Genetically Engineered Probiotic Limosilactobacillus reuteri Releasing IL-22 (LR-IL-22) Modifies the Tumor Microenvironment, Enabling Irradiation in Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and In Vivo Animal Model
2.2. Ovarian Cancer Cell Lines
2.3. Irradiation
2.4. Gavage Administration of the LR and LR-IL-22 or Intraperitoneal Delivery of the IL-22 Protein
2.5. Dose–Response Curves of the Number of Gavaged L. reuteri Producing IL-22 (LR-IL-22)
2.6. RNA-seq
2.7. Statistical Methods
3. Results
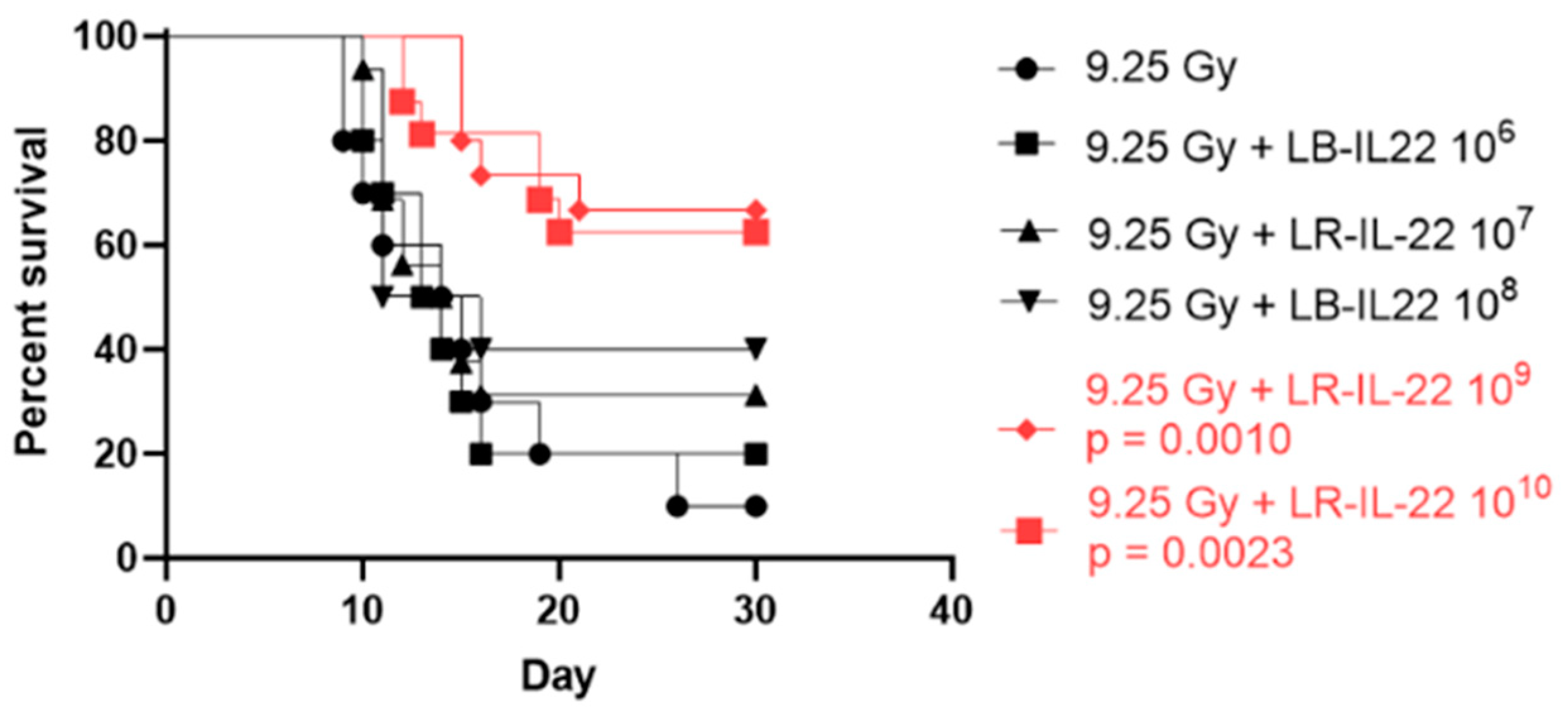
3.1. Survival Percentage Depended on the Bacterial Dose Administered for Effective Radiation Mitigation
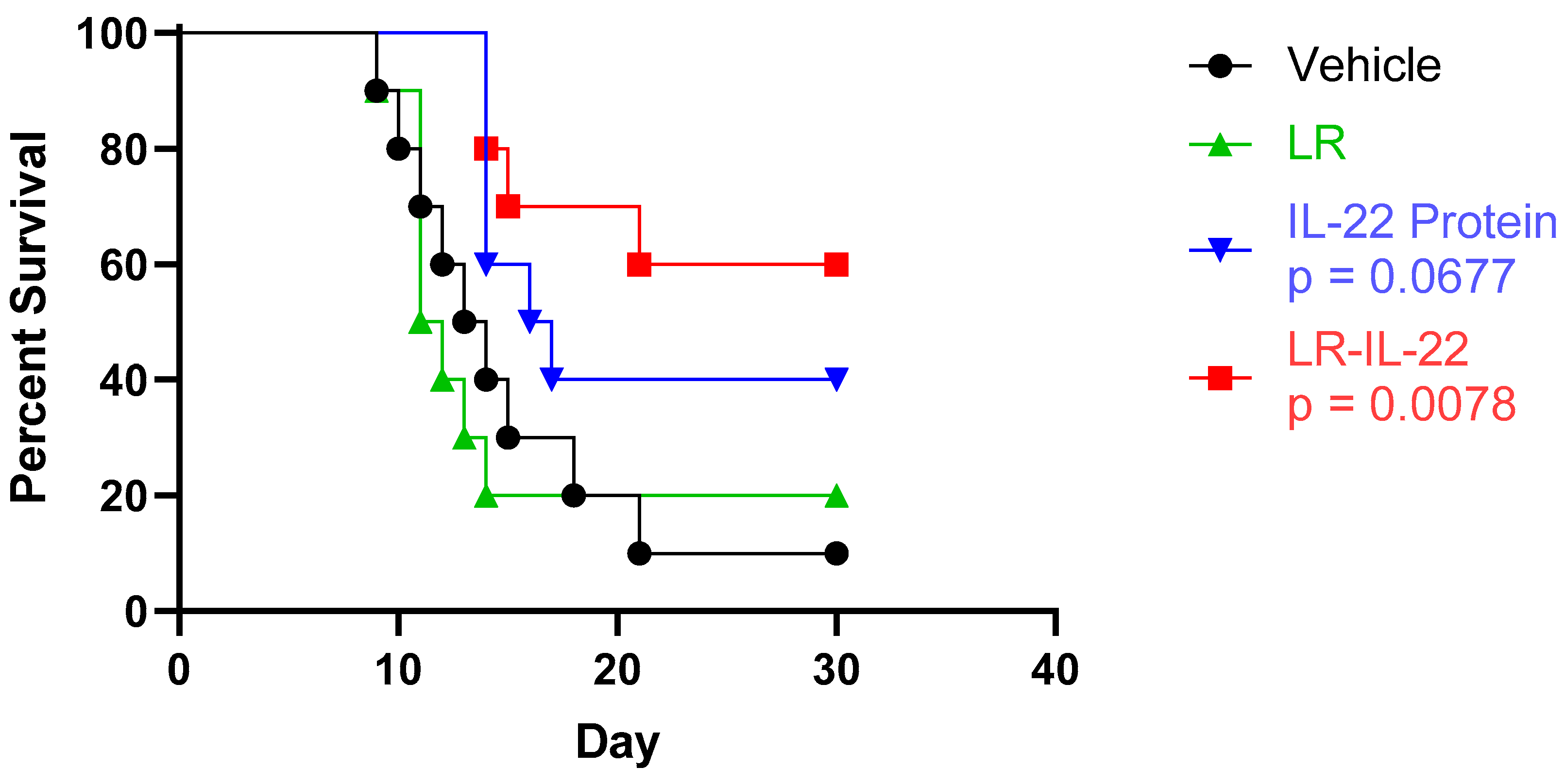
3.2. Increased Overall Survival Was Observed upon Administration of LR-IL-22 but Not IL22 or LR Alone
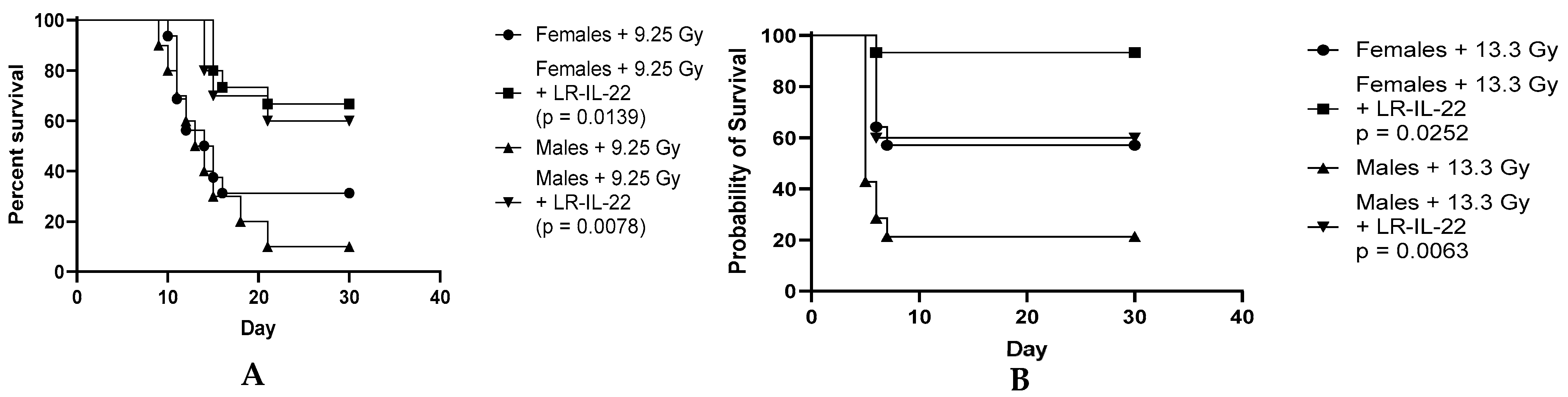
3.3. Effect of the LR-IL-22 Gavage on Survival in the Male and Female Mice Following Irradiation
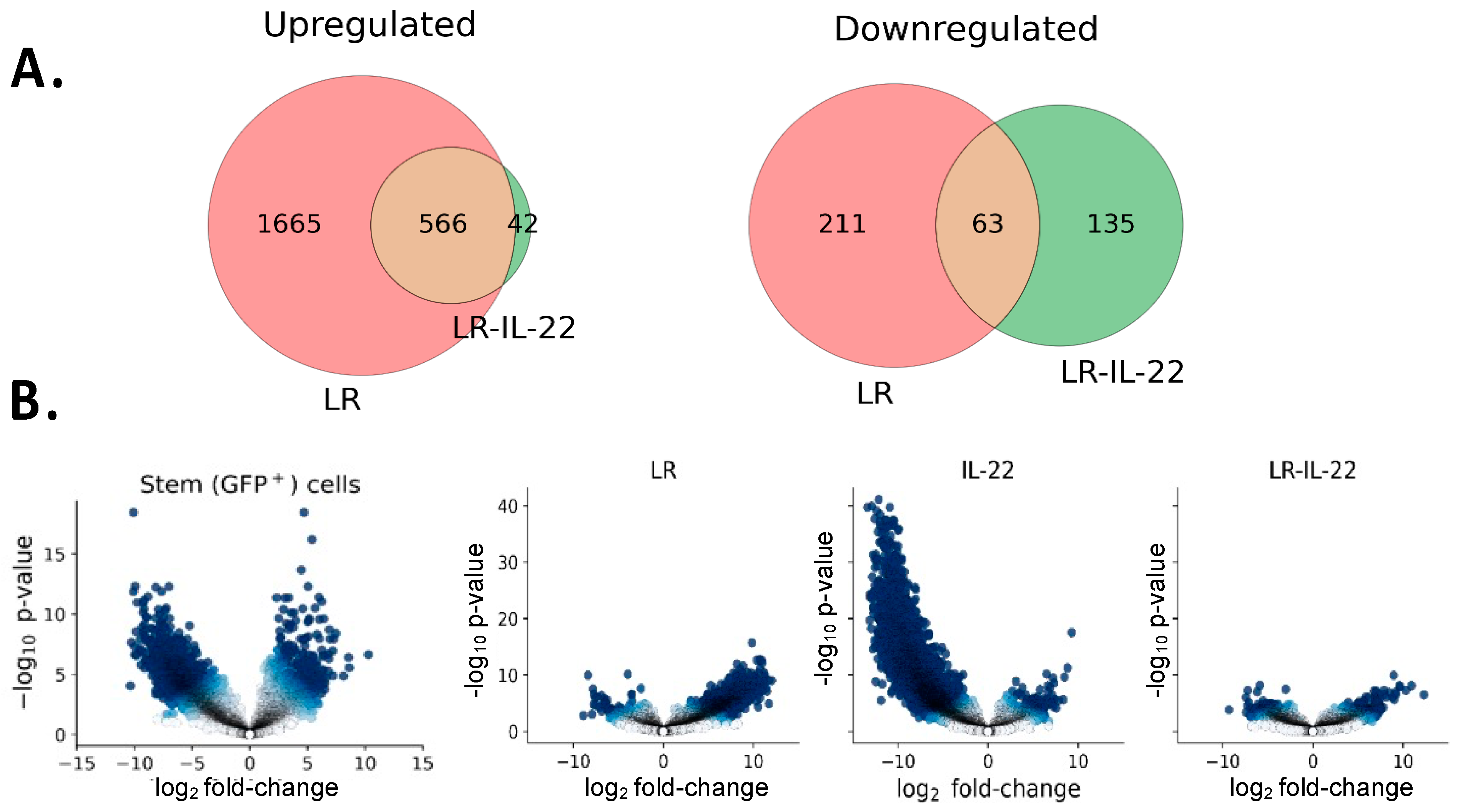
3.4. Differential Gene Expression in Irradiated Stem Cells in Mice Treated with and without TBI and LR-IL-22
3.4.1. Genes Downregulated by LR-IL-22
3.4.2. Genes Upregulated by LR-IL-22
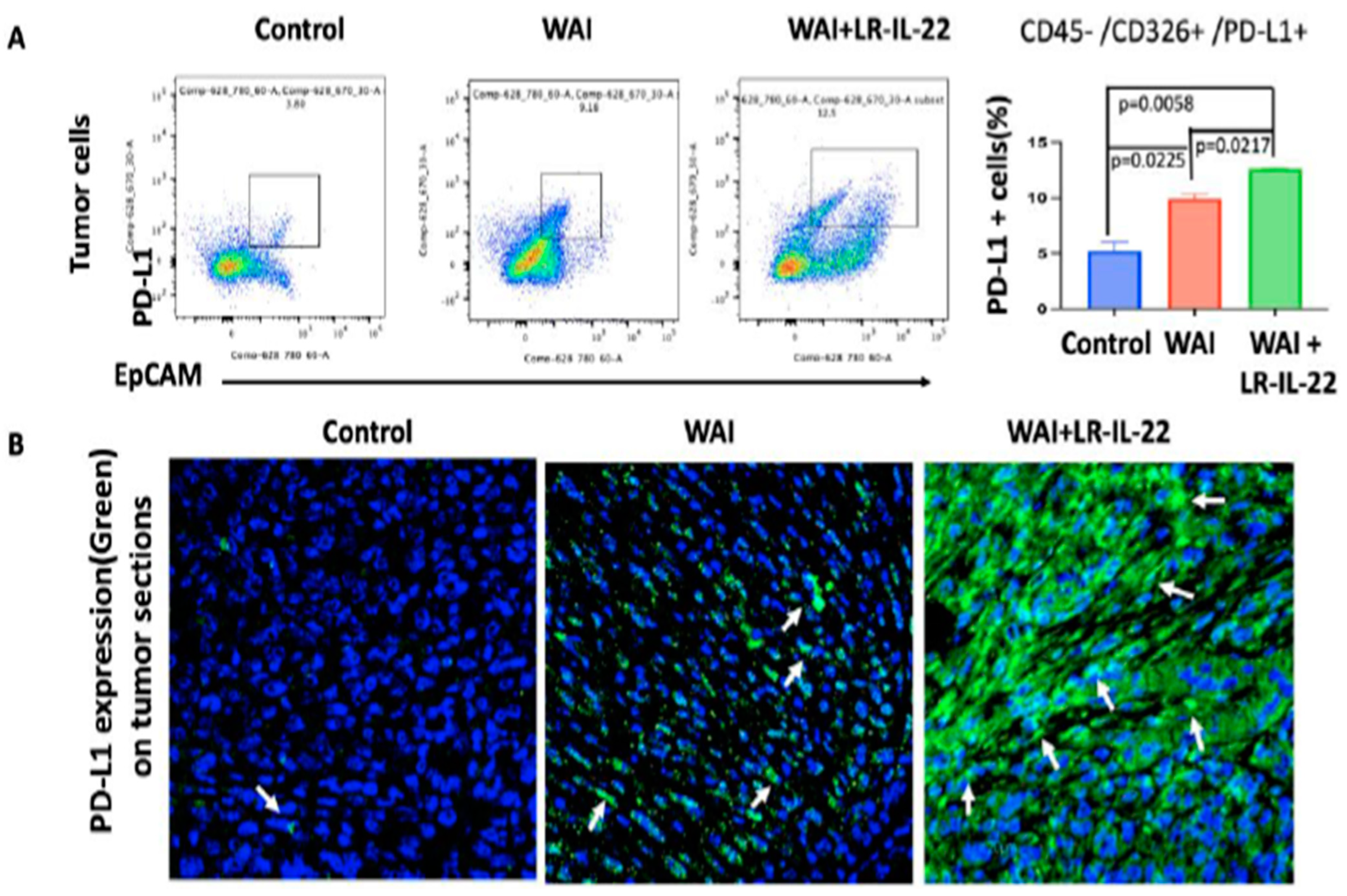
