The Rationale for Combining Hypofractionated Radiation and Hyperthermia
Simple Summary
Abstract
1. Introduction
2. Combining Heat with Radiation
3. Pre-Clinical Studies with Hypofractionation and Hyperthermia
3.1. In Vitro Studies
3.2. In Vivo Rodent Studies
3.3. Larger Animal (Canine and Feline) Studies
3.4. Normal Tissue Response to Hyperthermia and Hypofractionated Radiation
3.5. Conclusions from the Pre-Clinical In Vivo and Larger Animal Studies
4. Clinical Studies with Hypofractionation and Hyperthermia
5. General Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D. Large-dose fractionation (hypofractionation). Cancer 1985, 55, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.M.; Pompos, A.; Timmerman, R.; Jiang, S.; Story, M.D.; Pistenmaa, D.; Choy, H. The Role of Hypofractionated Radiation Therapy with Photons, Protons, and Heavy Ions for Treating Extracranial Lesions. Front. Oncol. 2015, 5, 302. [Google Scholar] [CrossRef] [PubMed]
- Lacas, B.; Bourhis, J.; Overgaard, J.; Zhang, Q.; Grégoire, V.; Nankivell, M.; Zackrisson, B.; Szutkowski, Z.; Suwiński, R.; Poulsen, M.; et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): An updated meta-analysis. Lancet Oncol. 2017, 18, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Myrehaug, S.; Hudson, J.; Soliman, H.; Ruschin, M.; Tseng, C.-L.; Detsky, J.; Husain, Z.; Keith, J.; Atenafu, E.G.; Maralani, P.; et al. Hypofractionated Stereotactic Radiation Therapy for Intact Brain Metastases in 5 Daily Fractions: Effect of Dose on Treatment Response. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 342–350. [Google Scholar] [CrossRef]
- Zhang-Velten, E.; Sanford, N.N. The Evolving Role of Hypofractionated Radiotherapy in Older Adults with Gastrointestinal Cancers. Semin. Radiat. Oncol. 2022, 32, 159–167. [Google Scholar] [CrossRef]
- Ritter, A.R.; Prasad, R.N.; Jhawar, S.R.; Bazan, J.G.; Gokun, Y.; Vudatala, S.; Diaz, D.A. Hypofractionated Radiation Therapy: A Cross-sectional Survey Study of US Radiation Oncologists. Am. J. Clin. Oncol. 2024, 47, 434–438. [Google Scholar] [CrossRef]
- Vens, C.; Koritzinsky, M.; Wouters, B.G. Irradiation-induced damage and the DNA damage response. In Basic Clinical Radiobiology; Joiner, M.C., van der Kogel, A.J., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 9–20. [Google Scholar]
- Dewey, W.C.; Hopwood, L.E.; Sapareto, S.A.; Gerweck, L.E. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef]
- Overgaard, J.; Bichel, P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology 1977, 123, 511–514. [Google Scholar] [CrossRef]
- Heacock, C.S.; Brown, S.L.; Bamburg, J.R. In vitro inactivation of actin by heat. Natl. Cancer Inst. Monogr. 1982, 61, 73–75. [Google Scholar]
- Lindegaard, J.C.; Overgaard, J. Factors of importance for the development of the step-down heating effect in a C3H mammary carcinoma in vivo. Int. J. Hyperth. 1987, 3, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Overgaard, J. Can mild hyperthermia improve tumour oxygenation? Int. J. Hyperth. 1997, 13, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Kim, M.-S.; Cho, L.C.; Dusenbery, K.; Sperduto, P.W. Radiobiological basis of SBRT and SRS. Int. J. Clin. Oncol. 2014, 19, 570–578. [Google Scholar] [CrossRef]
- Song, C.W.; Cho, L.C.; Yuan, J.; Dusenbery, K.E.; Griffin, R.J.; Levitt, S.H. Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 18–19. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Horsman, M.R.; Vaupel, P. Pathophysiological Basis for the Formation of the Tumor Microenvironment. Front. Oncol. 2016, 6, 66. [Google Scholar] [CrossRef]
- Folkman, J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986, 46, 467–473. [Google Scholar]
- Siemann, D.W.; Horsman, M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Kallman, R.F.; Rockwell, S. Effects of Radiation on Animal Tumor Models. In Radiotherapy, Surgery, and Immunotherapy; Becker, F.F., Ed.; Springer: Boston, MA, USA, 1977; Volume 6, pp. 225–279. [Google Scholar]
- Tozer, G.; Suit, H.D.; Barlai-Kovach, M.; Brunengraber, H.; Biaglow, J. Energy metabolism and blood perfusion in a mouse mammary adenocarcinoma during growth and following X irradiation. Radiat. Res. 1987, 109, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L. Radiation-induced reoxygenation in the SCCVII murine tumour: Evidence for a decrease in oxygen consumption and an increase in tumour perfusion. Radiother. Oncol. 1994, 32, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bussink, J.; Kaanders, J.H.; Rijken, P.F.; Raleigh, J.A.; Van der Kogel, A.J. Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiat. Res. 2000, 153, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Lasnitzki, I. A quantitative analysis of the direct and indirect action of X radiation on malignant cells. Br. J. Radiol. 1947, 20, 240–247. [Google Scholar] [CrossRef]
- Merwin, R.; Algire, G.H.; Kaplan, H.S. Transparent-chamber observations of the response of a transplantable mouse mammary tumor to local roentgen irradiation. J. Natl. Cancer Inst. 1950, 11, 593–627. [Google Scholar]
- Clement, J.J.; Tanaka, N.; Song, C.W. Tumor reoxygenation and postirradiation vascular changes. Radiology 1978, 127, 799–803. [Google Scholar] [CrossRef]
- Clement, J.J.; Song, C.W.; Levitt, S.H. Changes in functional vascularity and cell number following X-irradiation of a murine carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1976, 1, 671–678. [Google Scholar] [CrossRef]
- Reinhold, H.S.; Buisman, G.H. Radiosensitivity of capillary endothelium. Br. J. Radiol. 1973, 46, 54–57. [Google Scholar] [CrossRef]
- Goda, F.; Bacic, G.; O’Hara, J.A.; Gallez, B.; Swartz, H.M.; Dunn, J.F. The relationship between partial pressure of oxygen and perfusion in two murine tumors after X-ray irradiation: A combined gadopentetate dimeglumine dynamic magnetic resonance imaging and in vivo electron paramagnetic resonance oximetry study. Cancer Res. 1996, 56, 3344–3349. [Google Scholar]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef]
- Fuks, Z.; Kolesnick, R. Engaging the vascular component of the tumor response. Cancer Cell 2005, 8, 89–91. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Broerse, J.J. Experimental radiotherapy of a rat rhabdomyosarcoma with 15 meV neutrons and 300 kV x-rays. II. Effects of ractionated treatments, applied five times a week for several weeks. Eur. J. Cancer 1970, 6, 89–109. [Google Scholar] [CrossRef]
- Brown, J.M.; Carlson, D.J.; Brenner, D.J. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Park, H.J.; Griffin, R.J.; Hui, S.; Levitt, S.H.; Song, C.W. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 2012, 177, 311–327. [Google Scholar] [CrossRef]
