Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities
Simple Summary
Abstract
1. Introduction
2. Advantages of Nanocarriers in Drug Delivery
3. Nanocarriers for Targeted Therapy
3.1. Strategies for Ensuring Safe Delivery of Targeted Drugs in Cancer Therapy
3.1.1. Overcoming Lack of Selectivity with Nanoparticle Drug Delivery Systems
3.1.2. Combating Multidrug Resistance Using Nanoparticle Drug Delivery Systems
3.1.3. Improving Aqueous Solubility with Nanoparticle Drug Delivery Systems
4. The Synthesis of Metal Oxide Nanocarriers
5. Targeted Drug Delivery Systems for Cancer Cells
5.1. Active Targeting
5.2. Passive Targeting
6. Titanium Dioxide-Based Nanocarrier
7. Metal Oxide Nanocarriers for Targeted Cancer Therapy
7.1. Iron Oxide-Based Nanocarrier
7.1.1. Magnetically Guided Drug Delivery
7.1.2. Vectorized Magnetic Nanocarriers
7.1.3. MRI-Guided Drug Delivery Utilizing IONs
7.1.4. Drug Delivery Systems Activated by External Stimuli
7.2. Zinc Oxide-Based Nanocarrier
7.3. Copper Oxide-Based Nanocarrier
8. Metal Oxide Nanoparticles for Theranostic Applications
9. Barriers to Effective Drug Release in Nanocarrier Systems
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Soldato, D.; Arecco, L.; Agostinetto, E.; Franzoi, M.A.; Mariamidze, E.; Begijanashvili, S.; Brunetti, N.; Spinaci, S.; Solinas, C.; Vaz-Luis, I.; et al. The Future of Breast Cancer Research in the Survivorship Field. Oncol. Ther. 2023, 11, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, M.B.; Deangelis, L.M. Chapter 28—Neurologic Complications of Chemotherapy and Radiation Therapy. In Aminoff’s Neurology and General Medicine, 5th ed.; Aminoff, M.J., Josephson, S.A., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 591–609. ISBN 978-0-12-407710-2. [Google Scholar]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Davodabadi, F.; Sajjadi, S.F.; Sarhadi, M.; Mirghasemi, S.; Nadali Hezaveh, M.; Khosravi, S.; Kamali Andani, M.; Cordani, M.; Basiri, M.; Ghavami, S. Cancer Chemotherapy Resistance: Mechanisms and Recent Breakthrough in Targeted Drug Delivery. Eur. J. Pharmacol. 2023, 958, 176013. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef]
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.A.; Boulaiz, H. Smart Drug-Delivery Systems for Cancer Nanotherapy. Curr. Drug Targets 2018, 19, 339–359. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Joshi, P.B.; Chavali, M.S. Updates on Biogenic Metallic and Metal Oxide Nanoparticles: Therapy, Drug Delivery and Cytotoxicity. Pharmaceutics 2023, 15, 1650. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J.; Raoufi, M.; Vermesh, O.; Mahmoudi, M. Targeted Superparamagnetic Iron Oxide Nanoparticles for Early Detection of Cancer: Possibilities and Challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 287–307. [Google Scholar] [CrossRef]
- Chen, C.; Ge, J.; Gao, Y.; Chen, L.; Cui, J.; Zeng, J.; Gao, M. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles: A next Generation Contrast Agent for Magnetic Resonance Imaging. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1740. [Google Scholar] [CrossRef]
- Hu, Y.; Mignani, S.; Majoral, J.-P.; Shen, M.; Shi, X. Construction of Iron Oxide Nanoparticle-Based Hybrid Platforms for Tumor Imaging and Therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef] [PubMed]
- Vats, M.; Mishra, S.K.; Baghini, M.S.; Chauhan, D.S.; Srivastava, R.; De, A. Near Infrared Fluorescence Imaging in Nano-Therapeutics and Photo-Thermal Evaluation. Int. J. Mol. Sci. 2017, 18, 924. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current Status and Future Perspectives in Aspect of Drug Delivery and Pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef]
- Misra, K.P.; Misra, R.D.K. ZnO-Based Quantum Dots for Biosensing, Cancer Imaging and Therapy: An Overview. Biomed. Mater. Devices 2023, 1, 99–107. [Google Scholar] [CrossRef]
- Khan, M.J.; Ahmad, A.; Khan, M.A.; Siddiqui, S. Zinc Oxide Nanoparticle Induces Apoptosis in Human Epidermoid Carcinoma Cells Through Reactive Oxygen Species and DNA Degradation. Biol. Trace Elem. Res. 2021, 199, 2172–2181. [Google Scholar] [CrossRef]
- Patel, S.; Sahai, S.K. 4—Traditional Cancer Therapies and Perioperative Implications. In Perioperative Care of the Cancer Patient; Hagberg, C., Gottumukkala, V., Riedel, B., Nates, J., Buggy, D., Eds.; Elsevier: New Delhi, India, 2023; pp. 46–55. ISBN 978-0-323-69584-8. [Google Scholar]
- Ullah, M.; Shah, M.I.; Hasan, M.W.; Jamshed, M.; Mustafa, U.; Inam, M. Engineered Metal Nanoparticles for Precision Drug Delivery: Pioneering the Future of Medicine: Mini Review. J. Chin. Chem. Soc. 2024, 71, 1358–1367. [Google Scholar] [CrossRef]
- Erduran, M.; Çankaya, N.; Yalcin, S. Biobased Nanomaterials in Drug Delivery. In Biobased Nanomaterials: Applications in Biomedicine, Food Industry, Agriculture, and Environmental Sustainability; Ahmed, S., Ed.; Springer Nature: Singapore, 2024; pp. 173–222. ISBN 978-981-9705-42-9. [Google Scholar]
- Hosseinpour-Moghadam, R.; Taghizadeh, F.; Goshtasbi, N.; Merati, F.; Haeri, A. Chapter 23—Applications of Liposomes for Overcoming Cancer Drug Resistance. In Functionalized Nanomaterials for Cancer Research; Barabadi, H., Mostafavi, E., Mustansar Hussain, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 523–542. ISBN 978-0-443-15518-5. [Google Scholar]
- Rajkumar, S.; Prabaharan, M. Multi-Functional Nanocarriers Based on Iron Oxide Nanoparticles Conjugated with Doxorubicin, Poly(Ethylene Glycol) and Folic Acid as Theranostics for Cancer Therapy. Colloids Surf. B Biointerfaces 2018, 170, 529–537. [Google Scholar] [CrossRef]
