Risk Factors for Surgical Wound Infection and Fascial Dehiscence After Open Gynecologic Oncologic Surgery: A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Standard of Care
2.2. Statistical Analyses
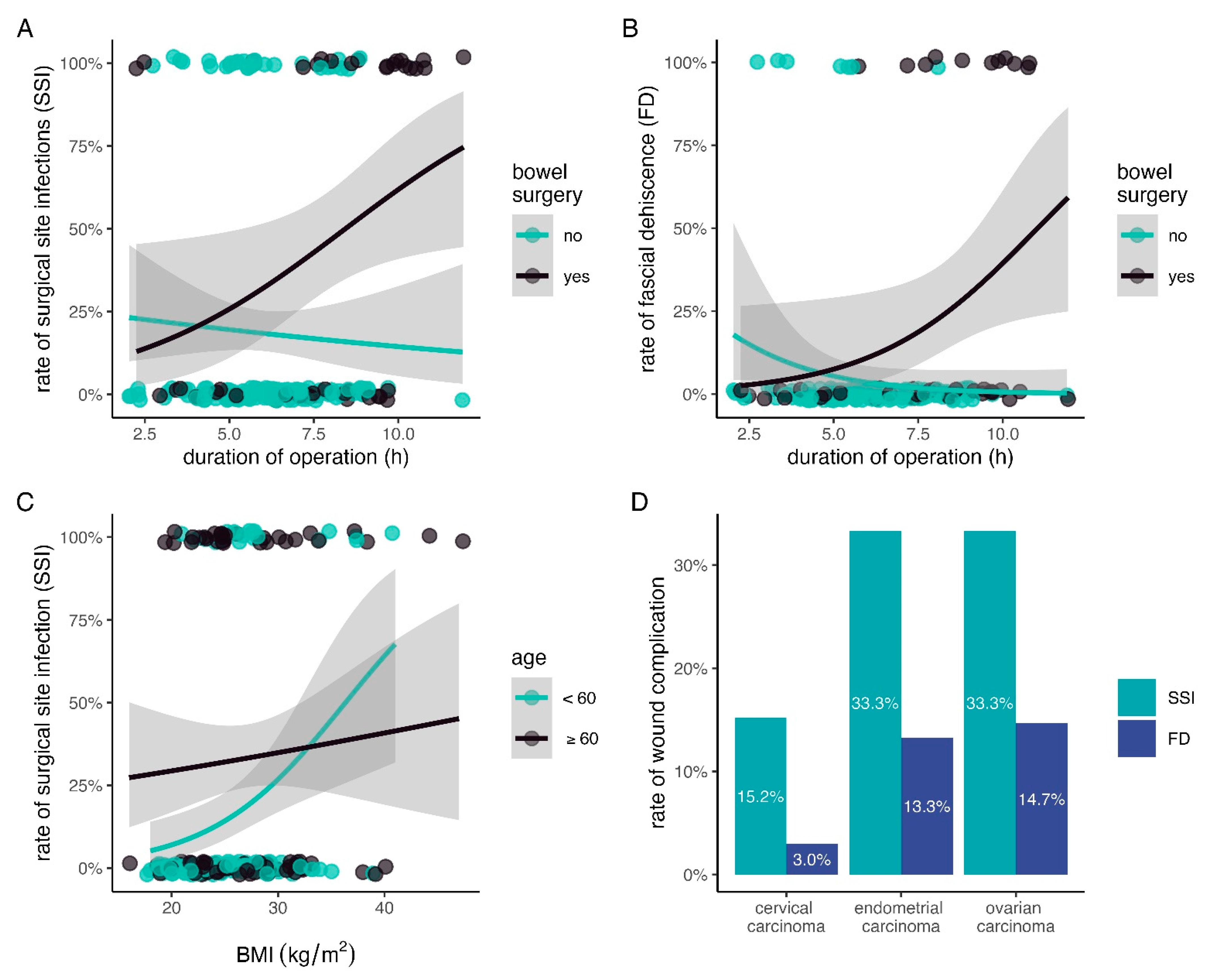
3. Results
4. Discussion
4.1. Summary of the Main Results
4.2. Results in the Context of the Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Surgical Side Infection Event (SSI). Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf (accessed on 2 December 2024).