3.5. Intraoral Administration (Gavage) of LR-IL-22 Induced PD-L1 Protein Release in Ovarian Cancer Cells, Mobilized CD8+ T Cells in the WAI Mice, and Improved Overall Survival When Combined with a PD-L1 Inhibitor
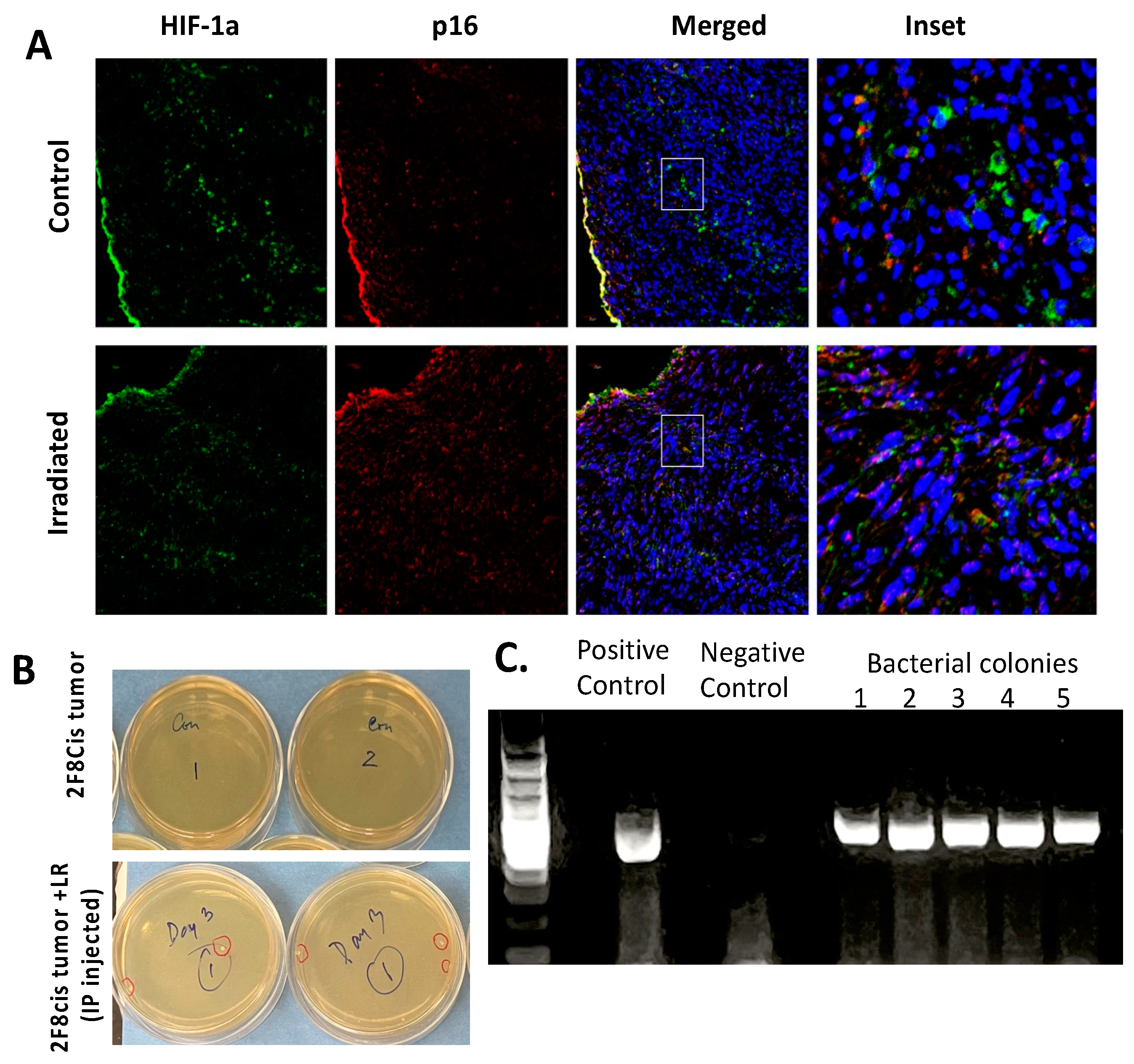
3.6. Migration of LR Bacteria into the 2F8cis Ovarian Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fields, E.C.; McGuire, A.P.; Lin, L.; Temkin, S.M. Radiation Treatment in Women with Ovarian Cancer: Past, Present, and Future. Front. Oncol. 2017, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Dembo, A.J. Epithelial ovarian cancer: The role of radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 835–845. [Google Scholar] [CrossRef] [PubMed]
- May, L.F.; Belinson, J.L.; Roland, T.A. Palliative benefit of radiation therapy in advanced ovarian cancer. Gynecol. Oncol. 1990, 37, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Gerszten, K.; Edwards, R.; Land, S.; D’Angelo, G.; Kelley, J., 3rd; Price, F. A phase I/II study of hypofractionated whole abdominal radiation therapy in patients with chemorsesistant ovarian carcinoma: Karnofsky score determines treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Hamade, D.F.; Espinal, A.; Yu, J.; Leibowitz, B.J.; Fisher, R.; Hou, W.; Shields, D.; van Pijkeren, J.