Retrospective Analysis of HLA Class II-Restricted Neoantigen Peptide-Pulsed Dendritic Cell Vaccine for Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Cancer Patients and Samples
2.2. Whole Exome Sequencing and RNA Sequencing (RNA-seq)
2.3. Read Mapping and Variant Calling
2.4. Neoantigen Prediction
2.5. Generation and Administration of DC Vaccines
2.6. Administration of Neo-P DC Vaccine Therapy
2.7. IFN-γ ELISpot Assay
2.8. Isolation of CD8-Positive T Cells and CD4-Positive T Cells
2.9. T-Cell Receptor (TCR) Sequencing Analysis
2.10. Statistical Analysis
3. Results
3.1. Treatment with Neo-P DC Vaccine for Postoperative Breast Cancer Patients
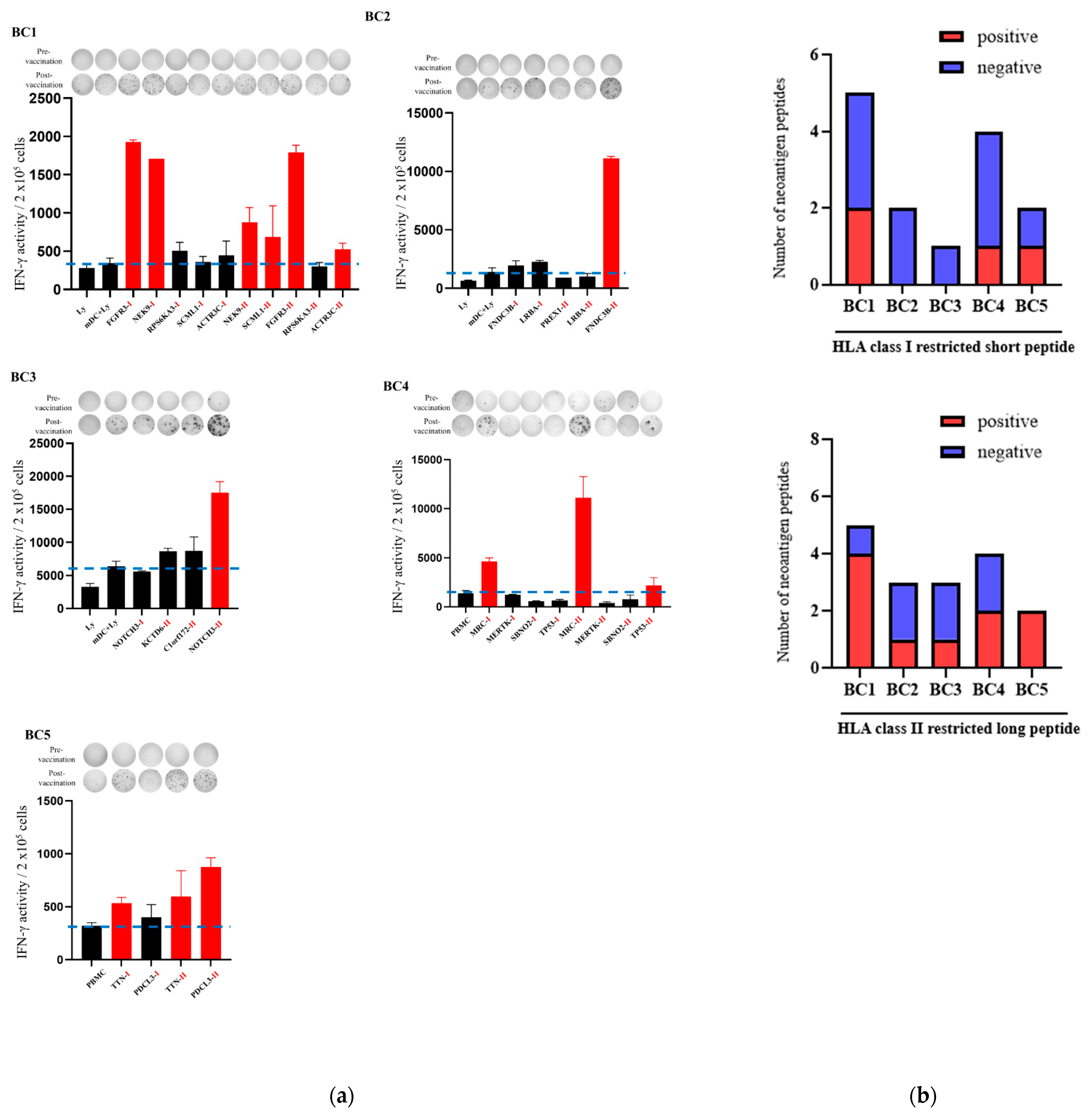
3.2. Immune Responses After Intranodal Neo-P DC Vaccine Administration
3.3. HLA Class II-Restricted Neoantigen Long Peptide Encompassing HLA Class I-Restricted Epitope-Pulsed DCs Activated CD8-Positive T Cells and CD4-Positive T Cells
3.4. TCR Repertoire Changes After Neo-P DC Vaccine
4. Discussion
- (1)
- The peripheral lymphocyte response was enhanced for both HLA class I-restricted neoantigens and HLA class II-restricted neoantigens. The increase in lymphocyte response was particularly marked for HLA class II-restricted neoantigens containing HLA class I affinity neoantigen epitopes.
