med 2019 [13] | APC, RASSF1A | 110 | 28 | 0.34 | 0.25–0.42 | 0.96 | 0.9–1 |
| Constâncio V et al. Clin Epigenetics 2019 [14] | RARβ2, SEPT9, SOX17 | 86 | 136 | 0.22 | 0.13–0.31 | 0.97 | 0.94–0.99 |
| Yang Z et al. Adv Clin Exp Med 2019 [23] | CDH13, WT1, CDKN2A, HOXA9, PITX2, CALCA, RASSF1A, DLEC1 | 39 | 11 | 0.72 | 0.55–0.85 | 0.91 | 0.59–1 |
| Powrózek T et al. Exp Lung Res 2016 [15] | RTEL1, PCDHGB6 | 55 | 80 | 0.47 | 0.33–0.60 | 0.95 | 0.87–0.98 |
| Powrózek T et al. Clin Transl Oncol 2016 [16] | DCLK1 | 46 | 95 | 0.39 | 0.34–0.44 | 0.92 | 0.9–0.94 |
| Powrózek T et al. Med Oncol 2014 [17] | SEPT9 | 47 | 100 | 0.53 | 0.48–0.58 | 0.92 | 0.90–0.94 |
| Ponomaryova AA et al. Lung Cancer 2013 [18] | RARβ2, RASSF1A | 60 | 32 | 0.85 | 0.76–0.94 | 0.75 | 0.6–0.9 |
| Vinayanuwattikun C et al. J Thorac Oncol 2011 [24] | SHP1P2 | 38 | 52 | 0.90 | 0.85–0.94 | 0.90 | 0.87–0.94 |
| Total cases | 651 | 604 | 0.62 | 0.47–0.77 | 0.90 | 0.85–0.94 |
| Gene | Reference | N° NSCLC Patients | N° Controls | Sensitivity | 95% CI Sensitivity | Specificity | 95% CI Specificity |