- Choi, D.H.; Oh, D.; Na, K.; Kim, H.; Choi, D.; Jung, Y.H.; Ahn, J.; Kim, J.; Kim, C.H.; Chung, S. Radiation induces acute and subacute vascular regression in a three-dimensional microvasculature model. Front. Oncol. 2023, 13, 1252014. [Google Scholar] [CrossRef]
- Song, C.W.; Griffin, R.J.; Lee, Y.-J.; Cho, H.; Seo, J.; Park, I.; Kim, H.K.; Kim, D.H.; Kim, M.-S.; Dusenbery, K.E.; et al. Reoxygenation and Repopulation of Tumor Cells after Ablative Hypofractionated Radiotherapy (SBRT and SRS) in Murine Tumors. Radiat. Res. 2019, 192, 159–168. [Google Scholar] [CrossRef]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A potent enhancer of radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef]
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperth. 2003, 19, 252–266. [Google Scholar] [CrossRef]
- Nielsen, O.S.; Henle, K.J.; Overgaard, J. Arrhenius analysis of survival curves from thermotolerant and step-down heated L1A2 cells in vitro. Radiat. Res. 1982, 91, 468–482. [Google Scholar] [CrossRef]
- Roizin-Towle, L.; Pirro, J.P. The response of human and rodent cells to hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 751–756. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Nygaard, T.G.; Burlett, M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res. 1979, 39, 966–972. [Google Scholar]
- Horsman, M.R.; Chaplin, D.J.; Overgaard, J. Combination of nicotinamide and hyperthermia to eliminate radioresistant chronically and acutely hypoxic tumor cells. Cancer Res. 1990, 50, 7430–7436. [Google Scholar]
- Overgaard, J. Effect of hyperthermia on the hypoxic fraction in an experimental mammary carcinoma in vivo. Br. J. Radiol. 1981, 54, 245–249. [Google Scholar] [CrossRef]
- Overgaard, J. The current and potential role of hyperthermia in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 535–549. [Google Scholar] [CrossRef]
- Li, G.C.; Kal, H.B. Effect of hyperthermia on the radiation response of two mammalian cell lines. Eur. J. Cancer 1977, 13, 65–69. [Google Scholar] [CrossRef]
- Dewey, W.C.; Freeman, M.L. Rationale for use of hyperthermia in cancer therapy. Ann. N. Y. Acad. Sci. 1980, 335, 372–378. [Google Scholar] [CrossRef]
- Freeman, M.L.; Holahan, E.V.; Highfield, D.P.; Raaphorst, G.P.; Spiro, I.J.; Dewey, W.C. The effect of pH on hyperthermic and x ray induced cell killing. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 211–216. [Google Scholar] [CrossRef]
- Mills, M.D.; Meyn, R.E. Hyperthermic potentiation of unrejoined DNA strand breaks following irradiation. Radiat. Res. 1983, 95, 327–338. [Google Scholar] [CrossRef]
- Stewart, F.A.; Denekamp, J. The therapeutic advantage of combined heat and X rays on a mouse fibrosarcoma. Br. J. Radiol. 1978, 51, 307–316. [Google Scholar] [CrossRef]
- Gillette, E.L.; Ensley, B.A. Effect of heating order on radiation response of mouse tumor and skin. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 209–213. [Google Scholar] [CrossRef]
- Overgaard, J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 1507–1517. [Google Scholar] [CrossRef]
- Hill, S.A.; Denekamp, J. The response of six mouse tumours to combined heat and X rays: Implications for therapy. Br. J. Radiol. 1979, 52, 209–218. [Google Scholar] [CrossRef]
- Myers, R.; Field, S.B. The response of the rat tail to combined heat and X rays. Br. J. Radiol. 1977, 50, 581–586. [Google Scholar] [CrossRef]
- Law, M.P.; Ahier, R.G.; Field, S.B. The response of mouse skin to combined hyperthermia and X-rays. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 32, 153–163. [Google Scholar] [CrossRef]
- Stewart, F.A.; Denekamp, J. Sensitization of mouse skin to X irradiation by moderate heating. Radiology 1977, 123, 195–200. [Google Scholar] [CrossRef]
- Hume, S.P.; Field, S.B. Hyperthermic sensitization of mouse intestine to damage by X rays: The effect of sequence and temporal separation of the two treatments. Br. J. Radiol. 1978, 51, 302–306. [Google Scholar] [CrossRef]
- Takata, M.; Sasaki, M.S.; Sonoda, E.; Morrison, C.; Hashimoto, M.; Utsumi, H.; Yamaguchi-Iwai, Y.; Shinohara, A.; Takeda, S. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998, 17, 5497–5508. [Google Scholar] [CrossRef]
- Jackson, S.P. Sensing and repairing DNA double-strand breaks. Carcinogenesis 2002, 23, 687–696. [Google Scholar] [CrossRef]
- Ihara, M.; Takeshita, S.; Okaichi, K.; Okumura, Y.; Ohnishi, T. Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int. J. Hyperth. 2014, 30, 102–109. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Dikomey, E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int. J. Radiat. Biol. 2001, 77, 399–408. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Lora-Michiels, M.; Viglianti, B.L.; Dewey, W.C.; Repacholi, M. Carcinogenic effects of hyperthermia. Int. J. Hyperth. 2003, 19, 236–251. [Google Scholar] [CrossRef]
- Roti Roti, J.L. Heat-induced alterations of nuclear protein associations and their effects on DNA repair and replication. Int. J. Hyperth. 2007, 23, 3–15. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Krawczyk, P.M.; Horsman, M.R.; Franken, N.A.P.; Crezee, J. Targeting therapy-resistant cancer stem cells by hyperthermia. Int. J. Hyperth. 2017, 33, 419–427. [Google Scholar] [CrossRef]
- Iwata, K.; Shakil, A.; Hur, W.J.; Makepeace, C.M.; Griffin, R.J.; Song, C.W. Tumour pO2 can be increased markedly by mild hyperthermia. Br. J. Cancer Suppl. 1996, 27, S217–S221. [Google Scholar]
- Vaupel, P.W.; Kelleher, D.K. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int. J. Hyperth. 2010, 26, 211–223. [Google Scholar] [CrossRef]
- Sen, A.; Capitano, M.L.; Spernyak, J.A.; Schueckler, J.T.; Thomas, S.; Singh, A.K.; Evans, S.S.; Hylander, B.L.; Repasky, E.A. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011, 71, 3872–3880. [Google Scholar] [CrossRef]
- Winslow, T.B.; Eranki, A.; Ullas, S.; Singh, A.K.; Repasky, E.A.; Sen, A. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: Analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int. J. Hyperth. 2015, 31, 693–701. [Google Scholar] [CrossRef]
- Song, C.W. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984, 44, 4721s–4730s. [Google Scholar]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Dodge, R.K.; Charles, H.C.; Samulski, T.V.; Prosnitz, L.R.; Dewhirst, M.W. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996, 56, 5347–5350. [Google Scholar]
- Vujaskovic, Z.; Song, C.W. Physiological mechanisms underlying heat-induced radiosensitization. Int. J. Hyperth. 2004, 20, 163–174. [Google Scholar] [CrossRef]
- Song, C.W.; Patten, M.S.; Chelstrom, L.M.; Rhee, J.G.; Levitt, S.H. Effect of multiple heatings on the blood flow in RIF-1 tumours, skin and muscle of C3H mice. Int. J. Hyperth. 1987, 3, 535–545. [Google Scholar] [CrossRef]