- (2)
- TCR repertoire analysis of three patients before and after vaccination showed that clonality increased in two of the three cases after Neo-P DC vaccination.
- (3)
- At the time of publication, there have been no relapses or adverse events in the five breast cancer patients.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Zhang, X.; Schuster, H.; Caron, E.; Ward, J.P.; Noguchi, T.; Ivanova, Y.; Hundal, J.; Arthur, C.D.; Krebber, W.J.; et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014, 515, 577–581. [Google Scholar] [CrossRef]
- McGranahan, N.; Furness, A.J.S.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef]
- Keenan, T.E.; Burke, K.P.; Van Allen, E.M. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019, 25, 389–402. [Google Scholar] [CrossRef]
- Puig-Saus, C.; Sennino, B.; Peng, S.; Wang, C.L.; Pan, Z.; Yuen, B.; Purandare, B.; An, D.; Quach, B.B.; Nguyen, D.; et al. Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy. Nature 2023, 615, 697–704. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Fang, Y.; Mo, F.; Shou, J.; Wang, H.; Luo, K.; Zhang, S.; Han, N.; Li, H.; Ye, S.; Zhou, Z.; et al. A pan-cancer clinical study of personalized neoantigen vaccine monotherapy in treating patients with various types of advanced solid tumors. Clin. Cancer Res. 2020, 26, 4511–4520. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Brightman, S.E.; Naradikian, M.S.; Miller, A.M.; Schoenberger, S.P. Harnessing neoantigen specific CD4 T cells for cancer immunotherapy. J. Leukoc. Biol. 2020, 107, 625–633. [Google Scholar] [CrossRef]
- Kreiter, S.; Vormehr, M.; van de Roemer, N.; Diken, M.; Löwer, M.; Diekmann, J.; Boegel, S.; Schrörs, B.; Vascotto, F.; Castle, J.C.; et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015, 520, 692–696. [Google Scholar] [CrossRef]
- Garg, A.D.; Vara Perez, M.; Schaaf, M.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Dendritic cell-based anticancer immunotherapy. Oncoimmunology 2017, 6, e1328341. [Google Scholar] [CrossRef]
- Saxena, M.; Bhardwaj, N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef]
- Kiyotani, K.; Chan, H.T.; Nakamura, Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Cancer Sci. 2018, 109, 542–549. [Google Scholar] [CrossRef]
- Morisaki, T.; Morisaki, T.; Kubo, M.; Onishi, H.; Hirano, T.; Morisaki, S.; Eto, M.; Monji, K.; Takeuchi, A.; Nakagawa, S.; et al. Efficacy of intranodal neoantigen peptide-pulsed dendritic cell vaccine monotherapy in patients with advanced solid tumors: A retrospective analysis. Anticancer Res. 2021, 41, 4101–4115. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Szolek, A.; Schubert, B.; Mohr, C.; Sturm, M.; Feldhahn, M.; Kohlbacher, O. OptiType: Precision HLA typing from next-generation sequencing data. Bioinformatics. 2014, 30, 3310–3316. [Google Scholar] [CrossRef]
- Bai, Y.; Ni, M.; Cooper, B.; Wei, Y.; Fury, W. Inference of high resolution HLA types using genome-wide RNA or DNA sequencing reads. BMC Genom. 2014, 15, 325. [Google Scholar] [CrossRef]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef]