- Kenig, J.; Richter, P.; Żurawska, S.; Lasek, A.; Zbierska, K. Risk Factors for Wound Dehiscence after Laparotomy–Clinical Control Trial. Pol. Przegl. Chir. 2012, 84, 565–573. [Google Scholar] [PubMed]
- Steiner, H.L.; Strand, E.A. Surgical-Site Infection in Gynecologic Surgery: Pathophysiology and Prevention. Am. J. Obs. Gynecol. 2017, 217, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, K.B.; Briggs, J.P.; Trivette, S.L.; Wilkinson, W.E.; Sexton, D.J. The Impact of Surgical-Site Infections in the 1990s: Attributable Mortality, Excess Length of Hospitalization, and Extra Costs. Infect. Control. Hosp. Epidemiol. 1999, 20, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Seaman, S.J.; Han, E.; Arora, C.; Kim, J.H. Surgical Site Infections in Gynecology: The Latest Evidence for Prevention and Management. Curr. Opin. Obs. Gynecol. 2021, 33, 296–304. [Google Scholar] [CrossRef]
- Chen, I.; Choudhry, A.J.; Schramm, D.; Cameron, D.W.; Leung, V.; Singh, S.S.; Hopkins, L.; Arendas, K.; Mallick, R. Type of Pelvic Disease as a Risk Factor for Surgical Site Infectionin Women Undergoing Hysterectomy. J. Minim. Invasive Gynecol. 2019, 26, 1149–1156. [Google Scholar] [CrossRef]
- Black, J.D.; de Haydu, C.; Fan, L.; Sheth, S.S. Surgical Site Infections in Gynecology. Obstet. Gynecol. Surv. 2014, 69, 501–510. [Google Scholar] [CrossRef]
- Pellegrini, J.E.; Toledo, P.; Soper, D.E.; Bradford, W.C.; Cruz, D.A.; Levy, B.S.; Lemieux, L.A. Consensus Bundle on Prevention of Surgical Site Infections After Major Gynecologic Surgery. J. Obs. Gynecol. Neonatal Nurs. 2017, 46, 100–113. [Google Scholar] [CrossRef]
- Davidson, C.; Enns, J.; Bennett, C.; Sangi-Haghpeykar, H.; Lundeen, S.; Eppes, C. Reducing Abdominal Hysterectomy Surgical Site Infections: A Multidisciplinary Quality Initiative. Am. J. Infect. Control 2020, 48, 1292–1297. [Google Scholar] [CrossRef]
- Guo, X.M.; Runge, M.; Miller, D.; Aaby, D.; Milad, M. A Bundled Intervention Lowers Surgical Site Infection in Hysterectomy for Benign and Malignant Indications. Int. J. Gynaecol. Obs. 2020, 150, 392–397. [Google Scholar] [CrossRef]
- Schiavone, M.B.; Moukarzel, L.; Leong, K.; Zhou, Q.C.; Afonso, A.M.; Iasonos, A.; Roche, K.L.; Leitao, M.M., Jr.; Chi, D.S.; Abu-Rustum, N.R.; et al. Surgical Site Infection Reduction Bundle in Patients with Gynecologic Cancer Undergoing Colon Surgery. Gynecol. Oncol. 2017, 147, 115–119. [Google Scholar] [CrossRef]
- Bruce, S.F.; Carr, D.N.; Burton, E.R.; Sorosky, J.I.; Shahin, M.S.; Naglak, M.C.; Edelson, M.I. Implementation of an Abdominal Closure Bundle to Reduce Surgical Site Infection in Patients on a Gynecologic Oncology Service Undergoing Exploratory Laparotomy. Gynecol. Oncol. 2018, 149, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Petca, A.; Rotar, I.C.; Borislavschi, A.; Petca, R.C.; Danau, R.A.; Dumitrascu, M.C.; Sandru, F.; Pacu, I. Adapting Surgical ‘Bundles’ to Prevent Surgical Site Infections in Obstetrics and Gynecology (Review). Exp. Ther. Med. 2022, 24, 695. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.; Neumayer, L.; Smout, R.; Horn, S.; Daley, J.; Henderson, W.; Khuri, S.; Program National Veterans Affairs Surgical Quality Improvement. Prognostic Models of Abdominal Wound Dehiscence after Laparotomy. J. Surg. Res. 2003, 109, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, B.P.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef]