- Predoi, D.; Balas, M.; Badea, M.A.; Ciobanu, S.C.; Buton, N.; Dinischiotu, A. Dextran-Coated Iron Oxide Nanoparticles Loaded with 5-Fluorouracil for Drug-Delivery Applications. Nanomaterials 2023, 13, 1811. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Luo, L.; Wang, Y.; Zhong, Y.; Dai, H.-B.; Sun, D.; Luo, M.-L.; Wu, W.; Wang, G.-X. Dual Tumor-Targeted Poly(Lactic-Co-Glycolic Acid)–Polyethylene Glycol–Folic Acid Nanoparticles: A Novel Biodegradable Nanocarrier for Secure and Efficient Antitumor Drug Delivery. Int. J. Nanomed. 2017, 12, 5745–5760. [Google Scholar] [CrossRef]
- Park, J.; Kadasala, N.R.; Abouelmagd, S.A.; Castanares, M.A.; Collins, D.S.; Wei, A.; Yeo, Y. Polymer–Iron Oxide Composite Nanoparticles for EPR-Independent Drug Delivery. Biomaterials 2016, 101, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Y.; Yan, C.; Song, L.; Wen, S.; Zang, F.; Chen, G.; Ding, Q.; Yan, C.; Gu, N. High-Performance PEGylated Mn–Zn Ferrite Nanocrystals as a Passive-Targeted Agent for Magnetically Induced Cancer Theranostics. Biomaterials 2014, 35, 9126–9136. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Lin, G.; Patton, V.K.; Wang, K.; Press, O.W.; Zhang, M. Gemcitabine and Chlorotoxin Conjugated Iron Oxide Nanoparticles for Glioblastoma Therapy. J. Mater. Chem. B 2016, 4, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.S.; Abdollahi, A.; Malek-Khatabi, A.; Ejarestaghi, N.M.; Atashi, A.; Yousefi, N.; Ebrahimnejad, P.; Elsawy, M.A.; Dinarvand, R. Recent Advances in PLGA-Based Nanofibers as Anticancer Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2023, 85, 104587. [Google Scholar] [CrossRef]
- Dang, B.-T.N.; Kwon, T.K.; Lee, S.; Jeong, J.-H.; Yook, S. Nanoparticle-Based Immunoengineering Strategies for Enhancing Cancer Immunotherapy. J. Control. Release 2024, 365, 773–800. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Nie, G. Multifunctional Biomolecule Nanostructures for Cancer Therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef]
- Maghsoudnia, N.; Eftekhari, R.B.; Sohi, A.N.; Zamzami, A.; Dorkoosh, F.A. Application of Nano-Based Systems for Drug Delivery and Targeting: A Review. J. Nanoparticle Res. 2020, 22, 245. [Google Scholar] [CrossRef]
- Sundar, D.S.; Antoniraj, M.G.; Kumar, C.S.; Mohapatra, S.S.; Houreld, N.N.; Ruckmani, K. Recent Trends of Biocompatible and Biodegradable Nanoparticles in Drug Delivery: A Review. Curr. Med. Chem. 2016, 23, 3730–3751. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Yurtdaş Kırımlıoğlu, G. Chapter 3—Drug Loading Methods and Drug Release Mechanisms of PLGA Nanoparticles. In Poly(Lactic-Co-Glycolic Acid) (PLGA) Nanoparticles for Drug Delivery; Kesharwani, P., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 55–86. ISBN 978-0-323-91215-0. [Google Scholar]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, M.Z.; Ghoneim, M.M.; Alshehri, S.; Imam, S.S.; Md, S.; Alhakamy, N.A.; Jain, K.; Ahmad, J. Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges. Pharmaceutics 2021, 13, 2039. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, X.; Wu, C.; Qiao, J.; Jin, H.; Li, H. A Protracted War against Cancer Drug Resistance. Cancer Cell Int. 2024, 24, 326. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zheng, J.; Zhang, Y.; Meng, D.; Wang, Y.; Xu, X.; Liang, N.; Shabiti, S.; Zhang, X.; Wang, Z.; et al. The Mechanisms of Multidrug Resistance of Breast Cancer and Research Progress on Related Reversal Agents. Bioorg. Med. Chem. 2023, 95, 117486. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Sethi, N.; Patel, P.; Shah, S.; Patel, K. Exploring the Potential of P-Glycoprotein Inhibitors in the Targeted Delivery of Anti-Cancer Drugs: A Comprehensive Review. Eur. J. Pharm. Biopharm. 2024, 198, 114267. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Garg, A.; Hussain, A.; Farid, A.; Kumar, P.; Taghizadeh-Hesary, F. Nanodelivery Systems: An Efficient and Target-Specific Approach for Drug-Resistant Cancers. Cancer Med. 2023, 12, 18797–18825. [Google Scholar] [CrossRef]
- Nyamba, I.; Sombié, C.B.; Yabré, M.; Zimé-Diawara, H.; Yaméogo, J.; Ouédraogo, S.; Lechanteur, A.; Semdé, R.; Evrard, B. Pharmaceutical Approaches for Enhancing Solubility and Oral Bioavailability of Poorly Soluble Drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Sood, R.; Tomar, D.; Kaushik, P.; Sharma, P.; Rani, N.; Guarve, K.; Dhankhar, S.; Garg, N. Enhanced Solubility and Increased Bioavailability with Engineered Nanocrystals. Curr. Drug Ther. 2024, 19, 638–647. [Google Scholar] [CrossRef]
- Jayapriya, P.; Pardhi, E.; Vasave, R.; Guru, S.K.; Madan, J.; Mehra, N.K. A Review on Stimuli-pH Responsive Liposomal Formulation in Cancer Therapy. J. Drug Deliv. Sci. Technol. 2023, 90, 105172. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric Micelles and Cancer Therapy: An Ingenious Multimodal Tumor-Targeted Drug Delivery System. Drug Deliv. Transl. Res. 2023, 13, 135–163. [Google Scholar] [CrossRef]
- Marabada, D.; Li, J.; Wei, S.; Huang, Q.; Wang, Z. Cyclodextrin Based Nanoparticles for Smart Drug Delivery in Colorectal Cancer. Chem. Biol. Drug Des. 2023, 102, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Virmani, T.; Kumar, G.; Sharma, A.; Pathak, K.; Akhtar, M.S.; Afzal, O.; Altamimi, A.S.A. Amelioration of Cancer Employing Chitosan, Its Derivatives, and Chitosan-Based Nanoparticles: Recent Updates. Polymers 2023, 15, 2928. [Google Scholar] [CrossRef] [PubMed]