- Howard-Flanders, P.; Moore, D. The time interval after pulsed irradiation within which injury to bacteria can be modified by dissolved oxygen. I. A search for an effect of oxygen 0.02 second after pulsed irradiation. Radiat. Res. 1958, 9, 422–437. [Google Scholar] [CrossRef]
- Lyng, H.; Monge, O.R.; Bøhler, P.J.; Rofstad, E.K. The relevance of tumour and surrounding normal tissue vascular density in clinical hyperthermia of locally advanced breast carcinoma. Int. J. Radiat. Biol. 1991, 60, 189–193. [Google Scholar] [CrossRef]
- Lyng, H.; Monge, O.R.; Bøhler, P.J.; Rofstad, E.K. Relationships between thermal dose and heat-induced tissue and vascular damage after thermoradiotherapy of locally advanced breast carcinoma. Int. J. Hyperth. 1991, 7, 403–415. [Google Scholar] [CrossRef]
- Henle, K.J.; Tomasovic, S.P.; Dethlefsen, L.A. Fractionation of combined heat and radiation in asynchronous CHO cells. I. Effects on radiation sensitivity. Radiat. Res. 1979, 80, 369–377. [Google Scholar] [CrossRef]
- Armour, E.P.; Wang, Z.; Corry, P.M.; Chen, P.Y.; Martinez, A. Hyperthermic enhancement of high dose-rate irradiation in 9L gliosarcoma cells. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 171–177. [Google Scholar] [CrossRef]
- Kötter, B.; Frey, B.; Winderl, M.; Rubner, Y.; Scheithauer, H.; Sieber, R.; Fietkau, R.; Gaipl, U.S. The in vitro immunogenic potential of caspase-3 proficient breast cancer cells with basal low immunogenicity is increased by hypofractionated irradiation. Radiat. Oncol. 2015, 10, 197. [Google Scholar] [CrossRef]
- Hader, M.; Savcigil, D.P.; Rosin, A.; Ponfick, P.; Gekle, S.; Wadepohl, M.; Bekeschus, S.; Fietkau, R.; Frey, B.; Schlücker, E.; et al. Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy. Cancers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Hader, M.; Streit, S.; Rosin, A.; Gerdes, T.; Wadepohl, M.; Bekeschus, S.; Fietkau, R.; Frey, B.; Schlücker, E.; Gekle, S.; et al. In Vitro Examinations of Cell Death Induction and the Immune Phenotype of Cancer Cells Following Radiative-Based Hyperthermia with 915 MHz in Combination with Radiotherapy. Cells 2021, 10, 1436. [Google Scholar] [CrossRef]
- Sengedorj, A.; Hader, M.; Heger, L.; Frey, B.; Dudziak, D.; Fietkau, R.; Ott, O.J.; Scheidegger, S.; Barba, S.M.; Gaipl, U.S.; et al. The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells. Cancers 2022, 14, 2050. [Google Scholar] [CrossRef]
- Mei, X.; Kok, H.P.; Rodermond, H.M.; van Bochove, G.G.W.; Snoek, B.C.; van Leeuwen, C.M.; Franken, N.A.P.; Ten Hagen, T.L.M.; Crezee, J.; Vermeulen, L.; et al. Radiosensitization by Hyperthermia Critically Depends on the Time Interval. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 817–828. [Google Scholar] [CrossRef]
- Hahn, E.W.; Alfieri, A.A.; Kim, J.H. The significance of fractionation regimens in radiation and combined hyperthermia using a murine fibrosarcoma. Cancer 1978, 42, 2596–2599. [Google Scholar] [CrossRef]
- Law, M.P. Some effects of fractionation on the response of the mouse ear to combined heat and X rays. Radiat. Res. 1979, 80, 360–368. [Google Scholar] [CrossRef]
- Stewart, F.A.; Denekamp, J. Fractionation studies with combined X rays and hyperthermia in vivo. Br. J. Radiol. 1980, 53, 346–356. [Google Scholar] [CrossRef]
- Overgaard, J. Fractionated radiation and hyperthermia: Experimental and clinical studies. Cancer 1981, 48, 1116–1123. [Google Scholar] [CrossRef]
- Baker, D.; Constable, W.; Sager, H.; Elkon, D. The effect of ultrasound-induced hyperthermia and X irradiation on skin. Radiat. Res. 1983, 96, 359–366. [Google Scholar] [CrossRef]
- Law, M.P.; Ahier, R.G.; Somaia, S. Thermal enhancement of radiation damage in previously irradiated skin. Br. J. Radiol. 1985, 58, 161–167. [Google Scholar] [CrossRef]
- Law, M.P.; Ahier, R.G. A long-term effect of prior irradiation on the thermal enhancement of radiation damage in the mouse ear. Int. J. Hyperth. 1987, 3, 167–175. [Google Scholar] [CrossRef]
- Baker, D.G.; Sager, H.T.; Constable, W.C. The response of a solid tumor to X-irradiation as modified by dose rate, fractionation, and hyperthermia. Cancer Investig. 1987, 5, 409–416. [Google Scholar] [CrossRef]
- Baker, D.G.; Constable, W.C.; Sager, H.; Kaiser, D.L. The effect of hyperthermia on radiation-induced carcinogenesis. Radiat. Res. 1988, 115, 448–460. [Google Scholar] [CrossRef]
- Zywietz, F.; Lierse, W. Changes in tumor vasculature under fractionated radiation-hyperthermia treatment. Recent Results Cancer Res. 1988, 107, 60–64. [Google Scholar] [CrossRef]
- Patrício, M.B.; Soares, J.; Vilhena, M. Morphologic and morphometric studies on tumor necrosis produced by radiotherapy, and hyperthermia singly and in combination. J. Surg. Oncol. 1989, 42, 5–10. [Google Scholar] [CrossRef]
- Koole, P.; Schipper, J. Treatment of the human retinoblastoma cell line Y-79 growing in the athymic mouse eye with fractionated hyperthermia and/or radiation. Int. J. Hyperth. 1990, 6, 203–211. [Google Scholar] [CrossRef]
- Zywietz, F. Vascular and cellular damage in a murine tumour during fractionated treatment with radiation and hyperthermia. Strahlenther. Onkol. 1990, 166, 493–501. [Google Scholar]
- Marino, C.; Cividalli, A. Combined radiation and hyperthermia: Effects of the number of heat fractions and their interval on normal and tumour tissues. Int. J. Hyperth. 1992, 8, 771–781. [Google Scholar] [CrossRef]
- Perez, C.A.; Patterson, J.H.; Emami, B. Evaluation of 45 degrees C hyperthermia and irradiation. I. Studies in a murine rhabdomyosarcoma model. Am. J. Clin. Oncol. 1993, 16, 469–476. [Google Scholar] [CrossRef]
- Nishimura, Y.; Urano, M. Timing and sequence of hyperthermia in fractionated radiotherapy of a murine fibrosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 605–611. [Google Scholar] [CrossRef]
- Nishimura, Y.; Urano, M. The effect of hyperthermia on reoxygenation during the fractionated radiotherapy of two murine tumors, FSa-II and MCa. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 141–148. [Google Scholar] [CrossRef]
- Zywietz, F. Simultaneous treatment of an experimental tumour with fractionated radiation and infrared-A-hyperthermia. Indian. J. Exp. Biol. 1996, 34, 833–837. [Google Scholar]
- Zywietz, F.; Reeker, W.; Kochs, E. Changes in tumor oxygenation during a combined treatment with fractionated irradiation and hyperthermia: An experimental study. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 155–162. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Patil, M.S.; Bhatt, M.B.; Bagewadikar, R.S.; Subramanian, M.; Rajan, R.; Kaklij, G.S.; Singh, B.B. Effect of whole body hyperthermia on radiation therapy of transplanted fibrosarcoma in Swiss mice. Int. J. Hyperth. 2001, 17, 428–438. [Google Scholar] [CrossRef]