- van Ramshorst, G.H.; Nieuwenhuizen, J.; Hop, W.C.; Arends, P.; Boom, J.; Jeekel, J.; Lange, J.F. Abdominal Wound Dehiscence in Adults: Development and Validation of a Risk Model. World J. Surg. 2010, 34, 20–27. [Google Scholar] [CrossRef]
- Tran, C.W.; McGree, M.E.; Weaver, A.L.; Martin, J.R.; Lemens, M.A.; Cliby, W.A.; Dowdy, S.C.; Bakkum-Gamez, J.N. Surgical Site Infection after Primary Surgery for Epithelial Ovarian Cancer: Predictors and Impact on Survival. Gynecol. Oncol. 2015, 136, 278–284. [Google Scholar] [CrossRef]
- Shi, L.; Gu, Q.; Zhang, F.; Li, D.; Ye, W.; Zhong, Y.; Shi, X. Predictive Factors of Surgical Site Infection after Hysterectomy for Endometrial Carcinoma: A Retrospective Analysis. BMC Surg. 2021, 21, 292. [Google Scholar] [CrossRef]
- Nugent, E.K.; Hoff, J.T.; Gao, F.; Massad, L.S.; Case, A.; Zighelboim, I.; Mutch, D.G.; Thaker, P.H. Wound Complications after Gynecologic Cancer Surgery. Gynecol. Oncol. 2011, 121, 347–352. [Google Scholar] [CrossRef]
- AlHilli, M.; Langstraat, C.; Tran, C.; Martin, J.; Weaver, A.; McGree, M.; Mariani, A.; Cliby, W.; Bakkum-Gamez, J. Risk Factors and Indications for 30-Day Readmission after Primary Surgery for Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 193–202. [Google Scholar] [CrossRef]
- Helgeland, J.; Tomic, O.; Hansen, T.M.; Kristoffersen, D.T.; Hassani, S.; Lindahl, A.K. Postoperative Wound Dehiscence after Laparotomy: A Useful Healthcare Quality Indicator? A Cohort Study Based on Norwegian Hospital Administrative Data. BMJ Open 2019, 9, e026422. [Google Scholar] [CrossRef]
- Robert Koch Institute. Prävention Postoperativer Wundinfektionen—Empfehlung Der Kommission Für Krankenhaushygiene Und Infektionsprävention (Krinko) Beim Robert Koch-Institut. Bundesgesundheitsblatt 2018, 61, 448–473. [Google Scholar] [CrossRef] [PubMed]
- Lachiewicz, M.P.; Moulton, L.J.; Jaiyeoba, O. Pelvic Surgical Site Infections in Gynecologic Surgery. Infect. Dis. Obs. Gynecol. 2015, 2015, 614950. [Google Scholar] [CrossRef] [PubMed]
- Boland, P.A.; Kelly, M.E.; Donlon, N.E.; Bolger, J.C.; Mehigan, B.J.; McCormick, P.H.; Larkin, J.O. Prophylactic Negative Pressure Wound Therapy for Closed Laparotomy Wounds: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Ir. J. Med. Sci. 2021, 190, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Desale, M.G.; Tanner, E.J., 3rd; Sinno, A.K.; Angarita, A.A.; Fader, A.N.; Stone, R.L.; Levinson, K.L.; Bristow, R.E.; Roche, K.L. Perioperative Fluid Status and Surgical Outcomes in Patients Undergoing Cytoreductive Surgery for Advanced Epithelial Ovarian Cancer. Gynecol. Oncol. 2016, 144, 61–64. [Google Scholar] [CrossRef]