- Safitri, N.; Rauf, N.; Tahir, D. Enhancing Drug Loading and Release with Hydroxyapatite Nanoparticles for Efficient Drug Delivery: A Review Synthesis Methods, Surface Ion Effects, and Clinical Prospects. J. Drug Deliv. Sci. Technol. 2023, 90, 105092. [Google Scholar] [CrossRef]
- Duncan, R.; Richardson, S.C.W. Endocytosis and Intracellular Trafficking as Gateways for Nanomedicine Delivery: Opportunities and Challenges. Mol. Pharm. 2012, 9, 2380–2402. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Triphati, A.; Pirzadah, T.B. Chapter 3—Synthesis Methods of Nanoparticles and Their Key Applications. In Synthesis of Bionanomaterials for Biomedical Applications; Ozturk, M., Roy, A., Bhat, R.A., Vardar-Sukan, F., Policarpo Tonelli, F.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–76. ISBN 978-0-323-91195-5. [Google Scholar]
- El-Khawaga, A.M.; Zidan, A.; El-Mageed, A.I.A.A. Preparation Methods of Different Nanomaterials for Various Potential Applications: A Review. J. Mol. Struct. 2023, 1281, 135148. [Google Scholar] [CrossRef]
- Shreyash, N.; Sonker, M.; Bajpai, S.; Tiwary, S.K. Review of the Mechanism of Nanocarriers and Technological Developments in the Field of Nanoparticles for Applications in Cancer Theragnostics. ACS Appl. Bio Mater. 2021, 4, 2307–2334. [Google Scholar] [CrossRef]
- Upadhyay, K.; Tamrakar, R.K.; Thomas, S.; Kumar, M. Surface Functionalized Nanoparticles: A Boon to Biomedical Science. Chem. Biol. Interact. 2023, 380, 110537. [Google Scholar] [CrossRef]
- Aminzai, M.T.; Yildirim, M.; Yabalak, E. Metallic Nanoparticles Unveiled: Synthesis, Characterization, and Their Environmental, Medicinal, and Agricultural Applications. Talanta 2024, 280, 126790. [Google Scholar] [CrossRef]
- Roberts, E.J.; Karadaghi, L.R.; Wang, L.; Malmstadt, N.; Brutchey, R.L. Continuous Flow Methods of Fabricating Catalytically Active Metal Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 27479–27502. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite Nanoparticles: Synthesis Methods—A Comparative Review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Nagaswarupa, H.P.; Darukesha, B.H.M.; Tejashwini, D.M. Fundamentals of Nanomaterial Synthesis. In Advances in Space Radiation Detection: Novel Nanomaterials and Techniques; Naik, R., Nagaswarupa, H.P., Darukesha, B.H.M., Tejashwini, D.M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 23–36. ISBN 978-3-031-74551-5. [Google Scholar]
- Liu, H.; Wang, S.; Yang, J.; Zhuo, R.; Zhao, J.; Liu, L.; Li, Y. The Application of Supercritical Fluid Technology in the Synthesis of Metal and Metal Oxide Nanoparticles. CrystEngComm 2024, 26, 5675–5693. [Google Scholar] [CrossRef]
- Zhang, X.; Ng, H.L.H.; Lu, A.; Lin, C.; Zhou, L.; Lin, G.; Zhang, Y.; Yang, Z.; Zhang, H. Drug Delivery System Targeting Advanced Hepatocellular Carcinoma: Current and Future. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 853–869. [Google Scholar] [CrossRef]
- Hejmady, S.; Pradhan, R.; Alexander, A.; Agrawal, M.; Singhvi, G.; Gorain, B.; Tiwari, S.; Kesharwani, P.; Dubey, S.K. Recent Advances in Targeted Nanomedicine as Promising Antitumor Therapeutics. Drug Discov. Today 2020, 25, 2227–2244. [Google Scholar] [CrossRef]
- Chen, S.; Cao, R.; Xiang, L.; Li, Z.; Chen, H.; Zhang, J.; Feng, X. Research Progress in Nucleus-Targeted Tumor Therapy. Biomater. Sci. 2023, 11, 6436–6456. [Google Scholar] [CrossRef]
- Torchilin, V.P. Passive and Active Drug Targeting: Drug Delivery to Tumors as an Example. In Drug Delivery; Schäfer-Korting, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–53. ISBN 978-3-642-00477-3. [Google Scholar]
- Yadav, K.S.; Mishra, D.K.; Deshpande, A.; Pethe, A.M. Chapter 7—Levels of Drug Targeting. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Advances in Pharmaceutical Product Development and Research; Academic Press: Cambridge, MA, USA, 2019; pp. 269–305. ISBN 978-0-12-817909-3. [Google Scholar]
- Banushi, B.; Joseph, S.R.; Lum, B.; Lee, J.J.; Simpson, F. Endocytosis in Cancer and Cancer Therapy. Nat. Rev. Cancer 2023, 23, 450–473. [Google Scholar] [CrossRef]
- Dhivya, R.; Ranjani, J.; Bowen, P.K.; Rajendhran, J.; Mayandi, J.; Annaraj, J. Biocompatible Curcumin Loaded PMMA-PEG/ZnO Nanocomposite Induce Apoptosis and Cytotoxicity in Human Gastric Cancer Cells. Mater. Sci. Eng. C 2017, 80, 59–68. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.-H. Cerium Oxide Decorated 5-Fluorouracil Loaded Chitosan Nanoparticles for Treatment of Hepatocellular Carcinoma. Int. J. Biol. Macromol. 2022, 216, 52–64. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Liao, Z.; Li, Y.; Qi, X.; Qian, Y.; Fenniri, H.; Zhao, P.; Shen, J. Zinc Oxide End-Capped Fe3O4@mSiO2 Core-Shell Nanocarriers as Targeted and Responsive Drug Delivery System for Chemo-/Ions Synergistic Therapeutics. Drug Deliv. 2019, 26, 732–743. [Google Scholar] [CrossRef]
- Bertrand, N.; Leroux, J.-C. The Journey of a Drug-Carrier in the Body: An Anatomo-Physiological Perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef]
- Salahpour Anarjan, F. Active Targeting Drug Delivery Nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- Raja, G.; Cao, S.; Kim, D.-H.; Kim, T.-J. Mechanoregulation of Titanium Dioxide Nanoparticles in Cancer Therapy. Mater. Sci. Eng. C 2020, 107, 110303. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.M.P.; Tonelli, F.C.P.; Cordeiro, H.G. TiO2 Nanoparticles in Cancer Therapy as Nanocarriers in Paclitaxel’s Delivery and Nanosensitizers in Phototherapies and/or Sonodynamic Therapy. Curr. Pharm. Biotechnol. 2024, 25, 133–143. [Google Scholar] [CrossRef]