|---|---|---|---|---|---|---|---|
| RASSF1A | Markou A et al. Clin Epigenetics 2022 [21] | 42 | 12 | 0.24 | 0.07–0.50 | 0.92 | 0.74–0.99 |
| Nunes SP et al. J Clin Med 2019 [13] | 110 | 28 | 0.18 | 0.11–0.25 | 1 | 1–1 | |
| Yang Z et al. Adv Clin Exp Med 2019 [23] | 39 | 11 | 0.41 | 0.26–0.58 | 1 | 0.72–1 | |
| Ponomaryova AA et al. Lung Cancer 2013 [18] | 60 | 32 | 0.66 | 0.54–0.78 | 0.57 | 0.40–0.74 | |
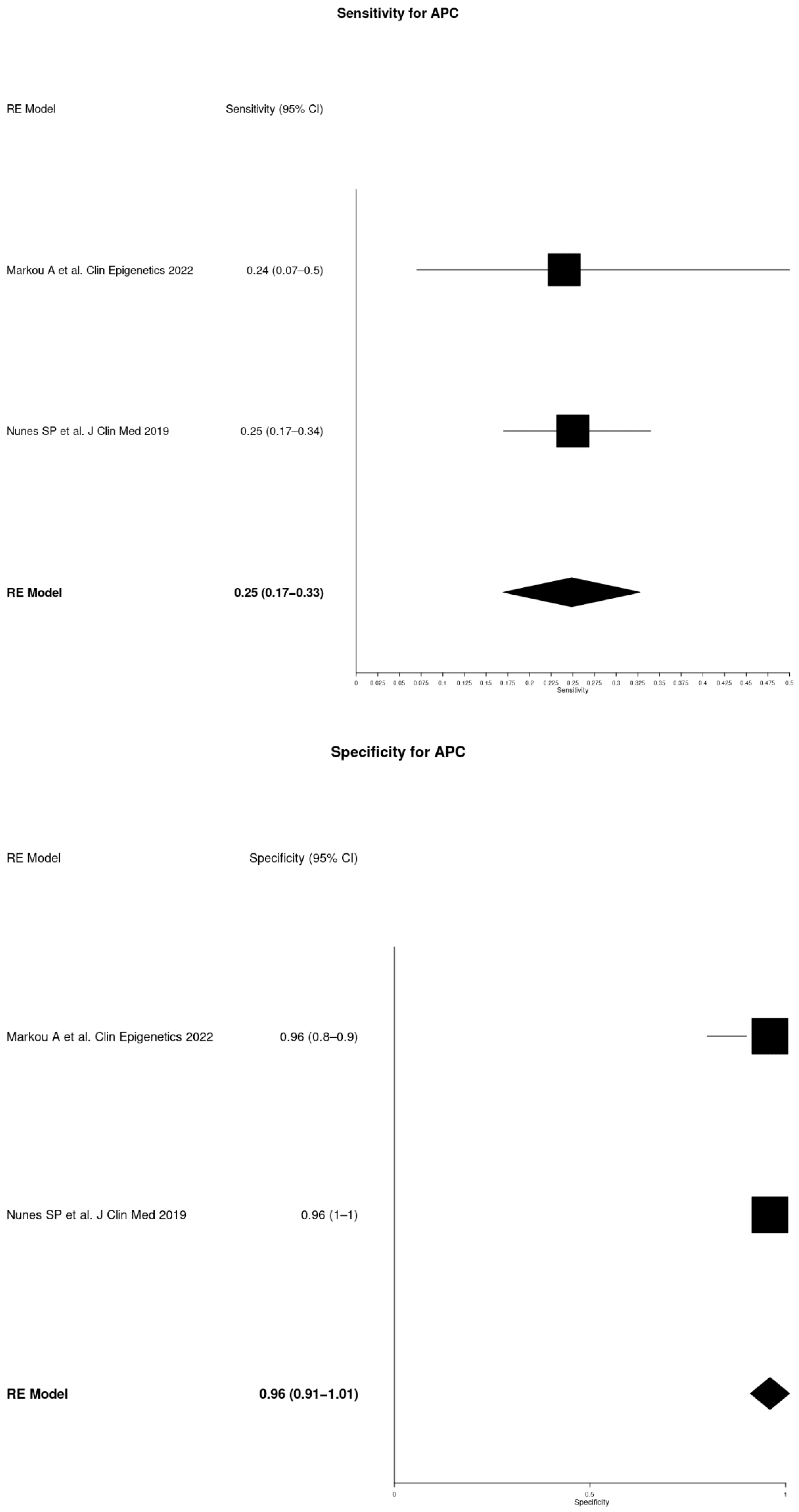
| APC | Markou A et al. Clin Epigenetics 2022 [21] | 42 | 12 | 0.24 | 0.07–0.50 | 0.96 | 0.80–1 |
| Nunes SP et al. J Clin Med 2019 [13] | 110 | 28 | 0.25 | 0.17–0.34 | 0.96 | 0.90–1 | |
| SOX17 | Zhao Y. et al. Adv Sci (Weinh) 2023 [20] | 39 | 33 | 0.64 | 0.47–0.79 | 0.88 | 0.72–0.97 |
| Constâncio V et al. Clin Epigenetics 2019 [14] | 86 | 136 | 0.24 | 0.15–0.33 | 0.96 | 0.92–0.99 | |
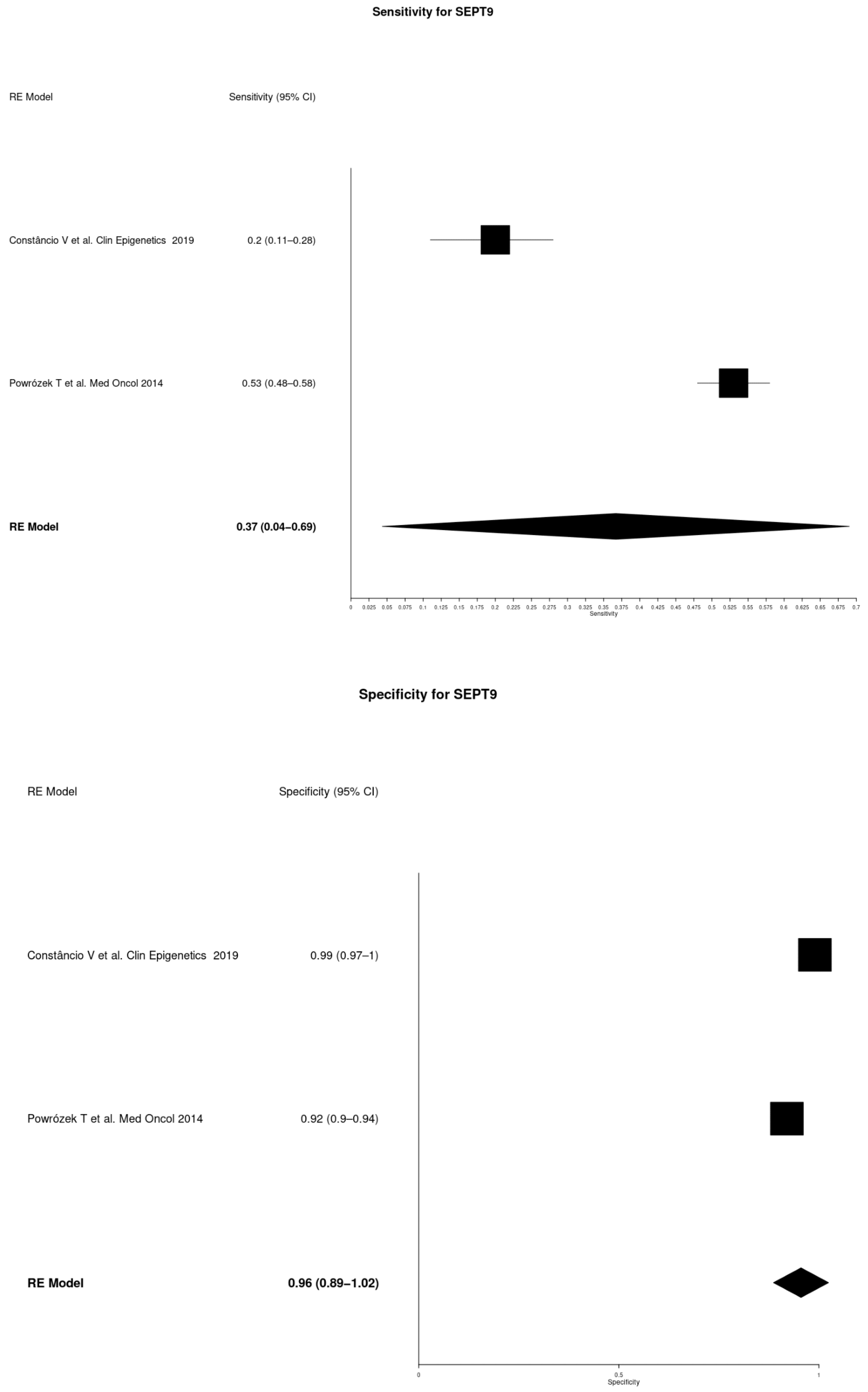
| SEPT9 | Constâncio V et al. Clin Epigenetics 2019 [14] | 86 | 136 | 0.20 | 0.11–0.28 | 0.99 | 0.97–1 |