- Hehr, T.; Budach, W.; Lamprecht, U.; Belka, C.; Classen, J.; Trübenbach, J.; Wehrmann, M.; Dietz, K.; Bamberg, M. Experimental thermoradiotherapy in malignant hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1374–1380. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.M.; Jamshidi-Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef]
- Dings, R.P.M.; Loren, M.L.; Zhang, Y.; Mikkelson, S.; Mayo, K.H.; Corry, P.; Griffin, R.J. Tumour thermotolerance, a physiological phenomenon involving vessel normalisation. Int. J. Hyperth. 2011, 27, 42–52. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Xi, X.; Hu, B.; Zhao, L.; Liao, Y.; Tang, J. Effects of magnetic induction hyperthermia and radiotherapy alone or combined on a murine 4T1 metastatic breast cancer model. Int. J. Hyperth. 2011, 27, 563–572. [Google Scholar] [CrossRef]
- Wittenborn, T.R.; Horsman, M.R. Targeting tumour hypoxia to improve outcome of stereotactic radiotherapy. Acta Oncol. 2015, 54, 1385–1392. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Quinn, T.J.; Scandiuzzi, L.; Basu, I.; Partanen, A.; Tomé, W.A.; Macian, F.; Guha, C. Low-Intensity Focused Ultrasound Induces Reversal of Tumor-Induced T Cell Tolerance and Prevents Immune Escape. J. Immunol. 2016, 196, 1964–1976. [Google Scholar] [CrossRef]
- van Oorschot, B.; Granata, G.; Di Franco, S.; ten Cate, R.; Rodermond, H.M.; Todaro, M.; Medema, J.P.; Franken, N.A.P. Targeting DNA double strand break repair with hyperthermia and DNA-PKcs inhibition to enhance the effect of radiation treatment. Oncotarget 2016, 7, 65504–65513. [Google Scholar] [CrossRef]
- Hoopes, P.J.; Wagner, R.J.; Song, A.; Osterberg, B.; Gladstone, D.J.; Bursey, A.A.; Fiering, S.N.; Giustini, A.J. The effect of hypofractionated radiation and magnetic nanoparticle hyperthermia on tumor immunogenicity and overall treatment response. Proc. SPIE Int. Soc. Opt. Eng. 2017, 10066, 105–110. [Google Scholar] [CrossRef][Green Version]
- Oei, A.L.; Korangath, P.; Mulka, K.; Helenius, M.; Coulter, J.B.; Stewart, J.; Velarde, E.; Crezee, J.; Simons, B.; Stalpers, L.J.A.; et al. Enhancing the abscopal effect of radiation and immune checkpoint inhibitor therapies with magnetic nanoparticle hyperthermia in a model of metastatic breast cancer. Int. J. Hyperth. 2019, 36, 47–63. [Google Scholar] [CrossRef]
- Prasad, B.; Kim, S.; Cho, W.; Kim, J.K.; Kim, Y.A.; Kim, S.; Wu, H.G. Quantitative Estimation of the Equivalent Radiation Dose Escalation using Radiofrequency Hyperthermia in Mouse Xenograft Models of Human Lung Cancer. Sci. Rep. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Skalina, K.A.; Singh, S.; Chavez, C.G.; Macian, F.; Guha, C. Low Intensity Focused Ultrasound (LOFU)-mediated Acoustic Immune Priming and Ablative Radiation Therapy for in situ Tumor Vaccines. Sci. Rep. 2019, 9, 15516. [Google Scholar] [CrossRef]
- Duval, K.E.A.; Petryk, J.D.; Crary-Burney, M.A.; Demidenko, E.; Wagner, R.J.; Hoopes, P.J. MNP hyperthermia and hypofractionated radiation activate similar immunogenetic and cytotoxic pathways. Int. J. Hyperth. 2020, 37, 929–937. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- De Vico, G.; Maiolino, P. Canine and Feline Models for Cancer. In Sourcebook of Models for Biomedical Research; Conn, P.M., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 677–682. [Google Scholar]
- Dewhirst, M.W.; Connor, W.G.; Sim, D.A. Preliminary results of a phase III trial of spontaneous animal tumors to heat and/or radiation: Early normal tissue response and tumor volume influence on initial response. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1951–1961. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Sim, D.A.; Wilson, S.; DeYoung, D.; Parsells, J.L. Correlation between initial and long-term responses of spontaneous pet animal tumors to heat and radiation or radiation alone. Cancer Res. 1983, 43, 5735–5741. [Google Scholar]
- Dewhirst, M.W.; Sim, D.A.; Sapareto, S.; Connor, W.G. Importance of minimum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res. 1984, 44, 43–50. [Google Scholar]
- Dewhirst, M.W.; Sim, D.A. Analysis of prognostic variables which influence early and long-term response of pet animal tumors to radiation alone and radiation plus heat. Front. Radiat. Ther. Oncol. 1984, 18, 47–55. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Sim, D.A. The utility of thermal dose as a predictor of tumor and normal tissue responses to combined radiation and hyperthermia. Cancer Res. 1984, 44, 4772s–4780s. [Google Scholar]
- Dewhirst, M.W.; Sim, D.A.; Forsyth, K.; Grochowski, K.J.; Wilson, S.; Bicknell, E. Local control and distant metastases in primary canine malignant melanomas treated with hyperthermia and/or radiotherapy. Int. J. Hyperth. 1985, 1, 219–234. [Google Scholar] [CrossRef]
- Gillette, E.L.; McChesney, S.L.; Dewhirst, M.W.; Scott, R.J. Response of canine oral carcinomas to heat and radiation. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1861–1867. [Google Scholar] [CrossRef]
- Thompson, J.M.; Dhoodhat, Y.A.; Bleehen, N.M.; Gorman, N.T. Microwave hyperthermia in the treatment of spontaneous canine tumours: An analysis of treatment parameters and tumour response. Int. J. Hyperth. 1988, 4, 383–399. [Google Scholar] [CrossRef]
- Denman, D.L.; Legorreta, R.A.; Kier, A.B.; Elson, H.R.; White, M.L.; Ralph Buncher, C.; Cooper Lewis, G.; Born, A.M.; Sundararaman, S.; Aron, B.S. Therapeutic responses of spontaneous canine malignancies to combinations of radiotherapy and hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 415–422. [Google Scholar] [CrossRef]
- Gillette, S.M.; Dewhirst, M.W.; Gillette, E.L.; Thrall, D.E.; Page, R.L.; Powers, B.E.; Withrow, S.J.; Rosner, G.; Wong, C.; Sim, D.A. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia: A randomized phase II study. Int. J. Hyperth. 1992, 8, 309–320. [Google Scholar] [CrossRef]
- Frew, D.G.; Dobson, J.M.; Stenning, S.P.; Bleehen, N.M. Response of 145 spontaneous canine head and neck tumours to radiation versus radiation plus microwave hyperthermia: Results of a randomized phase III clinical study. Int. J. Hyperth. 1995, 11, 217–230. [Google Scholar] [CrossRef]
- van der Zee, J.; Treurniet-Donker, A.D.; The, S.K.; Helle, P.A.; Seldenrath, J.J.; Meerwaldt, J.H.; Wijnmaalen, A.J.; van den Berg, A.P.; van Rhoon, G.C.; Broekmeyer-Reurink, M.P.; et al. Low dose reirradiation in combination with hyperthermia: A palliative treatment for patients with breast cancer recurring in previously irradiated areas. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 1407–1413. [Google Scholar] [CrossRef]