- Du, Y.; Ren, W.; Li, Y.; Zhang, Q.; Zeng, L.; Chi, C.; Wu, A.; Tian, J. The Enhanced Chemotherapeutic Effects of Doxorubicin Loaded PEG Coated TiO2 Nanocarriers in an Orthotopic Breast Tumor Bearing Mouse Model. J. Mater. Chem. B 2015, 3, 1518–1528. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, K.; Tan, Z.; Liu, Y.; Zhao, Z.; Zhu, G.; Ma, K.; Cui, C.; Wang, L.; Kang, T. Overcoming Drug Resistance with Functional Mesoporous Titanium Dioxide Nanoparticles Combining Targeting, Drug Delivery and Photodynamic Therapy. J. Mater. Chem. B 2018, 6, 7750–7759. [Google Scholar] [CrossRef]
- Kim, S.; Im, S.; Park, E.-Y.; Lee, J.; Kim, C.; Kim, T.; Kim, W.J. Drug-Loaded Titanium Dioxide Nanoparticle Coated with Tumor Targeting Polymer as a Sonodynamic Chemotherapeutic Agent for Anti-Cancer Therapy. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102110. [Google Scholar] [CrossRef] [PubMed]
- Rivera Rodriguez, M.A.; Campos-Ibarra, V.; Rasu Chettiar, A.-D.; Marasamy, L.; Manisekaran, R. Anticancer Effect of Surface Functionalized Nano Titanium Dioxide with 5-Fluorouracil on Oral Cancer Cell Line—An in Vitro Study. Micro Nano Lett. 2024, 19, e70004. [Google Scholar] [CrossRef]
- Bhullar, S.; Goyal, N.; Gupta, S. FericipXT-Coated PEGylated Rutile TiO2 Nanoparticles in Drug Delivery: In Vitro Assessment of Imatinib Release. RSC Adv. 2024, 14, 23886–23901. [Google Scholar] [CrossRef]
- Eshaghi, M.M.; Pourmadadi, M.; Rahdar, A.; Díez-Pascual, A.M. Improving Quercetin Anticancer Activity through a Novel Polyvinylpyrrolidone/Polyvinyl Alcohol/TiO2 Nanocomposite. J. Drug Deliv. Sci. Technol. 2023, 81, 104304. [Google Scholar] [CrossRef]
- Devanand Venkatasubbu, G.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Folate Targeted PEGylated Titanium Dioxide Nanoparticles as a Nanocarrier for Targeted Paclitaxel Drug Delivery. Adv. Powder Technol. 2013, 24, 947–954. [Google Scholar] [CrossRef]
- Heidari Khoee, M.; Khoee, S.; Lotfi, M. Synthesis of Titanium Dioxide Nanotubes with Liposomal Covers for Carrying and Extended Release of 5-FU as Anticancer Drug in the Treatment of HeLa Cells. Anal. Biochem. 2019, 572, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; et al. Smart Nanocarriers as an Emerging Platform for Cancer Therapy: A Review. Molecules 2022, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Liang, P.-C.; Chen, Y.-C.; Chiang, C.-F.; Mo, L.-R.; Wei, S.-Y.; Hsieh, W.-Y.; Lin, W.-L. Doxorubicin-Modified Magnetic Nanoparticles as a Drug Delivery System for Magnetic Resonance Imaging-Monitoring Magnet-Enhancing Tumor Chemotherapy. Int. J. Nanomed. 2016, 11, 2021–2037. [Google Scholar] [CrossRef]
- El Ghany, Y.I.A.; Tawfik, M.M.; El Bous, M.; Gomaa, I.; Moustafa, A.M.Y.; Hosny, N.M. Sesuvium sesuvioides (Fenzl) Mediated Synthesis of Zinc Oxide and Copper Oxide Nanoparticles and Their Potential Cytotoxic and Apoptotic Effects. In The First International Conference & Expo on Green Sciences; El-Dossoki, F., Hassan, M., Shehata, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 309–330. [Google Scholar]
- Truong, T.T.; Mondal, S.; Doan, V.H.M.; Tak, S.; Choi, J.; Oh, H.; Nguyen, T.D.; Misra, M.; Lee, B.; Oh, J. Precision-Engineered Metal and Metal-Oxide Nanoparticles for Biomedical Imaging and Healthcare Applications. Adv. Colloid Interface Sci. 2024, 332, 103263. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, X.; Zhao, J.; Ding, J.; Feng, S.-S. Multimodality Treatment of Cancer with Herceptin Conjugated, Thermomagnetic Iron Oxides and Docetaxel Loaded Nanoparticles of Biodegradable Polymers. Biomaterials 2012, 33, 7519–7529. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Saeed, M.; Ren, W.; Wu, A. Therapeutic Applications of Iron Oxide Based Nanoparticles in Cancer: Basic Concepts and Recent Advances. Biomater. Sci. 2018, 6, 708–725. [Google Scholar] [CrossRef]
- Su, C. Environmental Implications and Applications of Engineered Nanoscale Magnetite and Its Hybrid Nanocomposites: A Review of Recent Literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Pourradi, N.M.A.; Babaei, H.; Hamishehkar, H.; Baradaran, B.; Shokouhi-Gogani, B.; Shanehbandi, D.; Ghorbani, M.; Azarmi, Y. Targeted Delivery of Doxorubicin by Thermo/pH-Responsive Magnetic Nanoparticles in a Rat Model of Breast Cancer. Toxicol. Appl. Pharmacol. 2022, 446, 116036. [Google Scholar] [CrossRef] [PubMed]
- Rarokar, N.; Yadav, S.; Saoji, S.; Bramhe, P.; Agade, R.; Gurav, S.; Khedekar, P.; Subramaniyan, V.; Wong, L.S.; Kumarasamy, V. Magnetic Nanosystem a Tool for Targeted Delivery and Diagnostic Application: Current Challenges and Recent Advancement. Int. J. Pharm. X 2024, 7, 100231. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; Brown, S.D.; Holden, M.R.; Craig, G.E.; Plumb, J.A.; Brown, R.E.; Schreiter, N.; Chrzanowski, W.; Wheate, N.J. Cisplatin Drug Delivery Using Gold-Coated Iron Oxide Nanoparticles for Enhanced Tumour Targeting with External Magnetic Fields. Inorg. Chim. Acta 2012, 393, 328–333. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmad, M.; Akhtar, M.S.; Shaari, A.; Riaz, S.; Naseem, S.; Masood, M.; Saeed, M.A. Magnetic Properties of Polyvinyl Alcohol and Doxorubicine Loaded Iron Oxide Nanoparticles for Anticancer Drug Delivery Applications. PLoS ONE 2016, 11, e0158084. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Kara, N.; Ayoub, N.; Ilgu, H.; Fotiadis, D.; Ilgu, M. Aptamers Targeting Membrane Proteins for Sensor and Diagnostic Applications. Molecules 2023, 28, 3728. [Google Scholar] [CrossRef]