-P.; Mukherjee, A.; Epperly, M.W.; et al. Lactobacillus reuteri Releasing IL-22 (LR-IL-22) Facilitates Intestinal Radioprotection for Whole-Abdomen Irradiation (WAI) of Ovarian Cancer. Radiat. Res. 2022, 198, 89–105. [Google Scholar] [CrossRef]
- Budiu, R.A.; Elishaev, E.; Brozick, J.; Lee, M.; Edwards, R.P.; Kalinski, P.; Vlad, A.M. Immunobiology of human mucin 1 in a preclinical ovarian tumor model. Oncogene 2013, 32, 3664–3675. [Google Scholar] [CrossRef] [PubMed]
- Mony, J.T.; Zhang, L.; Ma, T.; Grabosch, S.; Tirodkar, T.S.; Brozick, J.; Tseng, G.; Elishaev, E.; Edwards, R.P.; Huang, X.; et al. Anti-PD-L1 prolongs survival and triggers T cell but not humoral anti-tumor immune responses in a human MUC1- expressing preclinical ovarian cancer model. Cancer Immunol. Immunother. 2015, 64, 1095–1108. [Google Scholar] [CrossRef]
- Carey, M.S.; Dembo, A.J.; Simm, J.E.; Fyles, A.W.; Treger, T.; Bush, R.S. Testing the validity of a prognostic classification in patients with surgically optimal ovarian carcinoma: A 15-year review. Int. J. Gynecol. Cancer 1993, 3, 24–35. [Google Scholar] [CrossRef]