| Powrózek T et al. Med Oncol 2014 [17] | 47 | 100 | 0.53 | 0.48–0.58 | 0.92 | 0.90–0.94 | |
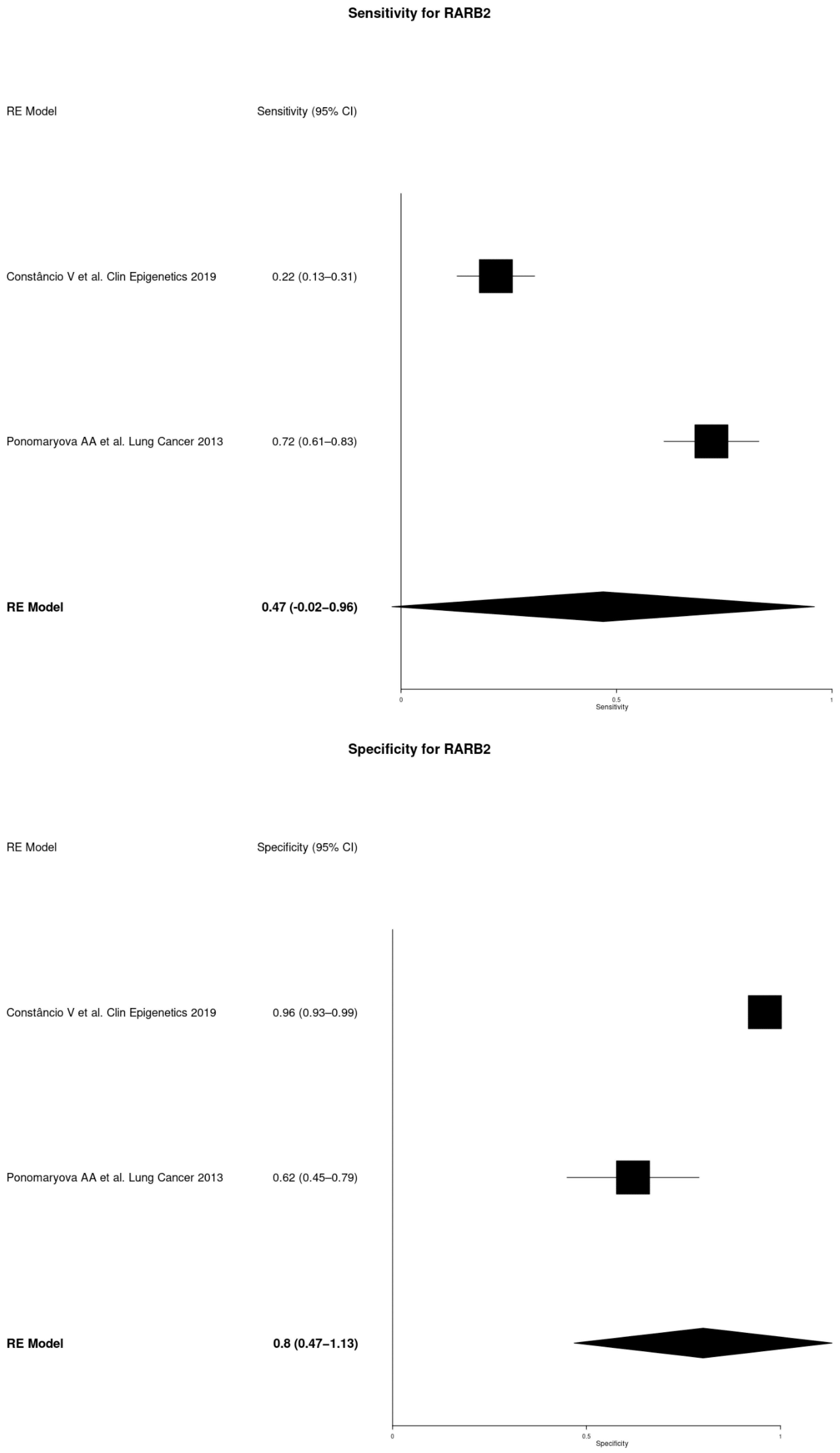
| RARβ2 | Constâncio V et al. Clin Epigenetics 2019 [14] | 86 | 136 | 0.22 | 0.13–0.31 | 0.96 | 0.93–0.99 |
| Ponomaryova AA et al. Lung Cancer 2013 [18] | 60 | 32 | 0.72 | 0.61–0.83 | 0.62 | 0.45–0.79 |
| Gene | Sensitivity | 95% CI Sensitivity | Specificity | 95% CI Specificity |
|---|---|---|---|---|
| RASSF1A | 0.37 | 0.16–0.59 | 0.83 | 0.58–1.09 |
| APC | 0.25 | 0.17–0.33 | 0.96 | 0.91–1.01 |
| SOX17 | 0.43 | 0.04–0.83 | 0.94 | 0.88–1.01 |
| SEPT9 | 0.37 | 0.04–0.69 | 0.96 | 0.89–1.02 |
| RARβ2 | 0.47 | −0.02–0.96 | 0.80 | 0.47–1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffeo, D.; Rina, A.; Serio, V.B.; Markou, A.; Powrózek, T.; Constâncio, V.; Nunes, S.P.; Jerónimo, C.; Calvo, A.; Mari, F.; et al. The Evidence Base for Circulating Tumor DNA-Methylation in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3641. https://doi.org/10.3390/cancers16213641
Maffeo D, Rina A, Serio VB, Markou A, Powrózek T, Constâncio V, Nunes SP, Jerónimo C, Calvo A, Mari F, et al. The Evidence Base for Circulating Tumor DNA-Methylation in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(21):3641. https://doi.org/10.3390/cancers16213641
Chicago/Turabian StyleMaffeo, Debora, Angela Rina, Viola Bianca Serio, Athina Markou, Tomasz Powrózek, Vera Constâncio, Sandra P. Nunes, Carmen Jerónimo, Alfonso Calvo, Francesca Mari, and et al. 2024. "The Evidence Base for Circulating Tumor DNA-Methylation in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis" Cancers 16, no. 21: 3641. https://doi.org/10.3390/cancers16213641
APA StyleMaffeo, D., Rina, A., Serio, V. B., Markou, A., Powrózek, T., Constâncio, V., Nunes, S. P., Jerónimo, C., Calvo, A., Mari, F., Frullanti, E., Rosati, D., & Palmieri, M. (2024). The Evidence Base for Circulating Tumor DNA-Methylation in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancers, 16(21), 3641. https://doi.org/10.3390/cancers16213641