- Sivam, N.S.; Suresh, S.; Hadke, M.S.; Kate, V.; Ananthakrishnan, N. Results of the Smead-Jones Technique of Closure of Vertical Midline Incisions for Emergency Laparotomies—A Prospective Study of 403 Patients. Trop. Gastroenterol. 1995, 16, 62–67. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S.; Studivant, R.X. Applied Logistic Regression; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Calcagno, V.; de Mazancourt, C. Glmulti: An R Package for Easy Automated Model Selection with (Generalized) Linear Models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Kupper, L.L.; Morgenstern, H. Epidemiologic Research: Principles and Quantitative Methods; John Wiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Iyer, R.; Gentry-Maharaj, A.; Nordin, A.; Burnell, M.; Liston, R.; Manchanda, R.; Das, N.; Desai, R.; Gornall, R.; Beardmore-Gray, A.; et al. Predictors of Complications in Gynaecological Oncological Surgery: A Prospective Multicentre Study (Ukgosoc—Uk Gynaecological Oncology Surgical Outcomes and Complications). Br. J. Cancer 2015, 112, 475–484. [Google Scholar] [CrossRef]
- Fox, N.S.; Melka, S.; Miller, J.; Bender, S.; Silverstein, M.; Saltzman, D.H.; Rebarber, A. Suture Compared with Staple Closure of Skin Incision for High-Order Cesarean Deliveries. Obs. Gynecol. 2018, 131, 523–528. [Google Scholar] [CrossRef]
- Tomita, K.; Chiba, N.; Ochiai, S.; Yokozuka, K.; Gunji, T.; Hikita, K.; Ozawa, Y.; Okihara, M.; Sano, T.; Tsutsui, R.; et al. Superficial Surgical Site Infection in Hepatobiliary-Pancreatic Surgery: Subcuticular Suture Versus Skin Staples. J. Gastrointest. Surg. 2018, 22, 1385–1393. [Google Scholar] [CrossRef]
- Tsujinaka, T.; Yamamoto, K.; Fujita, J.; Endo, S.; Kawada, J.; Nakahira, S.; Shimokawa, T.; Kobayashi, S.; Yamasaki, M.; Akamaru, Y.; et al. Subcuticular Sutures Versus Staples for Skin Closure after Open Gastrointestinal Surgery: A Phase 3, Multicentre, Open-Label, Randomised Controlled Trial. Lancet 2013, 382, 1105–1112. [Google Scholar] [CrossRef]
- Riley, R.D.; Snell, K.I.; Ensor, J.; Burke, D.L.; Harrell, F.E., Jr.; Moons, K.G.; Collins, G.S. Minimum Sample Size for Developing a Multivariable Prediction Model: Part Ii-Binary and Time-to-Event Outcomes. Stat. Med. 2019, 38, 1276–1296. [Google Scholar] [CrossRef]
| Characteristic | Complete Cohort | No Wound Complication | Wound Complication | |
|---|---|---|---|---|
| N = 204 | N = 154 | SSI: N = 50 | FD: N = 18 | |
| age (years) | 57.5 (42.0–67.0) | 54 (42.0–64.0) | 65.0 (50.5–73.8) | 65.5 (61.3–74.0) |
| BMI (kg/m2) | 25.0 (22.0–29.0) | 25.0 (22.0–29.0) | 26.0 (24.0–30.8) | 25.0 (23.0–27.8) |
| diabetes | 16 (7.8%) | 6 (3.9%) | 10 (20.0%) | 3 (16.7%) |
| cervical carcinoma | 99 (48.5%) | 84 (54.5%) | 15 (30.0%) | 3 (16.7%) |
| endometrial carcinoma | 30 (14.7%) | 20 (13.0%) | 10 (20.0%) | 4 (22.2%) |
| ovarian carcinoma | 75 (36.8%) | 50 (32.5%) | 25 (50.0%) | 11 (61.1%) |