- van der Zee, J.; van der Holt, B.; Rietveld, P.J.; Helle, P.A.; Wijnmaalen, A.J.; van Putten, W.L.; van Rhoon, G.C. Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br. J. Cancer 1999, 79, 483–490. [Google Scholar] [CrossRef]
- Sridhar, P.S.; Abilash, G.H.; Roopesh, K.; Senapati, M.R.; Madhusudhan, N.; Kallur, K.; Kundavai, S.; Shubha, S.A.; Gurunath, K.; Rita, S.; et al. SBRT and Hyperthermia in Hepatocellular Carcinoma—Tertiary Cancer Centre Experience. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E228–E229. [Google Scholar] [CrossRef]
- Notter, M.; Piazena, H.; Vaupel, P. Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: A retrospective study of 73 patients. Int. J. Hyperth. 2017, 33, 227–236. [Google Scholar] [CrossRef]
- Notter, M.; Thomsen, A.R.; Nitsche, M.; Hermann, R.M.; Wolff, H.A.; Habl, G.; Münch, K.; Grosu, A.-L.; Vaupel, P. Combined wIRA-Hyperthermia and Hypofractionated Re-Irradiation in the Treatment of Locally Recurrent Breast Cancer: Evaluation of Therapeutic Outcome Based on a Novel Size Classification. Cancers 2020, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Notter, M.; Stutz, E.; Thomsen, A.R.; Vaupel, P.; Kok, H.P.; Ivkov, R.; Crezee, H. Radiation-Associated Angiosarcoma of the Breast and Chest Wall Treated with Thermography-Controlled, Contactless wIRA-Hyperthermia and Hypofractionated Re-Irradiation. Cancers 2021, 13, 3911. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Stutz, E.; Puric, E.; Eberle, B.; Meister, A.; Marder, D.; Timm, O.; Rogers, S.; Wyler, S.; Bodis, S. A Pilot Study of Radiotherapy and Local Hyperthermia in Elderly Patients With Muscle-Invasive Bladder Cancers Unfit for Definitive Surgery or Chemoradiotherapy. Front. Oncol. 2019, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hahn, E.W.; Ahmed, S.A. Combination hyperthermia and radiation therapy for malignant melanoma. Cancer 1982, 50, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, G.; Nervi, C.; Cividalli, A.; Lovisolo, G.A. Problem of sequence and fractionation in the clinical application of combined heat and radiation. Cancer Res. 1984, 44, 4857s–4863s. [Google Scholar]
- Gonzalez Gonzalez, D.; van Dijk, J.D.; Blank, L.E.; Rümke, P. Combined treatment with radiation and hyperthermia in metastatic malignant melanoma. Radiother. Oncol. 1986, 6, 105–113. [Google Scholar] [CrossRef]
- Arcangeli, G.; Benassi, M.; Cividalli, A.; Lovisolo, G.A.; Mauro, F. Radiotherapy and Hyperthermia. Analysis of clinical results and identification of prognostic variables. Cancer 1987, 60, 950–956. [Google Scholar] [CrossRef]
- Howard, G.C.; Sathiaseelan, V.; Freedman, L.; Bleehen, N.M. Hyperthermia and radiation in the treatment of superficial malignancy: An analysis of treatment parameters, response and toxicity. Int. J. Hyperth. 1987, 3, 1–8. [Google Scholar] [CrossRef]
- Lindholm, C.E.; Kjellen, E.; Nilsson, P.; Hertzman, S. Microwave-induced hyperthermia and radiotherapy in human superficial tumours: Clinical results with a comparative study of combined treatment versus radiotherapy alone. Int. J. Hyperth. 1987, 3, 393–411. [Google Scholar] [CrossRef]
- Overgaard, J.; Overgaard, M. Hyperthermia as an adjuvant to radiotherapy in the treatment of malignant melanoma. Int. J. Hyperth. 1987, 3, 483–501. [Google Scholar] [CrossRef]
- Berdov, B.A.; Menteshashvili, G.Z. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int. J. Hyperth. 1990, 6, 881–890. [Google Scholar] [CrossRef]
- Masunaga, S.I.; Hiraoka, M.; Akuta, K.; Nishimura, Y.; Nagata, Y.; Jo, S.; Takahashi, M.; Abe, M.; Terachi, T.; Oishi, K. Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int. J. Hyperth. 1994, 10, 31–40. [Google Scholar] [CrossRef]
- Perez, C.A.; Pajak, T.; Emami, B.; Hornback, N.B.; Tupchong, L.; Rubin, P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am. J. Clin. Oncol. 1991, 14, 133–141. [Google Scholar] [CrossRef]
- Overgaard, J.; Gonzalez Gonzalez, D.; Hulshof, M.C.; Arcangeli, G.; Dahl, O.; Mella, O.; Bentzen, S.M. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef]
- Vernon, C.; Hand, J.; Field, S.; Machin, D.; Whaley, J.; Zee, J.; Vanputten, W.; Van Rhoon, G.; Vandijk, J. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar] [CrossRef]
- Chi, M.S.; Yang, K.L.; Chang, Y.C.; Ko, H.L.; Lin, Y.H.; Huang, S.C.; Huang, Y.Y.; Liao, K.W.; Kondo, M.; Chi, K.H. Comparing the Effectiveness of Combined External Beam Radiation and Hyperthermia Versus External Beam Radiation Alone in Treating Patients With Painful Bony Metastases: A Phase 3 Prospective, Randomized, Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 78–87. [Google Scholar] [CrossRef]
- Kapp, D.S.; Petersen, I.A.; Cox, R.S.; Hahn, G.M.; Fessenden, P.; Prionas, S.D.; Lee, E.R.; Meyer, J.L.; Samulski, T.V.; Bagshaw, M.A. Two or six hyperthermia treatments as an adjunct to radiation therapy yield similar tumor responses: Results of a randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1481–1495. [Google Scholar] [CrossRef]
- Emami, B.; Myerson, R.J.; Cardenes, H.; Paris, K.G.; Perez, C.A.; Straube, W.; Leybovich, L.; Mildenberger, M.; Kuske, R.R.; Devineni, V.R.; et al. Combined hyperthermia and irradiation in the treatment of superficial tumors: Results of a prospective randomized trial of hyperthermia fractionation (1/wk vs. 2/wk). Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 145–152. [Google Scholar] [CrossRef]
- Overgaard, J.; Nielsen, O.S. The importance of thermotolerance for the clinical treatment with hyperthermia. Radiother. Oncol. 1983, 1, 167–178. [Google Scholar] [CrossRef]
- Law, M.P. The response of normal tissues to hyperthermia. In Hyperthermia and Oncology: Thermal Effects on Cells and Tissues; Urano, M., Douple, E., Eds.; CRC Press/Taylor and Francis Group: Utrecht, The Netherlands, 1988; Volume 1, pp. 121–159. [Google Scholar]
- Nielsen, O.S. Influence of thermotolerance on the interaction between hyperthermia and radiation in L1A2 cells in vitro. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1983, 43, 665–673. [Google Scholar] [CrossRef]
- Nielsen, O.S.; Overgaard, J.; Kamura, T. Influence of thermotolerance on the interaction between hyperthermia and radiation in a solid tumour in vivo. Br. J. Radiol. 1983, 56, 267–273. [Google Scholar] [CrossRef] [PubMed]
- van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef] [PubMed]
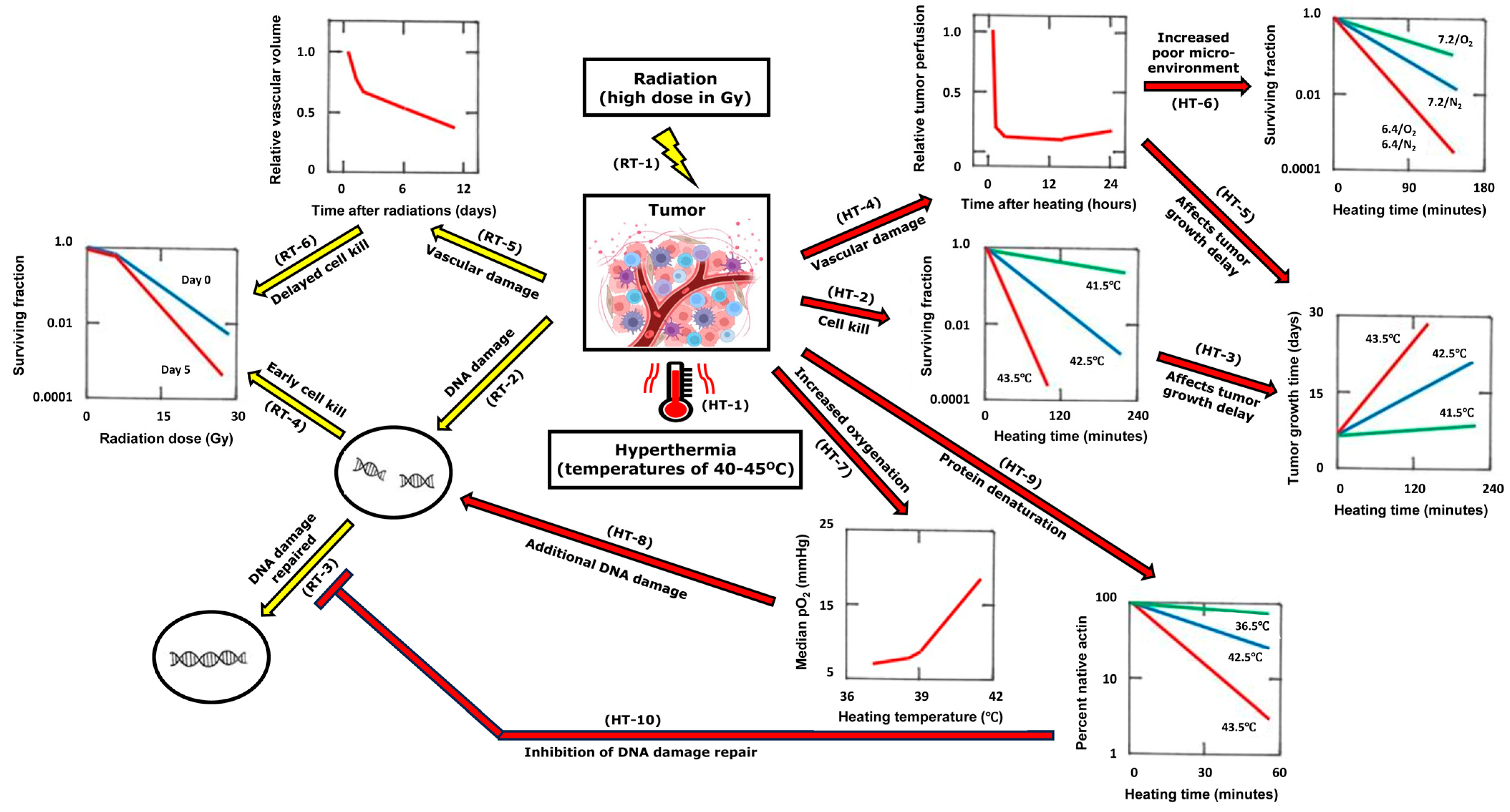
| Cell/Tumor Type | Radiation (RT) | Hyperthermia (HT) | RT-HT Sequence/Interval | Endpoints | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| CHO Cells | 1st RT = 4 Gy; 2nd RT = various doses (0/2/4/8/24 h interval) | Water bath; 45 °C for 10 min (0–2 HT fx) | HT 2.5 min before 1st RT and/or 2nd RT | Clonogenic cell survival | Cell-killing kinetics of fractionated RT and HT are more complex and not always the same as single treatments | [78] |