- Nagesh, P.K.B.; Johnson, N.R.; Boya, V.K.N.; Chowdhury, P.; Othman, S.F.; Khalilzad-Sharghi, V.; Hafeez, B.B.; Ganju, A.; Khan, S.; Behrman, S.W.; et al. PSMA Targeted Docetaxel-Loaded Superparamagnetic Iron Oxide Nanoparticles for Prostate Cancer. Colloids Surf. B Biointerfaces 2016, 144, 8–20. [Google Scholar] [CrossRef]
- Aires, A.; Ocampo, S.M.; Simões, B.M.; Rodríguez, M.J.; Cadenas, J.F.; Couleaud, P.; Spence, K.; Latorre, A.; Miranda, R.; Somoza, Á.; et al. Multifunctionalized Iron Oxide Nanoparticles for Selective Drug Delivery to CD44-Positive Cancer Cells. Nanotechnology 2016, 27, 065103. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic Iron Oxide Nanoparticles Conjugated with Folic Acid for Dual Target-Specific Drug Delivery and MRI in Cancer Theranostics. Mater. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Q.; Peng, J.; Su, J.; Lu, X.; Zhao, Y.; Qian, Z. Doxorubicin-Conjugated Heparin-Coated Superparamagnetic Iron Oxide Nanoparticles for Combined Anticancer Drug Delivery and Magnetic Resonance Imaging. J. Biomed. Nanotechnol. 2016, 12, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Abri Aghdam, M.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent Advances on Thermosensitive and pH-Sensitive Liposomes Employed in Controlled Release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Munnier, E.; Cohen-Jonathan, S.; Linassier, C.; Douziech-Eyrolles, L.; Marchais, H.; Soucé, M.; Hervé, K.; Dubois, P.; Chourpa, I. Novel Method of Doxorubicin–SPION Reversible Association for Magnetic Drug Targeting. Int. J. Pharm. 2008, 363, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, P.; Liu, C.-H.; Zhi, X.-T. Doxorubicin-Loaded Mesoporous Magnetic Nanoparticles to Induce Apoptosis in Breast Cancer Cells. Biomed. Pharmacother. 2015, 69, 355–360. [Google Scholar] [CrossRef]
- Quinto, C.A.; Mohindra, P.; Tong, S.; Bao, G. Multifunctional Superparamagnetic Iron Oxide Nanoparticles for Combined Chemotherapy and Hyperthermia Cancer Treatment. Nanoscale 2015, 7, 12728–12736. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Du, M.; Vermorken, A.; Cui, Y.; Zhang, L.; Guo, L.; Ma, L.; Chen, M. Cetuximab and Doxorubicin Loaded Dextran-Coated Fe3O4 Magnetic Nanoparticles as Novel Targeted Nanocarriers for Non-Small Cell Lung Cancer. J. Magn. Magn. Mater. 2019, 481, 122–128. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Kumari, S.; Sarkar, A.; Kumaran, S.S.; Chaturvedi, S.; Jain, T.K.; Agrawal, G. Imaging Application and Radiosensitivity Enhancement of Pectin Decorated Multifunctional Magnetic Nanoparticles in Cancer Therapy. Int. J. Biol. Macromol. 2021, 189, 443–454. [Google Scholar] [CrossRef]
- Dou, J.; Mi, Y.; Daneshmand, S.; Heidari Majd, M. The Effect of Magnetic Nanoparticles Containing Hyaluronic Acid and Methotrexate on the Expression of Genes Involved in Apoptosis and Metastasis in A549 Lung Cancer Cell Lines. Arab. J. Chem. 2022, 15, 104307. [Google Scholar] [CrossRef]
- Gonbadi, P.; Jalal, R.; Akhlaghinia, B.; Ghasemzadeh, M.S. Tannic Acid-Modified Magnetic Hydrotalcite-Based MgAl Nanoparticles for the in Vitro Targeted Delivery of Doxorubicin to the Estrogen Receptor-Overexpressing Colorectal Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 68, 103026. [Google Scholar] [CrossRef]
- Hormozi, N.; Esmaeili, A. Synthesis and Correction of Albumin Magnetic Nanoparticles with Organic Compounds for Absorbing and Releasing Doxorubicin Hydrochloride. Colloids Surf. B Biointerfaces 2019, 182, 110368. [Google Scholar] [CrossRef]
- Solak, K.; Mavi, A.; Yılmaz, B. Disulfiram-Loaded Functionalized Magnetic Nanoparticles Combined with Copper and Sodium Nitroprusside in Breast Cancer Cells. Mater. Sci. Eng. C 2021, 119, 111452. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Płóciennik, A.; Machnicka, B. Functionalized Magnetic Fe3O4 Nanoparticles for Targeted Methotrexate Delivery in Ovarian Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 9098. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.A.; Kamel, A.M.; El-Ghaffar, M.A.A. Biodegradable Functionalized Magnetite Nanoparticles as Binary-Targeting Carrier for Breast Carcinoma. BMC Chem. 2023, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Sadri, E.; Khoee, S.; Moayeri, S.; Haji Ali, B.; Pirhajati Mahabadi, V.; Shirvalilou, S.; Khoei, S. Enhanced Anti-Tumor Activity of Transferrin/Folate Dual-Targeting Magnetic Nanoparticles Using Chemo-Thermo Therapy on Retinoblastoma Cancer Cells Y79. Sci. Rep. 2023, 13, 22358. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Gupta, J.; Hassan, P.A.; Barick, K.C. Multifunctional ZnO Nanostructures: A next Generation Nanomedicine for Cancer Therapy, Targeted Drug Delivery, Bioimaging, and Tissue Regeneration. Nanotechnology 2023, 34, 282003. [Google Scholar] [CrossRef]
- Singh, T.A.; Das, J.; Sil, P.C. Zinc Oxide Nanoparticles: A Comprehensive Review on Its Synthesis, Anticancer and Drug Delivery Applications as Well as Health Risks. Adv. Colloid Interface Sci. 2020, 286, 102317. [Google Scholar] [CrossRef]
- Gholami, A.; Pourmadadi., M.; Abdouss., H.; Amiri., Z.; Abdouss., M.; Rahdar, A.; Behzadmehr., R.; Pandey, S. Formulation of Double Microemulsion Based on pH-Responsive PEG/PVA/Zinc Oxide as a Potential Nano-Platform for Drug Delivery: Green Synthesis, and Physico-Chemical Characterization. J. Mol. Liq. 2024, 410, 125563. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Phua, S.Z.F.; Lim, W.Q.; Zhao, Y. ZnO–DOX@ZIF-8 Core–Shell Nanoparticles for pH-Responsive Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 2223–2229. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots–Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef]