- Morisaki, T.; Morisaki, T.; Kubo, M.; Morisaki, S.; Nakamura, Y.; Onishi, H. Lymph nodes as anti-tumor immunotherapeutic tools: Intranodal-tumor-specific antigen-pulsed dendritic cell vaccine immunotherapy. Cancers 2022, 14, 2438. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Kiyotani, K.; Yap, K.L.; Campanile, A.; Antic, T.; Yew, P.Y.; Steinberg, G.; Park, J.H.; Nakamura, Y.; O’Donnell, P.H. Low T-cell receptor diversity, high somatic mutation burden, and high neoantigen load as predictors of clinical outcome in muscle-invasive bladder cancer. Eur. Urol. Focus. 2016, 2, 445–452. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Boyadzhyan, A.; Sasaninia, K.; Rai, V. Targeting neoantigens in cancer: Possibilities and opportunities in breast cancer. Antibodies 2024, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Miceli, A.P.; Kizhvatov, I.; DuPage, M.; Luoma, A.M.; Meng, W.; Lichti, C.F.; Esaulova, E.; Vomund, A.N.; et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019, 574, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Schiffman, K.; Disis, M.L. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J. Clin. Investig. 2001, 107, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Ohkuri, T.; Homma, S.; Ohtake, J.; Wakita, D.; Togashi, Y.; Kitamura, H.; Todo, S.; Nishimura, T. First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer-hybrid epitope long peptide of MAGE-A4 cancer antigen. Cancer Sci. 2012, 103, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Hunder, N.N.; Wallen, H.; Cao, J.; Hendricks, D.W.; Reilly, J.Z.; Rodmyre, R.; Jungbluth, A.; Gnjatic, S.; Thompson, J.A.; Yee, C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008, 358, 2698–2703. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Yossef, R.; Cafri, G.; Paria, B.C.; Lowery, F.J.; Jafferji, M.; Good, M.L.; Sachs, A.; Copeland, A.R.; Kim, S.P.; et al. Antigen Experienced T Cells from Peripheral Blood Recognize p53 Neoantigens. Clin. Cancer Res. 2020, 266, 1267–1276. [Google Scholar] [CrossRef]
- Palermo, B.; Del Bello, D.; Sottini, A.; Serana, F.; Ghidini, C.; Gualtieri, N.; Ferraresi, V.; Catricalà, C.; Belardelli, F.; Proietti, E.; et al. Dacarbazine treatment before peptide vaccination enlarges T-cell repertoire diversity of melan-a-specific, tumor-reactive CTL in melanoma patients. Cancer Res. 2010, 70, 7084–7092. [Google Scholar] [CrossRef]
- Connerotte, T.; Van Pel, A.; Godelaine, D.; Tartour, E.; Schuler-Thurner, B.; Lucas, S.; Thielemans, K.; Schuler, G.; Coulie, P.G. Functions of Anti-MAGE T-cells induced in melanoma patients under different vaccination modalities. Cancer Res. 2008, 68, 3931–3940. [Google Scholar] [CrossRef]
- Poran, A.; Scherer, J.; Bushway, M.E.; Besada, R.; Balogh, K.N.; Wanamaker, A.; Williams, R.G.; Prabhakara, J.; Ott, P.A.; Hu-Lieskovan, S.; et al. Combined TCR repertoire profiles and blood cell phenotypes predict melanoma patient response to personalized neoantigen therapy plus anti-PD-1. Cell Rep. Med. 2020, 1, 100141. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- El-Sayes, N.; Vito, A.; Mossman, K. Tumor heterogeneity: A great barrier in the age of cancer immunotherapy. Cancers 2021, 13, 806. [Google Scholar] [CrossRef]



| ID | Age/Sex | Subtype | Biopsy Site | Adjuvant Therapy | Metastasis | Stage | Years Since Diagnosis |
|---|---|---|---|---|---|---|---|
| BC1 | 51/F | Luminal | Breast | ET | none | I | 0 |
| BC2 | 57/F | Luminal | Breast | ET | none | I | 0 |
| BC3 | 63/F | Luminal | LN | ET, CT | LN | IV | 6 |
| BC4 | 35/F | TNBC | Breast | CT | LN | II | 0 |
| BC5 | 37/F | Luminal | Breast | CT | none | II | 0 |
| ID | No. of Exonic Mutations | No. of nsSNVs | No. of HLA Class I Neoantigens | No. of HLA Class II Neoantigens |