| 9L gliosarcoma | 5 × 5 Gy (Within 3 days) | Water bath; 41 °C various times | 8 h before 1st RT to end of RT (~60 h) | Clonogenic cell survival | Effect of WBH so not relevant | [79] |
| MCF-7 and MDA-MB-231 breast | 4 × 4 Gy or 6 × 3 Gy | HT chamber; 41 °C for 1 h | HT 4 h before or after 1st and last RT | Apoptosis and necrosis | HT before RT increases MDA-MD-231 apoptosis independent of RT schemes; HT after RT increases MCF-7 necrosis slightly only for 4 × 4 Gy RT | [80] |
| MCF-7 and MDA-MB-231 breast | 2 × 5 Gy (days 0 + 3) or 5 × 2 Gy (24 h intervals) | Warm water and microwaves; 39, 41, 44 °C for 1 h | HT-2 h-1st RT | Apoptosis and necrosis | HT temperature dependent benefit, independent of RT scheme; microwaves better than warm water | [81] |
| MCF-7 and MDA-MB-231 breast | 2 × 5 Gy (days 0 + 3) or 5 × 2 Gy (24 h intervals) | Microwaves; 39, 41, 44 °C for 1 h | HT-2 h-1st RT | Apoptosis and necrosis | HT temperature-dependent benefit, independent of RT scheme | [82] |
| MCF-7 and MDA-MB-231 breast | 2 × 5 Gy (24 h intervals) | Microwaves; 39, 41, 44 °C for 1 h | HT-2 h before/after 1st RT | Apoptosis and necrosis | No sequence dependence; HT temperature-dependent benefit | [83] |
| Organoids from cervical cancer patients | 3 × 4 Gy (24 h intervals) | Water bath; 42 °C for 1 h | HT 0–8 h before or after 1st RT | Organoid number ratio from day 8 to day 1 | Shorter interval had the greatest HT effect | [84] |
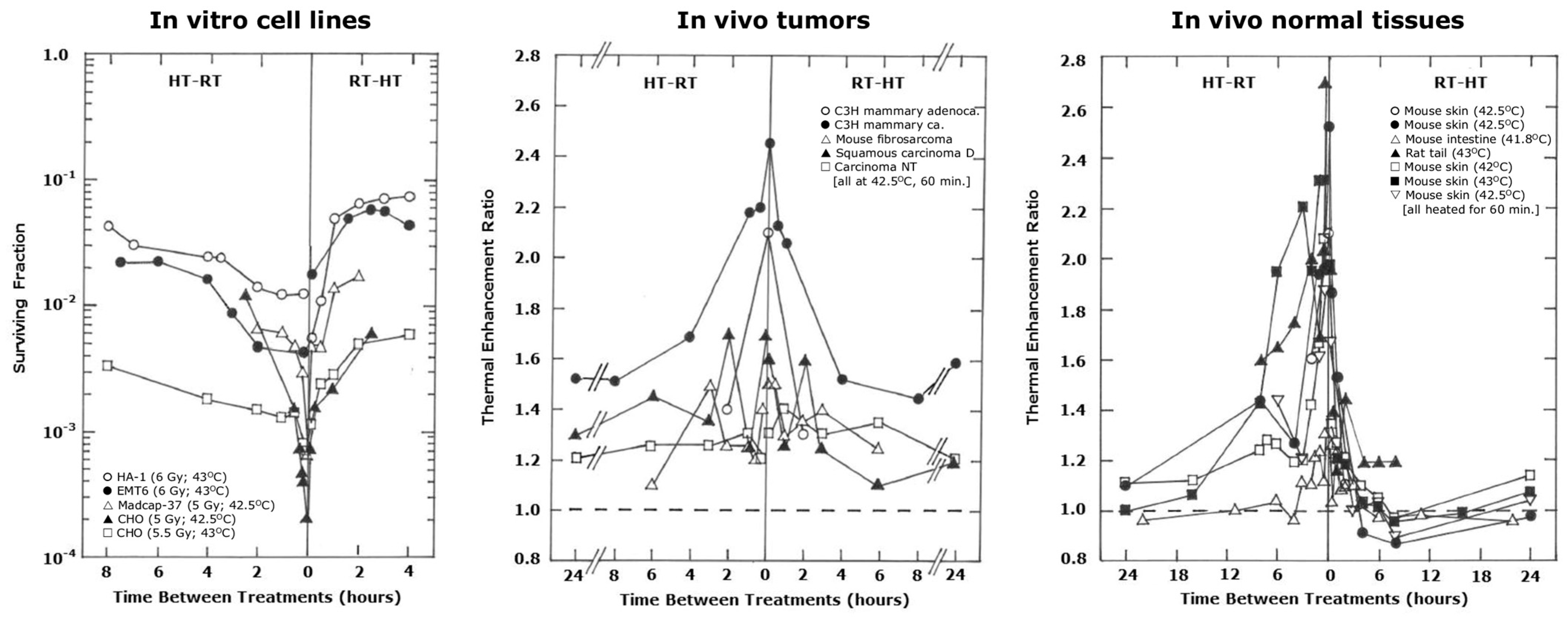
| Tumor and/or Normal Tissue (NT) | Radiation (RT) | Hyperthermia (HT) | RT-HT Sequence/Interval | Endpoints | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| Fibrosarcoma (mice) | 4 × 4.5–9 Gy (1–4 day intervals) | Water bath; 4 × 43.1 °C/15 min | RT- < 2 min-HT | Ex vivo cell survival, tumor growth, mouse survival | RT+HT superior to RT alone for all endpoints | [85] |
| Mouse ear | Various doses in 1/2/5/10 fx (24 h interval) | Water bath; 42.5 °C/30 min or 43.5 °C/30 min | HT 6 min before/after RT (HT with all RT fx) | Acute normal skin | TER dependent on temperature; HT-RT(TER) > RT-HT(TER) | [86] |
| Fibrosarcoma (mice) and mouse leg | 15–50 Gy in 1/2/5 fx (24 h intervals) | Water bath; 42.5 °C/1 h | RT-0/3 h-HT (HT with all RT fx) | Tumor growth and acute normal skin | TGF for 1 fx RT seen with 3 h interval; no TGF for 2/5 fx RT | [87] |
| C3H mammary carcinoma (mice) and surrounding skin | Various doses in 1/5 fx (24 or 72 h intervals) | Water bath; 42.5 °C/1 h | RT-0/4 h-HT (HT with all or last RT fx) | Tumor control and acute normal skin | TGF seen with RT-4 h-HT; TGF improved with 72 h interval between RT fx; TGF seen with both all/last HT | [88] |
| Mouse leg | 1 × 10/15/20 Gy or 3 × 10 Gy (48 h intervals) | Ultrasound; 42.5–43 °C/0.5–1 h | HT ≤ 0 –1 h before or after RT | Acute normal skin | TER for 3 × 10 Gy RT < TER for 1 × 10 Gy RT; TER independent of sequence or HT duration | [89] |
| Mouse ear (Re-irradiation) | RT: 1 × 17 Gy or 10 × 3.4 Gy daily fx; reRT: various doses | Water bath; 43 °C/15 min | RT-3 to 12 months-reRT-6 min-HT | Acute normal skin, late ear deformity | Previous RT increases HT sensitivity; no effect of fractionation for all endpoints | [90] |
| Mouse ear (Re-irradiation) | RT: 1 × 19 Gy or 10 × 3.8 Gy daily fx; reRT: various doses | Water bath; 43 °C/12 min | RT-10 months-reRT; HT given 6 min before or after reRT | Acute normal skin, late ear deformity | Previous RT increases HT sensitivity; no effect of fractionation; HT-RT showed more effect than RT-HT for all endpoints | [91] |
| KHT sarcoma (mice) | 3 × 10 Gy (2/3/4 days intervals) given with low (0.19 Gy/min) and high (2.12 Gy/min) dose rates | Water bath; 42.5 °C/30 min Ultrasound; 42.5 °C/30 min | HT-0.1 h-RT | Tumor control and metastasis | HT enhanced RT with both endpoints; high dose rate gave better HT enhancement; HT by ultrasound had better enhancement with high dose rate RT only | [92] |
| Mouse leg | 1 × 30 Gy or 6 × 6 Gy (48 h intervals) | Water bath; 37–43 °C/45 min after all RT fx | RT-0.1 h-HT | Acute and late normal skin, carcinogenesis | HT effects seen for acute; late damage: RT+HT less than RT alone; no carcinogenic effect; no effect of fractionation | [93] |
| R1H rhabdomyosarcoma (rats) | 25 × 3 Gy (5 fx/week) | Microwaves; 2 × 43 °C/1 h (Monday/Friday) | RT-10 min-HT | Vascular changes | RT+HT showed more vascular damage than RT alone | [94] |
| Breast carcinoma Tx and Sarcoma 37 (mice) | 2 × 8.5 Gy (48 h interval) | No heat method stated; 2 × 43.5 °C/30 min (48 h interval) | HT after RT | Tumor necrosis | RT+HT showed more tumor necrosis than RT alone for both tumor models | [95] |
| Y-79 eye implanted retinoblastoma (mice) | 3–9 × 3 Gy (3 fx/week) | Coaxial heating; 1–3 × 43 °C/30 min or 1–3 × 45 °C/30 min | RT-15 min-HT | Tumor control | HT enhanced 3/6 × 3 Gy; 8/9 × 3 Gy alone too effective for HT effect to be seen | [96] |
| R1H rhabdomyosarcoma (rats) | 25 × 3 Gy (5 fx/week) | Microwaves; 2 × 43 °C/1 h (Monday/Friday) | RT-10 min-HT | Vascular changes | RT+HT showed more vascular damage than RT alone | [97] |
| C3H mammary carcinoma (mice) and mouse leg | Various doses in 20 fx given daily or 20 fx in 26 days (weekend gap) | Water bath; 43 °C/1 h (1 HT with 1st RT only, 4 HT every 5 or 7 days, 8 HT every Monday and Thursday) | RT-0 or 4 h-HT | Tumor control and acute normal skin | 4 HT fx with 0 or 4 h intervals showed the best TGF | [98] |
| RIF-1 rhabdomyosarcoma and surrounding skin (mice) | 10 × 4 Gy (72 h interval) | Radiofrequency; 10 × 45 °C/15 min or 10 × 43 °C/60 min (72 h intervals) | RT followed by HT | Tumor regression, regrowth, curability and acute normal skin | RT+HT > RT alone for all tumor endpoints; RT+HT has similar skin reaction to RT alone; | [99] |
| FSa-II fibrosarcoma (mice) and mouse leg | Various doses in 5 daily fx | Water bath; 43.5 °C/45 min | HT-24 h-1st RT; 1st RT- ≤ 2 min-HT; 5th RT- ≤ 2 min-HT; 5th RT-0.5 h-HT; 5th RT-4 h-HT; 5th RT-24 h-HT | Tumor growth and acute normal skin | HT enhanced RT in tumors and acute NT, so no TGF for any interval or sequence. | [100] |
| Various doses in 10 daily fx | HT-24 h-1st RT; 1st RT- ≤ 2 min-HT; 10th RT- ≤ 2 min-HT; 10th RT-24 h-HT | Tumor control and partial foot atrophy (late) | HT enhanced RT in tumors and late NT, so no TGF for any interval or sequence | |||
| FSa-II fibrosarcoma and MCa mammary carcinoma (mice) and mouse leg | Many doses in 2/5/10/20 fx (24 h intervals for 2/5/10 fx; 20 fx with 6/18 h intervals) | Water bath; 43.5 °C/45 min | HT-24 h-1st RT | Tumor control, pO2 and partial foot atrophy (late) | HT before RT did not affect pO2 or tumor growth; no TGF for either tumor model; MCa more HT sensitive than FSa-II | [101] |