- Tan, L.; Liu, J.; Zhou, W.; Wei, J.; Peng, Z. A Novel Thermal and pH Responsive Drug Delivery System Based on ZnO@PNIPAM Hybrid Nanoparticles. Mater. Sci. Eng. C 2014, 45, 524–529. [Google Scholar] [CrossRef]
- Mishra, P.; Ali Ahmad, M.F.; Al-Keridis, L.A.; Saeed, M.; Alshammari, N.; Alabdallah, N.M.; Tiwari, R.K.; Ahmad, A.; Verma, M.; Fatima, S.; et al. Methotrexate-Conjugated Zinc Oxide Nanoparticles Exert a Substantially Improved Cytotoxic Effect on Lung Cancer Cells by Inducing Apoptosis. Front. Pharmacol. 2023, 14, 1194578. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wu, C.; Jiang, H.; Li, Q.; Wang, X.; Chen, B. Synergistic Cytotoxic Effect of Different Sized ZnO Nanoparticles and Daunorubicin against Leukemia Cancer Cells under UV Irradiation. J. Photochem. Photobiol. B 2008, 93, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Al Dine, E.J.; Marchal, S.; Schneider, R.; Hamie, B.; Ghanbaja, J.; Roques-Carmes, T.; Hamieh, T.; Toufaily, J.; Gaffet, E.; Alem, H. A Facile Approach for Doxorubicine Delivery in Cancer Cells by Responsive and Fluorescent Core/Shell Quantum Dots. Bioconjugate Chem. 2018, 29, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, H.; Gong, X.; Li, Q.; Zhao, X. Synthesis, Characterization, and Cytotoxicity Assessment of N-Acetyl-l-Cysteine Capped ZnO Nanoparticles as Camptothecin Delivery System. Colloids Surf. B Biointerfaces 2019, 174, 476–482. [Google Scholar] [CrossRef]
- Peng, H.; Cui, B.; Li, G.; Wang, Y.; Li, N.; Chang, Z.; Wang, Y. A Multifunctional β-CD-Modified Fe3O4@ZnO:Er3+,Yb3+ Nanocarrier for Antitumor Drug Delivery and Microwave-Triggered Drug Release. Mater. Sci. Eng. C 2015, 46, 253–263. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, J.-S.; Zhang, P.; Chen, J.; Kong, J.-L.; Sun, L.-H.; Xiong, H.-M.; Möhwald, H. Self-Assembled ZnO Nanoparticle Capsules for Carrying and Delivering Isotretinoin to Cancer Cells. ACS Appl. Mater. Interfaces 2017, 9, 18474–18481. [Google Scholar] [CrossRef]
- Gomaa, S.; Nassef, M.; Tabl, G.; Zaki, S.; Abdel-Ghany, A. Doxorubicin and Folic Acid-Loaded Zinc Oxide Nanoparticles-Based Combined Anti-Tumor and Anti-Inflammatory Approach for Enhanced Anti-Cancer Therapy. BMC Cancer 2024, 24, 34. [Google Scholar] [CrossRef]
- Ostovar, S.; Pourmadadi, M.; Zaker, M.A. Co-Biopolymer of Chitosan/Carboxymethyl Cellulose Hydrogel Improved by Zinc Oxide and Graphene Quantum Dots Nanoparticles as pH-Sensitive Nanocomposite for Quercetin Delivery to Brain Cancer Treatment. Int. J. Biol. Macromol. 2023, 253, 127091. [Google Scholar] [CrossRef]
- Kaliyaperumal, V.; Rajasekaran, S.; Kanniah, R.; Gopal, D.; Ayyakannu Sundaram, G.; Kumar, A.S.K. Synthesis and Evaluation of Gelatin–Chitosan Biofilms Incorporating Zinc Oxide Nanoparticles and 5-Fluorouracil for Cancer Treatment. Materials 2024, 17, 3186. [Google Scholar] [CrossRef]
- Mosleh, A.M.; El-Sherif, A.A.; El-Sayed, A.A.; Fahmy, H.M. Characterization and Cytotoxicity Assessment of Synthesized Palladium (II) Complex-Encapsulated Zinc Oxide Nanoparticles for Cancer Treatment. Cell Biochem. Biophys. 2024, 82, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, C.; Li, A.; Wang, J.; Cai, X. Red Fluorescent ZnO Nanoparticle Grafted with Polyglycerol and Conjugated RGD Peptide as Drug Delivery Vehicles for Efficient Target Cancer Therapy. Mater. Sci. Eng. C 2019, 95, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, J.; Bian, C.; Zhu, C.; Chen, C.; Guo, Z.; Zhang, Z.; Agyekum, G.A.; Zhang, Z.; Cao, X. pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery. Materials 2020, 13, 3950. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. Chitosan-Cellulose Hydrogel Conjugated with L-Histidine and Zinc Oxide Nanoparticles for Sustained Drug Delivery: Kinetics and In-Vitro Biological Studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef]
- Abbasian, M.; Hasanzadeh, P.; Mahmoodzadeh, F.; Salehi, R. Novel Cationic Cellulose-Based Nanocomposites for Targeted Delivery of Methotrexate to Breast Cancer Cells. J. Macromol. Sci. Part A 2020, 57, 99–115. [Google Scholar] [CrossRef]
- Joshi, M.; Bhatt, P. Deciphering the Anticancer Activity of Biocompatible Zinc Oxide Nanoparticles Loaded with Methotrexate on Breast Cancer Cells. Bull. Mater. Sci. 2023, 46, 192. [Google Scholar] [CrossRef]
- Gebreslassie, Y.T.; Gebremeskel, F.G. Green and Cost-Effective Biofabrication of Copper Oxide Nanoparticles: Exploring Antimicrobial and Anticancer Applications. Biotechnol. Rep. 2024, 41, e00828. [Google Scholar] [CrossRef]
- Zaiki, Y.; Iskandar, A.; Wong, T.W. Functionalized Chitosan for Cancer Nano Drug Delivery. Biotechnol. Adv. 2023, 67, 108200. [Google Scholar] [CrossRef]
- Atloo, T.; Mohammadkhani, R.; Mohammadi, A.; Zaboli, K.A.; Kaboli, S.; Rahimi, H.; Nosrati, H.; Danafar, H. The Bovine Serum Albumin Coated Copper Oxide Nanoparticle for Curcumin Delivery in Biological Environment: In-Vitro Drug Release. J. Polym. Environ. 2022, 30, 3203–3208. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Venkatachalam, K.; Wang, M.-H. Folic Acid Functionalized Starch Encapsulated Green Synthesized Copper Oxide Nanoparticles for Targeted Drug Delivery in Breast Cancer Therapy. Int. J. Biol. Macromol. 2020, 164, 2073–2084. [Google Scholar] [CrossRef]