| operation time (hours) | 6.2 (5.1–7.8) | 6.2 (5.0–7.4) | 6.7 (5.3–8.8) | 7.9 (5.5–9.8) |
| bowel surgery performed * | 47 (23.0%) | 26 (16.9%) | 21 (42.0%) | 11 (61.1%) |
| neoadjuvant chemotherapy | 15 (7.4%) | 10 (6.5%) | 5 (10.0%) | 2 (11.1%) |
| ASA score 1 | 31 (15.2%) | 29 (18.8%) | 2 (4.0%) | 1 (5.6%) |
| ASA score 2 | 147 (72.1%) | 111 (72.1%) | 36 (72.0%) | 14 (77.8%) |
| ASA score 3 | 24 (11.8%) | 13 (8.4%) | 11 (22.0%) | 3 (16.7%) |
| ASA score 4 | 2 (1.0%) | 1 (0.6%) | 1 (2.0%) | 0 (0.0%) |
| active smoker | 47 (23.0%) | 36 (23.4%) | 11 (22.0%) | 2 (11.1%) |
| hemoglobin (mmol/L) before surgery | 8.1 (7.4–8.5) | 8.1 (7.5–8.5) | 8.1 (7.0–8.5) | 8.2 (7.8–8.9) |
| serum protein (g/L) before surgery | 72.0 (68.0–74.0) | 72.0 (68.0–74.0) | 72.0 (67.0–75.0) | 70.0 (66.3–74.0) |
| units of crystalloids given during surgery | 6 (5–9) | 6 (5–8) | 7 (5–10) | 10 (5–12) |
| laparotomy extended over umbilicus | 152 (74.9%) | 109 (71.2%) | 43 (86.0%) | 15 (83.3%) |
| skin closure with subcuticular suture | 48 (24.4%) | 43 (29.1%) | 5 (10.2%) | 2 (11.8%) |
| skin closure with stapler | 149 (75.6%) | 105 (70.9%) | 44 (89.8%) | 15 (88.2%) |
| Characteristic | Univariable Regression | Multivariable Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| operation time (per hours) | 1.21 | (1.03–1.42) | 0.024 | 0.98 | (0.74–1.28) | 0.88 |
| bowel surgery performed * | 3.56 | (1.76–7.23) | <0.001 | 4.59 | (1.24–18.2) | 0.024 |
| operation time (per hour, if bowel surgery performed) ‡ | 1.46 | (1.01–2.12) | 0.044 | 1.52 | (1.03–2.29) | 0.039 |
| age (decades) | 1.44 | (1.15–1.84) | 0.002 | 1.19 | (0.85–1.69) | 0.31 |
| BMI (per 10 kg/m2) | 2.39 | (1.32–4.45) | 0.005 | 2.48 | (1.12–5.70) | 0.028 |
| BMI (per 10 kg/m2 and per decade) ‡ | 0.59 | (0.36–0.94) | 0.027 | 0.48 | (0.26–0.83) | 0.013 |
| diabetes | 6.17 | (2.16–19.1) | <0.001 | 1.94 | (0.49–7.83) | 0.34 |
| laparotomy extended over umbilicus | 2.48 | (1.09–6.40) | 0.041 | 0.78 | (0.24–2.65) | 0.69 |
| hemoglobin (mmol/L) before surgery | 0.79 | (0.57–1.11) | 0.17 | 0.89 | (0.59–1.35) | 0.58 |
| units of crystalloids given during surgery | 1.07 | (0.97–1.18) | 0.15 | 0.87 | (0.72–1.03) | 0.10 |
| ASA score | ||||||
| 1 | ref. | ref. | ref. | ref. | ||
| 2 | 4.70 | (1.32–30.0) | 0.041 | 2.46 | (0.59–16.9) | 0.27 |
| 3 | 12.3 | (2.80–87.0) | 0.003 | 4.26 | (0.64–39.3) | 0.15 |
| 4 | 14.5 | (0.46–489) | 0.093 | 7.11 | (0.19–285) | 0.25 |
| type of cancer | ||||||
| cervical carcinoma | ref. | ref. | ref. | ref. | ||
| endometrial carcinoma | 2.80 | (1.08–7.14) | 0.031 | 1.57 | (0.46–5.20) | 0.46 |
| ovarian carcinoma | 2.80 | (1.36–5.92) | 0.006 | 0.68 | (0.17–2.49) | 0.57 |
| skin closure technique | ||||||
| subcuticular suture | ref. | ref. | ref. | ref. | ||
| stapler | 3.60 | (1.45–10.9) | 0.011 | 3.05 | (0.97–11.2) | 0.069 |
| Characteristic | Univariable Regression | Multivariable Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| operation time (per hour) | 1.31 | (1.03–1.68) | 0.03 | 0.6 | (0.34–0.98) | 0.06 |