| R1H rhabdomyosarcoma (rats) | 8 × 4 Gy (2 fx/week) | Infrared; 8 × 43 °C/1 h | Simultaneous RT-HT | Tumor growth | RT+HT better than RT alone | [102] |
| R1H rhabdomyosarcoma (rats) | 20 × 3 Gy (5 fx/week) | Microwaves; 8 × 43 °C/1 h (Monday/Friday) | RT-10 min-HT | Changes in pO2 | RT+HT induced larger decrease than RT alone | [103] |
| Fibrosarcoma (mice) | 1 × 20 Gy or 5 × 7.5 Gy (24 h intervals) | Temperature-controlled cage (WBH); 1 × 39 °C/1 h | HT-20 h-RT | Tumor growth | WBH + fx RT significantly better than RT alone for tumor growth; no WBH effect seen for 1 × 20 Gy | [104] |
| Morris hepatoma 3924A (rats) | 10 × 2.5–4.5 Gy (5 days/week) | Radiofrequency; 4× (22 min ≥ 40 °C + 10 min ≥ 41 °C) | HT-<10 min-RT (HT Tuesday/Thursday) | Tumor growth | HT effect better with higher RT dose per fx | [105] |
| FSa-II fibrosarcoma (mice) | 7 × 3 Gy (24 h intervals) | Water bath; 4–7 × 41.5 °C/1 h 24–48 h intervals | HT before/after RT | Tumor growth | Small benefit of daily HT+RT regardless of schedule; but on alternative days, HT before RT was best | [106] |
| B16F10 melanoma (mice) | 2 × 5 Gy (5 Gy each given on days 8 and 9 post-inoculation) | Water bath; 2 × 41.5 °C/1 h on days 7 and 8 post-inoculation | HT followed by RT on day 8 | Tumor growth | RT+HT better than RT alone | [107] |
| 4T1 breast (mice) | 2 × 10 Gy (24 h intervals) | Magnetic-induced HT by implanted thermoseeds; 2 × 41–45 °C/10 min | Simultaneous RT and HT | Tumor growth, metastasis, mouse survival | RT+HT better than RT alone for all endpoints | [108] |
| C3H mammary carcinoma (mice) | 3 × 15 Gy (3 fx/week) | Water bath; 41.5 °C/1 h | RT-4 h-HT (HT with all or last RT fx) | Tumor control | HT significantly enhanced RT, irrespective of 1 or 3 HT fx | [109] |
| Various B16 melanoma cells lines (mice) | 3 × 10 Gy (24 h intervals) | LOFU; temperature/time not stated | LOFU-24 h-RT | Tumor growth, metastasis, mouse survival | RT+HT better than RT for all endpoints | [110] |
| SiHa cervix tumors (mice) | 3 × 4 Gy (24 h intervals) | Water bath; 42 °C/1 h | HT was applied to 1st RT only (no interval/sequence stated) | Tumor growth, apoptosis | No HT effects seen in tumor growth, but significantly higher apoptosis in RT+HT | [111] |
| B16F10 melanoma (mice) | 3 × 5 Gy (48 h intervals) | Tumor implanted INP + AMF; 2 × 43 °C/30 min | Sequence/interval not stated | Mouse survival | RT+HT better than RT alone | [112] |
| 4T1 breast (mice) | 3 × 8 Gy (24 h intervals) | Tumor implanted INP + AMF; 1 × 43 °C/20 min or (1 × 45 °C/5 min + 43 °C/15 min) | HT before 1st RT only, but interval time not stated | Tumor growth, metastasis | HT significantly enhanced RT-induced growth delay with higher temperature; no temperature effect on metastasis | [113] |
| Lung cancer xenografts (mice) | 2 × 5 Gy (48 h intervals) | Radiofrequency; 2 × 42 °C/30 min | HT-≤4 h-RT | Tumor growth, apoptosis | RT+HT > RT alone for all endpoints | [114] |
| TPSA24 prostate adenocarcinoma (mice) | 2 × 10 Gy (48 h intervals) | LOFU; max. temp 45 °C no duration stated; (1.5 s at focal point) | LOFU-2 to 3 h-RT | Tumor growth, control, mouse survival | RT+HT > RT alone for all end points | [115] |
| B16F10 melanoma (mice) | 1 × 15 Gy or 3 × 8 Gy (24 h intervals) | Tumor implanted INP+AMF; 1 × 43 °C/30 min | INP-3 h-AMF-1 h-RT | Tumor growth | RT+HT better than RT alone independent of RT scheme | [116] |
| SiHa cervix tumors (mice) | 3 × 4 Gy (24 h intervals) | Water bath; 42 °C/1 h | HT 0/2/4/8 h before and after 1st RT only | Tumor growth | Best effect with shorter interval; sequence independent | [84] |
| Tumor Type | Radiation (RT) | Hyperthermia (HT) | RT-HT Sequence/Interval | Endpoints | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| Dogs (52) and cats (20) with various types of tumors; phase III(randomized) | 4.6 Gy/fx; 2 fx/week for 4 weeks | Radiofrequency 44° C/30 min; 1x/week | HT-10 min-RT | Tumor control and early/long-term NT response | Overall CR for RT+HT > RT alone; HT only not effective; RT+HT had better CR for larger tumors; similar early reaction in all groups; no late damage | [119] |
| Dogs and cats (130) with various types of tumors (randomized) | 4.6 Gy/fx; 2 fx/week for 4 weeks | High-frequency current or microwaves 44 °C/30 min; 1x/week | HT-10 min-RT | Tumor control | Overall CR for HT+RT > RT alone; HT prolonged response duration | [120] |
| CR for RT+HT better with high-frequency current heating and more uniform heating | [121] | |||||
| Tumor control and early/long-term NT response | HT enhanced early normal tissue response, but less than in tumor; HT did not enhance late NT response | [122] | ||||
| Dogs (166) and cats (70) with various types of tumors (randomized) | 4.6 Gy/fx; 2 fx/week for 4 weeks | High-frequency current or microwaves 44 °C/30 min; 1x/week | HT-10 min-RT | Tumor control and early/long-term NT response | Smaller tumors, high-frequency current heating method, and higher temperature minima significantly improve CR; early/late NT response similar in RT+HT and RT alone groups | [123] |
| Dogs (43) with primary malignant melanoma (randomized) | 4.6 Gy/fx; 2 fx/week for 4 weeks | High-frequency current or microwaves 42 °C/30 min; 1x/week 42 °C/60 min; 2x/week | HT-0 h-RT, or RT-2–3 h-HT | Tumor control | Overall CR for RT+HT > RT alone; higher control with higher temperature minima; uniform heating, smaller tumor volume, and no nodal metastasis improve CR | [124] |
| Dogs (38) with oral carcinomas (randomized) | 2.5–5 Gy/fx; 10 fx in 22 days | Low radiofrequency current or ultrasound ≥42 °C/30 min; 2x/week | HT 3 h after RT treatments on days 1, 3, 4, 6, 7, 9, and 10 | Tumor control and late NT response (necrosis) | No significant difference in the TCD50 value for RT alone (38 Gy) and RT+HT (33 Gy); no late NT necrosis found | [125] |
| Dogs (51) with various tumors phase I/II (non-randomized) | 9–10 Gy/fx; 1x/week for 4 weeks | Microwaves 1 or 2x 44 °C/30 min | RT-10–20 min-HT | Tumor control | 2xHT had significantly better CR than 1x HT | [126] |
| Dogs (113) with various tumors; phase III (randomized) | 3.5 Gy/fx; 3x/week for 14 fx | Microwaves 44 °C/30 min; 1x/week | RT-30 min-HT, or RT-4–5 h-HT | Tumor control and early/late NT response | Overall CR for RT+HT > RT alone regardless of interval; HT significantly enhanced early and late NT response | [127] |