- Goswami, U.; Dutta, A.; Raza, A.; Kandimalla, R.; Kalita, S.; Ghosh, S.S.; Chattopadhyay, A. Transferrin–Copper Nanocluster–Doxorubicin Nanoparticles as Targeted Theranostic Cancer Nanodrug. ACS Appl. Mater. Interfaces 2018, 10, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, K. Exploration of Polydopamine Capped Bimetallic Oxide (CuO-NiO) Nanoparticles Inspired by Mussels for Enhanced and Targeted Paclitaxel Delivery for Synergistic Breast Cancer Therapy. Appl. Surf. Sci. 2023, 626, 157266. [Google Scholar] [CrossRef]
- Singh, S.; Pal, K. Polyphenol Modified CuO Nanorods Capped by Kappa-Carrageenan for Controlled Paclitaxel Release in Furnishing Targeted Chemotherapy in Breast Carcinoma Cells. Int. J. Biol. Macromol. 2024, 255, 127893. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, K. Folic-Acid Adorned Alginate-Polydopamine Modified Paclitaxel/Zn-CuO Nanocomplex for pH Triggered Drug Release and Synergistic Antitumor Efficacy. Int. J. Biol. Macromol. 2023, 234, 123602. [Google Scholar] [CrossRef]
- Mohammadhassan, Z.; Mohammadkhani, R.; Mohammadi, A.; Zaboli, K.A.; Kaboli, S.; Rahimi, H.; Nosrati, H.; Danafar, H. Preparation of Copper Oxide Nanoparticles Coated with Bovine Serum Albumin for Delivery of Methotrexate. J. Drug Deliv. Sci. Technol. 2022, 67, 103015. [Google Scholar] [CrossRef]
- Singh, S.; Ghosh, C.; Roy, P.; Pal, K. Biosynthesis of Folic Acid Appended PHBV Modified Copper Oxide Nanorods for pH Sensitive Drug Release in Targeted Breast Cancer Therapy. Int. J. Pharm. 2022, 622, 121831. [Google Scholar] [CrossRef]
- Guo, L.-M.; Xu, X.-M.; Zhao, D.; Cai, X.-G.; Zhou, B. Biosynthesis, Characterization of PLGA Coated Folate-Mediated Multiple Drug Loaded Copper Oxide (CuO) Nanoparticles and It’s Cytotoxicity on Nasopharyngeal Cancer Cell Lines. AMB Express 2020, 10, 160. [Google Scholar] [CrossRef]
- Varukattu, N.B.; Vivek, R.; Rejeeth, C.; Thangam, R.; Ponraj, T.; Sharma, A.; Kannan, S. Nanostructured pH-Responsive Biocompatible Chitosan Coated Copper Oxide Nanoparticles: A Polymeric Smart Intracellular Delivery System for Doxorubicin in Breast Cancer Cells. Arab. J. Chem. 2020, 13, 2276–2286. [Google Scholar] [CrossRef]
- Gharehdaghi, Z.; Rahimi, R.; Naghib, S.M.; Molaabasi, F. Fabrication and Application of Copper Metal–Organic Frameworks as Nanocarriers for pH-Responsive Anticancer Drug Delivery. J. Iran. Chem. Soc. 2022, 19, 2727–2737. [Google Scholar] [CrossRef]
- Peng, N.; Ding, X.; Wang, Z.; Cheng, Y.; Gong, Z.; Xu, X.; Gao, X.; Cai, Q.; Huang, S.; Liu, Y. Novel Dual Responsive Alginate-Based Magnetic Nanogels for Onco-Theranostics. Carbohydr. Polym. 2019, 204, 32–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Liu, L.; Xu, J.; Jiang, H.; Li, D.; Lan, J.; Li, J.; Yang, J.; Tu, Q.; et al. ZnO-Based Multifunctional Nanocomposites to Inhibit Progression and Metastasis of Melanoma by Eliciting Antitumor Immunity via Immunogenic Cell Death. Theranostics 2020, 10, 11197–11214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wan, P.; Yang, M.; Han, F.; Wang, T.; Wang, Y.; Li, Y. Cell Membrane Camouflaged Cerium Oxide Nanocubes for Targeting Enhanced Tumor-Selective Therapy. J. Mater. Chem. B 2021, 9, 9524–9532. [Google Scholar] [CrossRef] [PubMed]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Kommineni, N.; Chaudhari, R.; Conde, J.; Tamburaci, S.; Cecen, B.; Chandra, P.; Prasad, R. Engineered Liposomes in Interventional Theranostics of Solid Tumors. ACS Biomater. Sci. Eng. 2023, 9, 4527–4557. [Google Scholar] [CrossRef]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the Efficacy of Nanoparticle-Based Drug Delivery Systems for Cancer Treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and Limitations of Current Techniques for Analyzing the Biodistribution of Nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Croitoru, G.-A.; Pîrvulescu, D.-C.; Niculescu, A.G.; Grumezescu, A.M.; Antohi, A.M.; Nicolae, C.-L. Metallic Nanomaterials—Targeted Drug Delivery Approaches for Improved Bioavailability, Reduced Side Toxicity, and Enhanced Patient Outcomes. Rom. J. Morphol. Embryol. 2024, 65, 145–158. [Google Scholar] [CrossRef]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent Advancements in Biocompatible Inorganic Nanoparticles towards Biomedical Applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface Functionalization of Polymeric Nanoparticles for Tumor Drug Delivery: Approaches and Challenges. Expert Opin. Drug Deliv. 2017, 14, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Frisch, E.; Clavier, L.; Belhamdi, A.; Vrana, N.E.; Lavalle, P.; Frisch, B.; Heurtault, B.; Gribova, V. Preclinical In Vitro Evaluation of Implantable Materials: Conventional Approaches, New Models and Future Directions. Front. Bioeng. Biotechnol. 2023, 11, 1193204. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, M.S.; Xi, C.E.; Bapuji, R.; Wotherspoon, H.; Kimmelman, J.; Bedford, P.; McIsaac, D.I.; Lalu, M.M.; Fergusson, D.A. Synthesizing Regulatory Guidance for Demonstrating Preclinical Efficacy and Translating Promising Cell Therapies to Early Phase Clinical Trials: A Scoping Review. BMC Med. 2024, 22, 487. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Üstündağ Okur, N.; Karavas, E.; Bikiaris, D.N. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef]
- Paliwal, A.; Jain, S.; Kumar, S.; Wal, P.; Khandai, M.; Khandige, P.S.; Sadananda, V.; Anwer, M.K.; Gulati, M.; Behl, T.; et al. Predictive Modelling in Pharmacokinetics: From in-Silico Simulations to Personalized Medicine. Expert Opin. Drug Metab. Toxicol. 2024, 20, 181–195. [Google Scholar] [CrossRef]