| bowel surgery performed * | 6.55 | (2.41–18.9) | <0.001 | 8.57 | (0.91–94.9) | 0.06 |
| operation time (per hour, if bowel surgery performed) ‡ | 2.38 | (1.28–4.43) | 0.006 | 2.48 | (1.34–5.1) | 0.007 |
| age (per decade) | 1.61 | (1.13–2.42) | 0.01 | 1.51 | (0.90–2.65) | 0.13 |
| diabetes | 2.66 | (0.57–9.44) | 0.16 | 1.08 | (0.17–5.71) | 0.93 |
| units of crystalloids given during surgery | 1.18 | (1.04–1.33) | 0.01 | 1.06 | (0.84–1.32) | 0.62 |
| ASA score | ||||||
| 1 | ref. | ref. | ref. | ref. | ||
| 2 | 3.16 | (0.6–58.3) | 0.28 | 0.49 | (0.05–10.7) | 0.56 |
| 3 | 4.29 | (0.51–90) | 0.22 | 0.31 | (0.02–9.33) | 0.44 |
| 4 | n/a | n/a | n/a | n/a | ||
| type of cancer | ||||||
| cervical carcinoma | ref. | ref. | ref. | ref. | ||
| endometrial carcinoma | 4.92 | (1.03–26.3) | 0.05 | 4.01 | (0.58–32.5) | 0.16 |
| ovarian carcinoma | 5.50 | (1.64–25.0) | 0.01 | 0.57 | (0.06–4.98) | 0.61 |
| skin closure technique | ||||||
| subcuticular suture | ref. | ref. | ref. | ref. | ||
| stapler | 2.57 | (0.69–16.7) | 0.22 | 1.4 | (0.28–10.6) | 0.71 |
| Publication (Year) | SSI (In %) | FD (In %) | Number of Patients | Significant Risk Factors |
|---|---|---|---|---|
| Nugent et al. (2011) [19] | 34 | n/a | 373 | BMI, pulmonary disease, albumin, prior abdominal surgery, length of surgery, pelvic drain placement, lysis of adhesions performed |
| Tran et al. (2015) * [17] | 10.8 | n/a | 888 | increasing BMI, increasing operative time, advanced tumor stage |
| Shi et al. (2021) ‡ [18] | 14.47 | n/a | 318 | FIGO stage IV, open surgery, duration of drainages ≥ 7 d, postoperative serum albumin < 30 g/L, postoperative blood sugar ≥ 10 mmol/L |
| Our publication (2024) | 24.5 | 8.8 | 204 | bowel surgery, operation time (if bowel surgery was performed), BMI (depending on the age of the patient) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagedorn, C.; Dornhöfer, N.; Aktas, B.; Weydandt, L.; Lia, M. Risk Factors for Surgical Wound Infection and Fascial Dehiscence After Open Gynecologic Oncologic Surgery: A Retrospective Cohort Study. Cancers 2024, 16, 4157. https://doi.org/10.3390/cancers16244157
Hagedorn C, Dornhöfer N, Aktas B, Weydandt L, Lia M. Risk Factors for Surgical Wound Infection and Fascial Dehiscence After Open Gynecologic Oncologic Surgery: A Retrospective Cohort Study. Cancers. 2024; 16(24):4157. https://doi.org/10.3390/cancers16244157
Chicago/Turabian StyleHagedorn, Carolin, Nadja Dornhöfer, Bahriye Aktas, Laura Weydandt, and Massimiliano Lia. 2024. "Risk Factors for Surgical Wound Infection and Fascial Dehiscence After Open Gynecologic Oncologic Surgery: A Retrospective Cohort Study" Cancers 16, no. 24: 4157. https://doi.org/10.3390/cancers16244157
APA StyleHagedorn, C., Dornhöfer, N., Aktas, B., Weydandt, L., & Lia, M. (2024). Risk Factors for Surgical Wound Infection and Fascial Dehiscence After Open Gynecologic Oncologic Surgery: A Retrospective Cohort Study. Cancers, 16(24), 4157. https://doi.org/10.3390/cancers16244157