| Dogs (64) with spontaneous soft tissue sarcomas; phase II (randomized) | 3.5–5.5 Gy/fx; 10 fx in 22 days | Ultrasound 42 °C/30 min; 2x/week | RT-3 h-HT | Tumor control and late NT response | Overall CR for RT+HT similar to RT alone but heat significantly prolonged local tumor control; late NT response similar in both arms | [128] |
| Dogs (145) with spontaneous head and neck tumors; phase III (randomized) | 9 Gy/fx; 1x/week for 4 weeks | Microwaves 2× 44 °C/30 min; | HT within 30 min of 1st and 2nd RT | Tumor control and early/late NT response | Overall CR for RT+HT similar to RT alone; similar early NT toxicity in both arms; but more late NT response (skin reactions) in RT+HT group | [129] |
| Study Characteristics | Radiation (RT) | Hyperthermia (HT) | RT-HT Sequence/Interval | Clinical Findings | Ref. |
|---|---|---|---|---|---|
| (a) Non-randomized studies | |||||
| Multiple recurrent malignant melanoma lesions (99) in 38 patients RT (54 lesions) RT+HT (45 lesions) | 13 × 3.3 Gy or 10 × 4 Gy; 2x/week 7 × 5.5 Gy or 6 × 6.6 Gy; 1x/week | Radiofrequency 42–43.5 °C for 30 min; 1 or 2x/week | HT-3–6 min-RT | Overall CR: HT+RT > RT alone; no enhanced normal tissue morbidity; 1x/week CR better than 2x/week CR indicating better control with higher RT dose/fx | [137] |
| Various superficial lesions (163) in 77 patients; 71 lesions received 5–6 Gy/fx RT (31 lesions) RT+HT (40 lesions) | 8 × 5 Gy; 2x/week | Microwaves or radiofrequency 42.5 °C for 45 min; 2x/week | RT-0 h-HT RT-4 h-HT | CR significantly better with higher HT temperature, higher RT dose/fx, and lower time interval between RT-HT; No increased skin damage at 45 °C due to skin around lesion cooling | [138] |
| 5 × 6 Gy; 2x/week | Microwaves or radiofrequency 45 °C for 30 min; 2x/week | RT-0 h-HT | |||
| Metastatic malignant melanoma lesions (49) in 24 patients RT (8 lesions) RT+HT (38 lesions) HT (3 lesions) | 3 × 6 Gy or 3 × 8 Gy; 1x/week 6 × 4 Gy or 6 × 5 Gy; 2x/week | Microwaves 43 °C for 1 h; 1 or 2x/week | RT- ≤ 30 min-HT | CR: RT+HT > RT alone; no CR for HT only lesions; higher Gy/fx showed better CR | [139] |
| Cutaneous and nodal malignant melanoma metastatic lesions (38) in 17 patients RT (17 lesions) RT+HT (21 lesions) | 8 × 5 Gy; 2x/week 5 × 6 Gy; 2x/week | Radiofrequency 42.5 °C for 45 min; (5 Gy/fx); 2x/week 45 °C for 30 min; (6 Gy/fx); 2x/week | RT-0 h-HT | CR: RT+HT greater than RT alone but not significant for both schedules; CR prolonged in both arms for 6–24 months | [140] |
| Superficial lesions (41) in 16 patients RT (21 lesions) RT+HT (20 lesions) | 6 × 4 Gy; 2 fx/week (Majority) | Radiofrequency 43 °C for 60 min; 2x/week | RT-30 min-HT | Overall response (CR+PR): RT+HT > RT alone, but CR is similar for both arms; increased skin reaction in HT group related to HT dose; also 3 late fibrosis in RT+HT arm | [141] |
| Superficial recurrent malignant lesions (56) in 18 patients RT (28 lesions) RT+HT (28 lesions) | 10 × 3 Gy; 5 fx/week | Microwaves 41–45 °C for 45 min; 2x/week | RT-0.5–1.5 h or 3–4 h-HT (HT with 2nd RT/week) | CR for RT+HT > RT alone (matched lesions); some local pain and normal tissue reactions but controlled | [142] |
| Cutaneous and nodal malignant melanoma metastatic lesions (115) in 36 patients RT (62 lesions) RT+HT (53 lesions) | 3 × 5–10 Gy; 3 fx in 8 days | Capacitive or radiofrequency 43 °C for 60 min; 3 fx in 8 days | RT< 0.5 h-HT (simultaneous) RT-3–4 h-HT (sequential) | TER simultaneous: 1.43 (tumor) and 1.42 (skin); TER sequential: 1.24 (tumor) and 1.02 (skin); therefore, TGF sequential (1.22) greater than TGF simultaneous (1.01) | [143] |
| Locally advanced rectal cancer RT (59 patients) RT+HT (56 patients) | 10 × 4 Gy; 3x/week | Electromagnetic 42–43 °C for 1 h; 4–5 HT fx in total | HT-10 min-RT HT started from 3rd RT fx | RT+HT significantly enhanced primary tumor regression and 5-year survival | [144] |
| Urinary bladder cancer (49 patients); phase I/II RT (21 patients) RT+HTlow (12 patients) RT+HThigh (16 patients) | 6 × 4 Gy; 3x/week | Capacitive heating 2x/week for 35–60 min; Intravesical average (Tav) = 41.5 °C, further classified into HTlow(<41.5 °C) and HThigh (≥41.5 °C) | HT immediately after RT | Tumor degradation and downstaging by thermoradiotherapy significantly higher when Tav ≥ 41.5 °C; local recurrence and survival similar in all three arms. | [145] |
| (b) Randomized studies | |||||
| Various superficial tumors (237 evaluable patients); phase III RT (117 patients) RT+HT (120 patients) | 8 × 4 Gy; 2x/week | Microwaves 42°C for 1 h; 2x/week | RT-0.25–0.5 h -HT | Overall CR: RT+HT similar to RT alone; significantly higher CR in smaller (<3 cm) tumors due to better heating of smaller tumors; acute/late toxicities comparable in both arms | [146] |
| Recurrent or metastatic malignant melanoma (128 tumors in 68 patients); phase III RT (65 tumors) RT+HT (63 tumors) | 3 × 8/9 Gy; 4-day intervals | Microwave or radiofrequency 3 × 43 °C for 1 h | RT- < 30 min-HT | RT+HT had a significantly better effect on CR than RT alone, with no effect on acute or late RT reactions | [147] |
| Superficial localized breast cancer (56 patients in ESHO trial protocol); phase III RT (27 patients) RT+HT (29 patients) | 8 × 4 Gy; 2x/week | Electromagnetic 42.5–43 °C for 30–60 min; 4–8 HT fx with ≥3 days between HT sessions | RT-0.5–1 h-HT | CR (ESHO only): RT+HT significantly greater than RT alone; odds ratio of 5.7 strongly in favor of RT+HT arm | [148] |
| Bone metastasis resulting in Brief pain inventory (BPI ≥ 4) (57 patients); phase III RT (28 patients) RT+HT (29 patients) | 10 × 3 Gy; 5x/week | Capacitive Normal rectal (42.5 °C) or esophageal (41.5 °C) temperatures reported, as bone metastasis temperatures are not measurable 2x/week for 40 min | RT- < 2 h-HT | Compared to RT alone, RT+HT significantly increased the pain control rate and extended response duration; pain control is attributed to complete control of bone metastatic lesions | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, P.M.; Folefac, C.A.; Overgaard, J.; Horsman, M.R. The Rationale for Combining Hypofractionated Radiation and Hyperthermia. Cancers 2024, 16, 3916. https://doi.org/10.3390/cancers16233916
Sinha PM, Folefac CA, Overgaard J, Horsman MR. The Rationale for Combining Hypofractionated Radiation and Hyperthermia. Cancers. 2024; 16(23):3916. https://doi.org/10.3390/cancers16233916
Chicago/Turabian StyleSinha, Priyanshu M., Charlemagne A. Folefac, Jens Overgaard, and Michael R. Horsman. 2024. "The Rationale for Combining Hypofractionated Radiation and Hyperthermia" Cancers 16, no. 23: 3916. https://doi.org/10.3390/cancers16233916
APA StyleSinha, P. M., Folefac, C. A., Overgaard, J., & Horsman, M. R. (2024). The Rationale for Combining Hypofractionated Radiation and Hyperthermia. Cancers, 16(23), 3916. https://doi.org/10.3390/cancers16233916