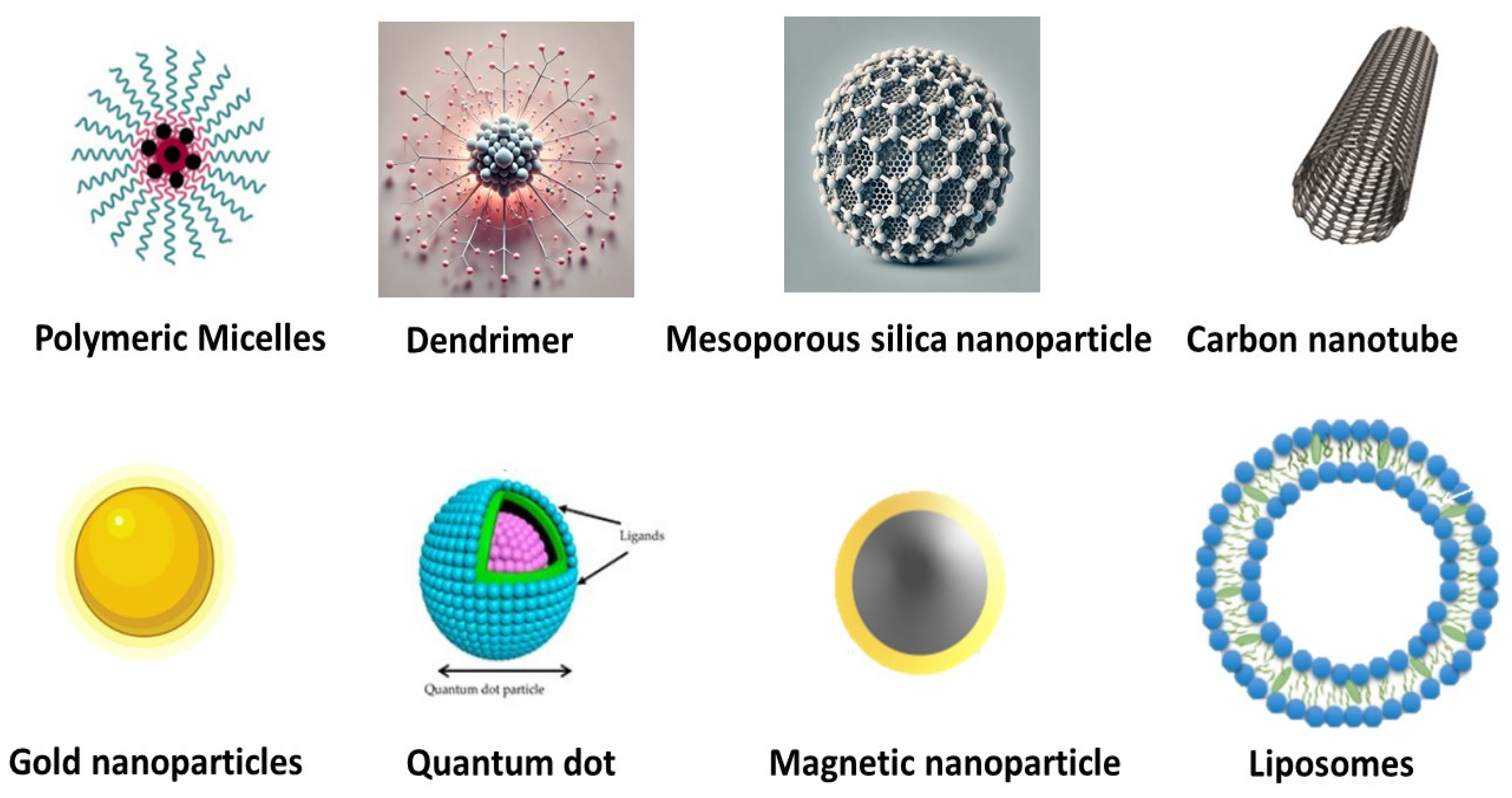
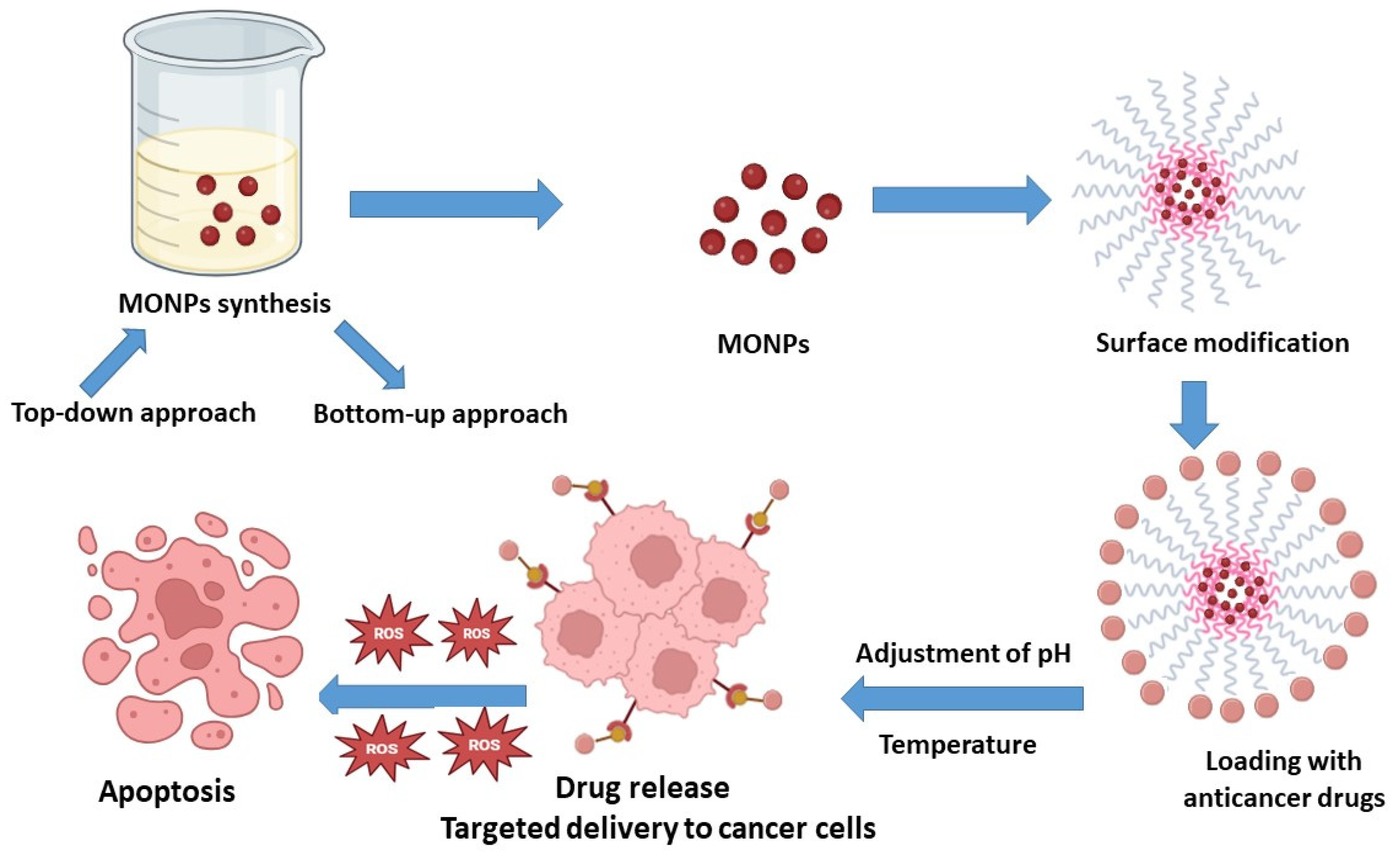
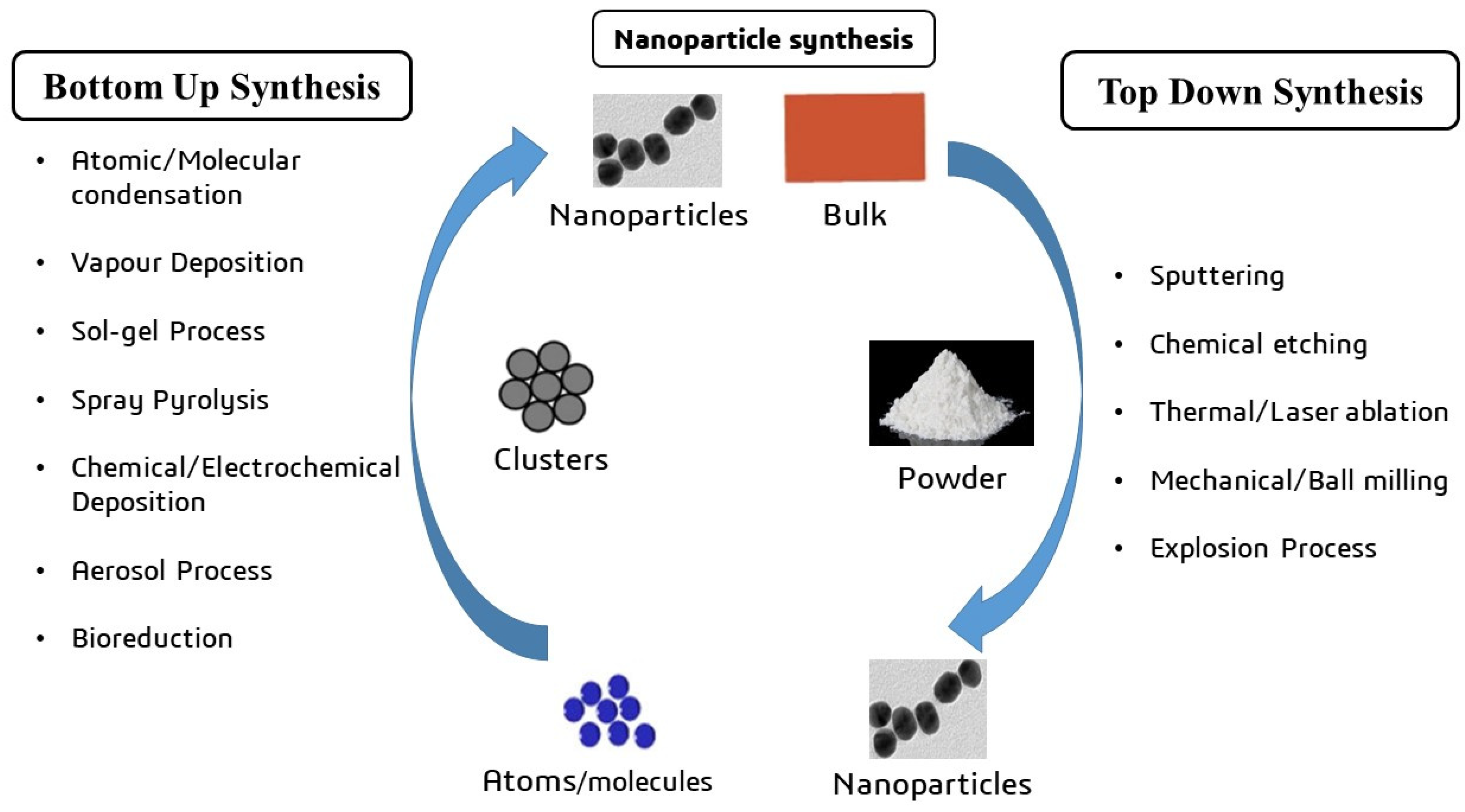
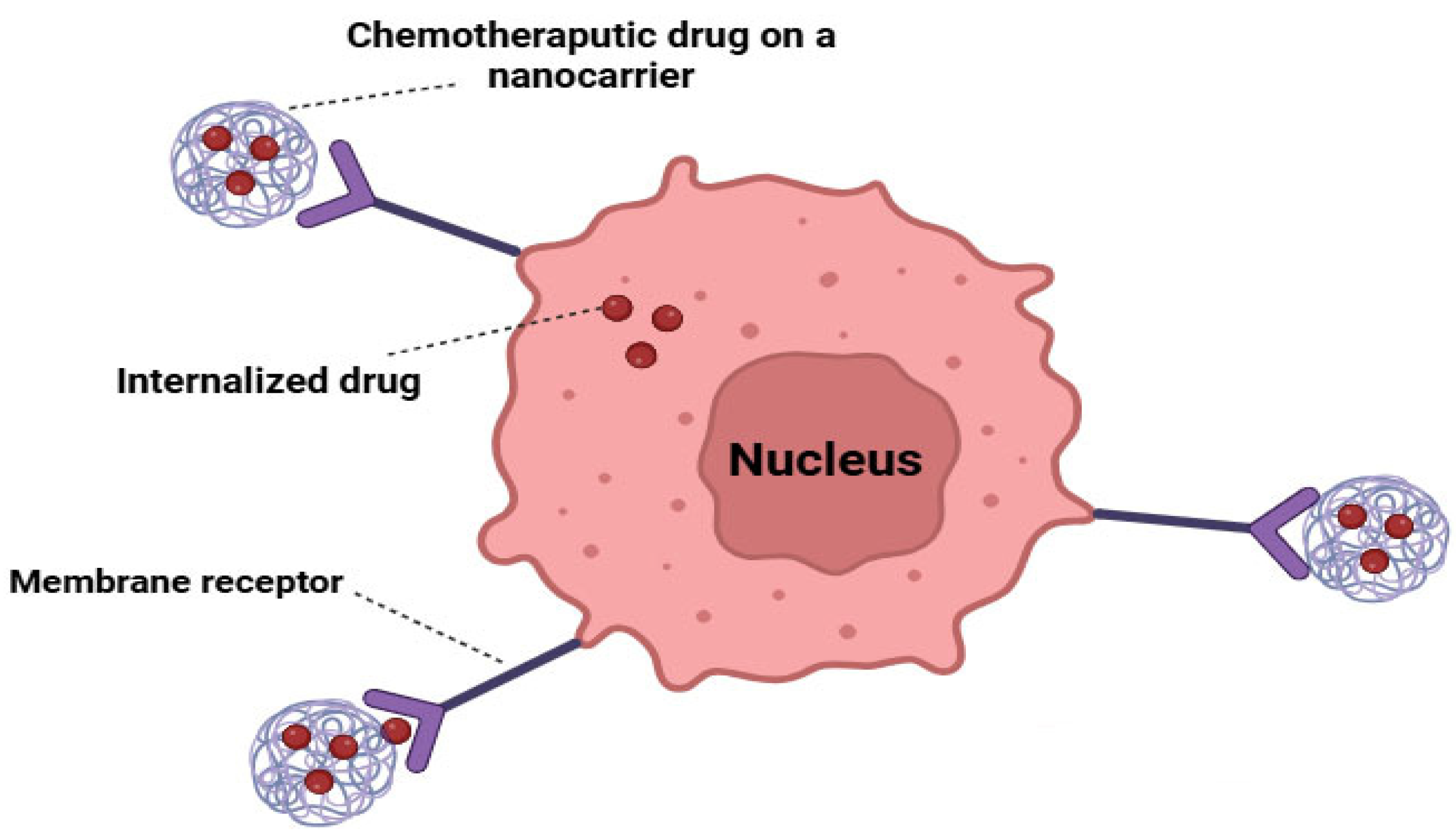

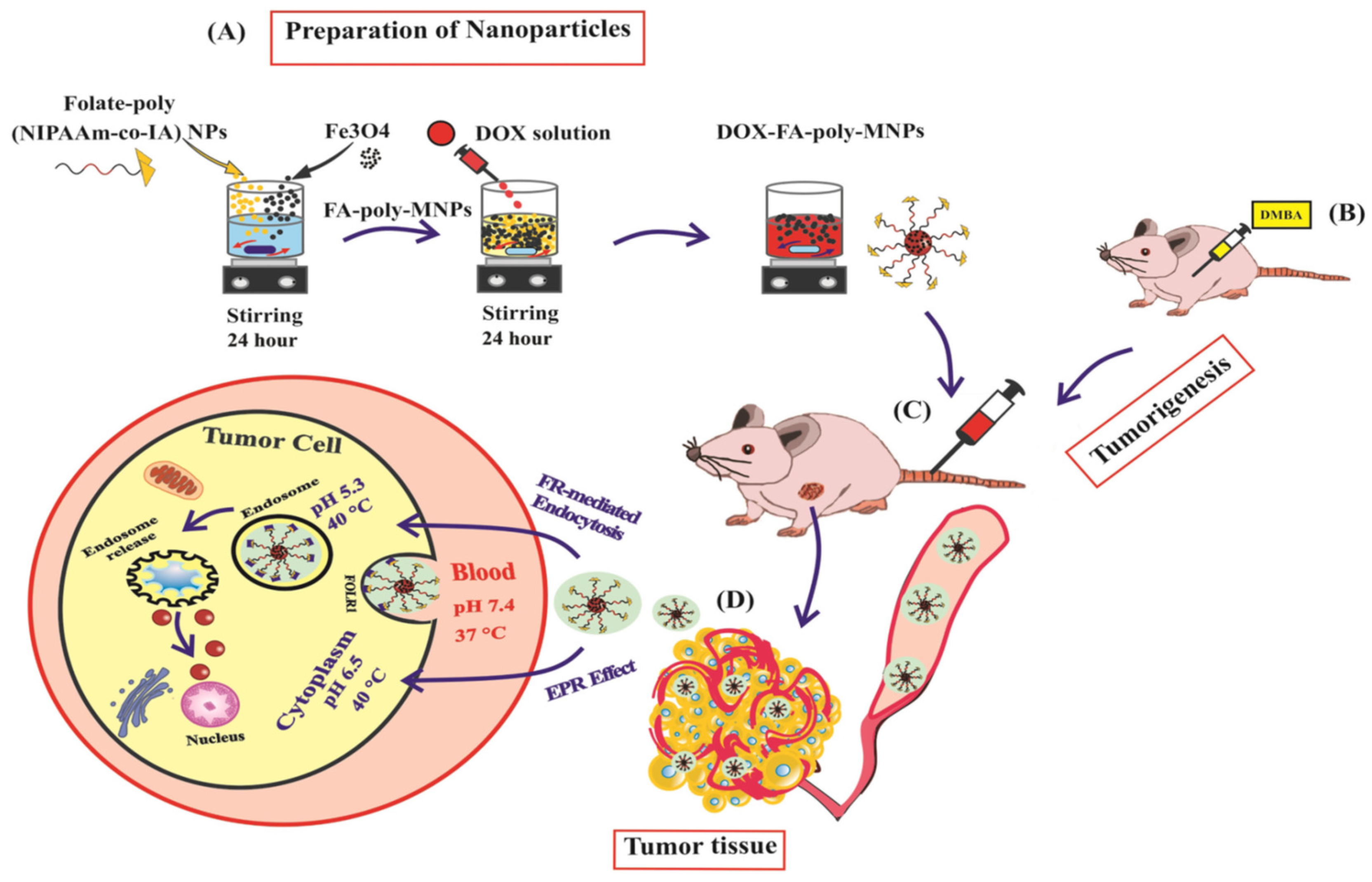

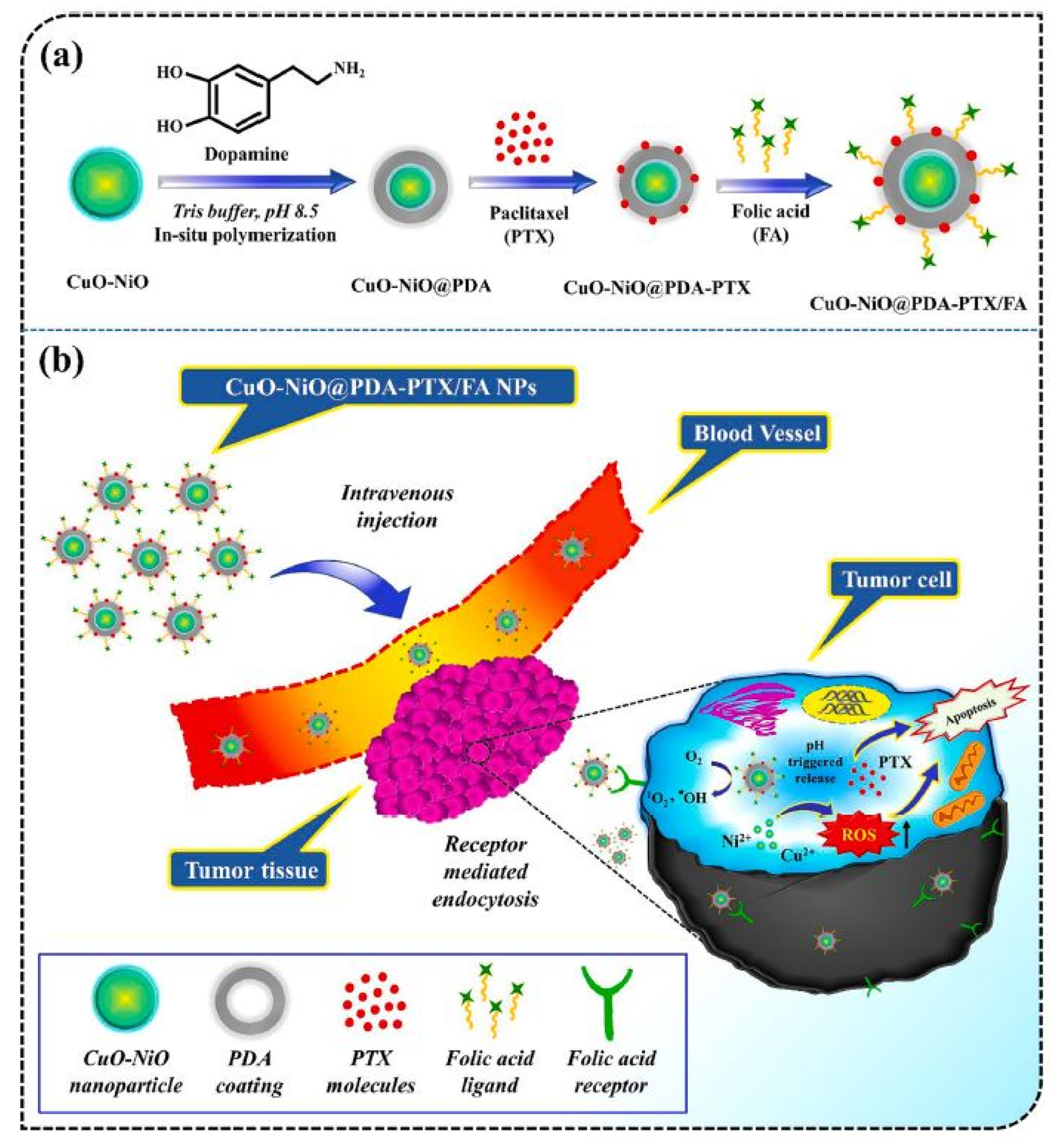
| S. No. | Nanocarrier | Active Drug/Therapeutic Agent | Targeted Cancer Type | Administration Method | Drug Release Within 24 h (%) | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| 1 | TiO2–pPBA | Doxorubicin | Breast cancer | In vitro, In vivo | 80% | Preclinical | [75] |
| 2 | TiO2 NPs–APTES | 5-Fluorouracil | Oral cancer | In vitro | 50% | Preclinical | [76] |
| 3 | PEG-coated TiO2 nanoparticles | Imatinib | - | In vitro | 99% (pH 4.4) | Preclinical | [77] |
| 4 | PVP/PVA/TiO2/QC | Quercetin | Human glioblastoma | In vitro | 57% (pH 5.4) | Preclinical | [78] |
| 5 | Folic acid–PEG–TiO2 | Paclitaxel | Liver Cancer | In vitro | 70% | Preclinical | [79] |
| 6 | TiO2–nanotubes | 5-fluorouracil | Cervical cancer | In vitro | – | Preclinical | [80] |
| 7 | TiO2–PEG | Doxorubicin | Breast tumor | In vitro, In vivo | 80% | Preclinical | [73] |
| 8 | Mesoporous TiO2 | Doxorubicin | Lung cancer | In vitro | 40% | Preclinical | [74] |
| S. No. | Nanocarrier | Active Drug/Therapeutic Agent | Targeted Cancer Type | Administration Method | Drug Release Within 24 h (%) | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| 1 | Folate–Fe3O4 | Doxorubicin | Breast cancer | In vivo | - | Preclinical | [92] |
| 2 | Dextran–Fe3O4 | Doxorubicin and cetuximab | lung cancer | In vivo | 20% | Preclinical | [106] |
| 3 | Fe3O4 NPs with Au shell and pectin | Curcumin | Cervical cancer | In vivo | 40% | Preclinical | [107] |
| 4 | Hyaluronic acid–Fe3O4 | Methotrexate | Lung cancer | In vitro | 8.41% | Preclinical | [108] |
| 5 | Tannic acid–Fe3O4 | Doxorubicin | Colon cancer | In vitro | 70% | Preclinical | [109] |
| 6 | Albumin–Fe3O4 | Doxorubicin | Breast cancer | In vitro | 83.35% | Preclinical | [110] |
| 7 | Fe3O4–mesoporous silica | Disulfiram | Breast cancer | In vitro | 100% | Preclinical | [111] |
| 8 | Fe3O4–APTES | Methotrexate | Ovarian cancer | In vitro | 95% | Preclinical | [112] |
| 9 | Cs/DOX/Cit–MNPs | Doxorubicin | Breast cancer | In vitro | 75% | Preclinical | [113] |
| 10 | PMNP-VCR-FA-TF | Vincristine | Eye cancer | In vitro, ex vivo | 64.71% (48 h) | Preclinical | [114] |
| S. No. | Nanocarrier | Active Drug/Therapeutic Agent | Targeted Cancer Type | Administration Method | Drug Release Within 24 h (%) | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| 1 | ZnONPs/DOX/FA | Doxorubicin | Colon and Breast cancer | In vitro, in vivo | - | Preclinical | [128] |
| 2 | CS/CMC/GQDs/ZnO@QC | Quercetin | Brain cancer | In vitro | 82% (72 h) | Preclinical | [129] |
| 3 | 5-Fu–β-CD/ZnO | 5-Fluorouracil | Skin cancer | In vitro | 72.5% | Preclinical | [130] |
| 4 | Pd(II)/ZnO | Palladium (II) | Breast cancer | In vitro | 44.3% | Preclinical | [131] |
| 5 | PEG/PVA/ZnONPs | Quercetin | Breast cancer | In vitro | 56% (pH = 5.4) | Preclinical | [118] |
| 6 | ZnO-PG-RGD | Doxorubicin | Cervical cancer | In vitro | 60% (pH 5.2) | Preclinical | [132] |
| 7 | MSCNs–ZnO | Doxorubicin | Breast cancer | In vitro | 35% (pH 5.0) | Preclinical | [133] |
| 8 | CH-HIS-ZnO | Quercetin | Skin cancer | In vitro | 72.41% (pH 5.0) | Preclinical | [134] |
| 9 | MCC-g-PMADQUAT-g-PAcZnO | Methotrexate | Breast cancer | In vitro | 32% (pH 5.4 and 40 °C) | Preclinical | [135] |
| 10 | MTX–ZnONPs | Methotrexate | Breast cancer | In vitro | 90% | Preclinical | [136] |
| S. No. | Nanocarrier | Active Drug/Therapeutic Agent | Targeted Cancer Type | Administration Method | Drug Release Within 24 h (%) | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| 1 | Ga@CuO-PTX@K-carr/FA | Paclitaxel | Breast cancer | In vitro | 50% (pH 5.0) | Preclinical | [143] |
| 2 | CuO-NiO@PDA-PTX/FA NPs | Paclitaxel | Breast cancer | In vitro | 40% (pH 5.0) | Preclinical | [142] |
| 3 | Zn-CuO@PTX/AlgPDA NPs | Paclitaxel | Breast cancer | In vitro | 45% (pH 5.0) | Preclinical | [144] |
| 4 | CuO@BSA-MTX NPS | Methotrexate | Breast cancer | In vitro | 65% (Proteinase K enzyme, pH 7.4) | Preclinical | [145] |
| 5 | CuO-PTX@PHBV-PEG | Paclitaxel | Breast cancer | In vitro | 38% (pH 5.0) | Preclinical | [146] |
| 6 | PLGA-CuONPs | Doxorubicin and docetaxel | Nasopharyngeal cancer | In vitro | 700 µg/ml | Preclinical | [147] |
| 7 | DOX-Cs-CuO NPs | Doxorubicin | Breast cancer | In vitro | 73% | Preclinical | [148] |
| 8 | Fe3O4@Cu3(BTC)2 | Doxorubicin | Breast cancer | In vitro | 85.5% (pH 5.0) | Preclinical | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassin, M.T.; Al-Otibi, F.O.; Al-Sahli, S.A.; El-Wetidy, M.S.; Mohamed, S. Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities. Cancers 2024, 16, 4234. https://doi.org/10.3390/cancers16244234
Yassin MT, Al-Otibi FO, Al-Sahli SA, El-Wetidy MS, Mohamed S. Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities. Cancers. 2024; 16(24):4234. https://doi.org/10.3390/cancers16244234
Chicago/Turabian StyleYassin, Mohamed Taha, Fatimah O. Al-Otibi, Sarah A. Al-Sahli, Mohammad S. El-Wetidy, and Sara Mohamed. 2024. "Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities" Cancers 16, no. 24: 4234. https://doi.org/10.3390/cancers16244234
APA StyleYassin, M. T., Al-Otibi, F. O., Al-Sahli, S. A., El-Wetidy, M. S., & Mohamed, S. (2024). Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities. Cancers, 16(24), 4234. https://doi.org/10.3390/cancers16244234