|---|---|---|---|---|
| BC1 | 21 | 12 | 56 | 758 |
| BC2 | 25 | 13 | 71 | 868 |
| BC3 | 62 | 41 | 208 | 1241 |
| BC4 | 52 | 25 | 72 | 1145 |
| BC5 | 337 | 22 | 53 | 191 |
| ID | Gene | Exp. Level | AA Change | HLA Class II Neoantigen | Affinity (nm), HLA Type | HLA Class I Peptide | Affinity (nm), HLA Type |
|---|---|---|---|---|---|---|---|
| BC1 | NEK9 | 37 | D542V | VQCGCVGTFLLTQSGKV | 169, DRB1:0405 | VQCGCVGTF | 33, HLA-B15:01 |
| SCML1 | 25 | R199Q | TAKVLCYYIDQLKQGKCF | 165, DRB1:0405 | QLKQGKCF | 151, HLA-B15:01 | |
| FGFR3 | 31 | G380R | AGSVYAGILSYRVGFFLF | 85, DRB1:0405 | SYRVGFFLF | 14, HLA-B24:02 | |
| RPS6KA3 | 47 | P342L | FSTIDWNKLYRREIHLPF | 52, DRB1:0405 | KLYRREIHLPF | 35, HLA-B15:01 | |
| ACTR3C | 5 | R32L | VLAKAASWTSRQVGELTL | 124, DRB1:0901 | RQVGELTL | 169, HLA-B15:01 | |
| BC2 | PREX1 | 49 | H1429Y | VFYYIEGSRQALKVIFYL | 17, DRB1:1201 | VANTNVFYY | 17, HLA-B35:01 |
| LRBA | 3 | I1375R L1376R | VMDNMVMACGGRRPLLSA | 35, DRB1:1201 | MVMACGGRR | 116, HLA-A11:01 | |
| FNDC3B | 41 | Y175C | QEIIPFCGMSTYITR | 87, DRB1:1201 | IPFCGMSTY | 6, HLA-B35:01 | |
| BC3 | NOTCH3 | 3895 | N1588H | SVVMLEIDHRLCLQS | 5, DRB1:0301 | VMLEIDHRL | 9, HLA-A02:01 |
| KCTD6 | 279 | S151F | TKVHFLLEGISNYFTKW | 16, DRB1:1501 | LTITTKVHF | 23, HLA-B58:01 | |
| C1orf172 | 419 | P203R | SLRSTFASSPR | 43, HLA-A33:03 | |||
| BC5 | MRC2 | 386 | R1437W | TAALILYWRRQSIER | 3, DRB1:1201 | TAALILYWR | 12, HLA-A33:03 |
| MERTK | 30 | S972L | RLVRNGVSWSHLSMLPLG | 38, DRB1:1201 | LVRNGVSWSHL | 89, HLA-B07:02 | |
| SBNO2 | 12 | V416M | VLDLQNKLPLARMVYASA | 10, DRB1:1201 | LPLARMVYASA | 176, HLA-B07:02 | |
| TP53 | 29 | Y126C | TCTCSPALNKMFCQLAKTCPV | 57, DRB1:1201 | TCTCSPAL | 394, HLA-C03:03 | |
| FAM178A | 1 | P552R | RTKSPPAAL | 107, HLA-B07:02 | |||
| BC5 | PDCL3 | 45 | D67N | EENERAIEMYRRRRLAEW | 68, DRB1:1454 | EENERAIEMY | 89, HLA-B44:03 |
| TTN | 3 | A16911S | YQFRIFAENRYGQSFSL | 285, DRB1:1454 | AENRYGQSFSL | 11, HLA-B40:01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morisaki, T.; Kubo, M.; Morisaki, S.; Umebayashi, M.; Tanaka, H.; Koya, N.; Nakagawa, S.; Tsujimura, K.; Yoshimura, S.; Kiyotani, K.; et al. Retrospective Analysis of HLA Class II-Restricted Neoantigen Peptide-Pulsed Dendritic Cell Vaccine for Breast Cancer. Cancers 2024, 16, 4204. https://doi.org/10.3390/cancers16244204
Morisaki T, Kubo M, Morisaki S, Umebayashi M, Tanaka H, Koya N, Nakagawa S, Tsujimura K, Yoshimura S, Kiyotani K, et al. Retrospective Analysis of HLA Class II-Restricted Neoantigen Peptide-Pulsed Dendritic Cell Vaccine for Breast Cancer. Cancers. 2024; 16(24):4204. https://doi.org/10.3390/cancers16244204
Chicago/Turabian StyleMorisaki, Takafumi, Makoto Kubo, Shinji Morisaki, Masayo Umebayashi, Hiroto Tanaka, Norihiro Koya, Shinichiro Nakagawa, Kenta Tsujimura, Sachiko Yoshimura, Kazuma Kiyotani, and et al. 2024. "Retrospective Analysis of HLA Class II-Restricted Neoantigen Peptide-Pulsed Dendritic Cell Vaccine for Breast Cancer" Cancers 16, no. 24: 4204. https://doi.org/10.3390/cancers16244204
APA StyleMorisaki, T., Kubo, M., Morisaki, S., Umebayashi, M., Tanaka, H., Koya, N., Nakagawa, S., Tsujimura, K., Yoshimura, S., Kiyotani, K., Nakamura, Y., Nakamura, M., & Morisaki, T. (2024). Retrospective Analysis of HLA Class II-Restricted Neoantigen Peptide-Pulsed Dendritic Cell Vaccine for Breast Cancer. Cancers, 16(24), 4204. https://doi.org/10.3390/cancers16244204