- Thermozier, S.; Hou, W.; Zhang, X.; Shields, D.; Fisher, R.; Bayir, H.; Kagan, V.; Yu, J.; Liu, B.; Bahar, I.; et al. Anti-ferroptosis drug, Baicalein, enhances total body irradiation mitigation by two other drugs that target apoptosis and necroptosis. Radiat. Res. 2020, 193, 435–450. [Google Scholar] [CrossRef]
- Zhang, X.; Fisher, R.; Hou, W.; Shields, D.; Epperly, M.W.; Wang, H.; Wei, L.; Leibowitz, B.J.; Yu, J.; Alexander, L.M.; et al. Second-generation probiotics producing IL-22 increase survival of mice after total body irradiation. In Vivo 2020, 34, 39–50. [Google Scholar] [CrossRef]
- Sun, Q.-Y.; Zhou, H.-H.; Mao, X.-Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2019, 29, 9. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Creagh, E.M. Caspase-4 and -5 Biology in the Pathogenesis of Inflammatory Bowel Disease. Front. Pharmacol. 2022, 31, 919567. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Z.; Wen, H. Protein O-GlcNAcylation Regulates Innate Immune Cell Function. Front. Immunol. 2022, 13, 805018. [Google Scholar] [CrossRef] [PubMed]
- Show, M.D.; Hill, C.M.; Anway, M.D.; Wright, W.W.; Zirkin, B.R. Phosphorylation of Mitogen-Activated Protein Kinase 8 (MAPK8) Is Associated with Germ Cell Apoptosis and Redistribution of the Bcl2-Modifying Factor (BMF). J. Androl. 2008, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Talebzadeh-Farrooji, M.; Osborne, M.J.; Prokop, J.W.; McDonald, P.C.; Karar, J.; Hou, Z.; He, M.; Kebebew, E.; Orntoft, T.; et al. LIMD2 is a small LIM-only protein overexpressed in metastatic lesions that regulates cell motility and tumor progression by directly binding to and activating the integrin-linked kinase. Cancer Res. 2014, 74, 1390–1403. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, W.; Liu, S.; Liu, Z.; Xu, P.; Fang, W. Regulatory network and targeted interventions for CCDC family in tumor pathogenesis. Cancer Lett. 2023, 565, 216225. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, J.; Xu, W.; Wang, Y.; Zheng, C.-C.; Li, B.; He, Q.-Y. KCTD12 promotes tumorigenesis by facilitating CDC25B/CDK1/Aurora A-dependent G2/M transition. Oncogene 2017, 36, 6177–6189. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Miao, C.; Dongol, S.; Li, Y.; Jin, C.; Dong, R.; Li, Y.; Yang, X.; Kong, B. CCNG1 (Cyclin G1) regulation by mutant-P53 via induction of Notch3 expression promotes high-grade serous ovarian cancer (HGSOC) tumorigenesis and progression. Cancer Med. 2019, 8, 351–362. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Song, H.-M.; Zhou, Q. Comprehensive analysis of the expression and prognosis for S100 in human ovarian cancer: A STROBE study. Medicine 2020, 99, e22777. [Google Scholar] [CrossRef]
- Azimi, A.; Pernemalm, M.; Stolt, M.F.; Hansson, J.; Lehtio, J.; Brage, S.E.; Johansson, C.H. Proteomics analysis of melanoma metastases: Association between S100A13 expression and chemotherapy resistance. Br. J. Cancer 2014, 110, 2489–2495. [Google Scholar] [CrossRef][Green Version]
- Birkenkamp-Demtröder, K.; Hahn, S.A.; Mansilla, F.; Thorsen, K.; Maghnouj, A.; Christensen, R.; Oster, B.; Orntoft, T.F. Keratin23 (KRT23) knockdown decreases proliferation and affects the DNA damage response of colon cancer cells. PLoS ONE 2013, 8, e73593. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Cao, Y.; Zhou, X.; Wei, B.; Zhan, L.; Sun, S.Y. Stimulative role of ST6GALNAC1 in proliferation, migration and invasion of ovarian cancer stem cells via the Akt signaling pathway. Cancer Cell. Int. 2019, 1986, 86. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Gao, F.; Zhang, S.; Lv, X.; Chen, Y.; Liu, Q. A review of the mechanism of DDIT4 serve as a mitochondrial related protein in tumor regulation. Sci. Prog. 2021, 104, 36850421997273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ni, C.; Lu, H. Polo-Like Kinase 2: From Principle to Practice. Front. Oncol. 2022, 12, 956225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, X.; Gao, B. Knockdown of S100A11 expression suppresses ovarian cancer cell growth and invasion. Exp. Ther. Med. 2015, 9, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kitaura, J.; Enomoto, Y.; Lu, Y.; Nishimura, K.; Isobe, M.; Ozaki, K.; Komeno, Y.; Nakahara, F.; Oki, T.; et al. Transforming growth factor-β-stimulated clone-22 is a negative-feedback regulator of Ras/Raf signaling: Implications for tumorigenesis. Cancer Sci. 2012, 103, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sinkala, M.; Nkhoma, P.; Mulder, N.; Martin, D.P. Integrated molecular characterisation of the MAPK pathways in human cancers reveals pharmacologically vulnerable mutations and gene dependencies. Commun. Biol. 2021, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Lee, Y.; Ting, H.; Liu, N.; Liu, S.; Lin, S.; Yeh, S.; Li, G.; Chang, C. Deficiency in TR4 nuclear receptor abrogates Gadd45a expression and increases cytotoxicity induced by ionizing radiation. Cell. Mol. Biol. Lett. 2012, 17, 309–322. [Google Scholar] [CrossRef]
- Steenbergen, R.; Nanowski, T.S.; Beigneux, A.; Kulinski, A.; Young, S.G.; Vance, J.E. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. Chem. Biol. Chem. 2005, 280, 40032–40040. [Google Scholar] [CrossRef]
- Zhong, H.; De Marzo, A.M.; Laughner, E.; Lim, M.; Hilton, D.A.; Zagzag, D.; Buechler, P.; Isaacs, W.B.; Semenza, G.L.; Simons, J.W. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999, 59, 5830–5835. [Google Scholar]
- Talks, K.L.; Turley, H.; Gatter, K.C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J.; Harris, A.L. The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages. Am. J. Pathol. 2000, 157, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Kunos, C.; Sill, M.; Buekers, T.; Walker, J.; Schilder, J.; Yamada, S.; Waggoner, S.E.; Mohiuddin, M.; Fracasso, P.M. Low-dose abdominal radiation as a docetaxel chemosensitizer for recurrent epithelial ovarian cancer: A phase 1 study of the Gynecologic Oncology Group. Gynecol. Oncol. 2011, 120, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.L.; Heong, V.; Tang, J.I.; Choo, B.A.; Kumarakulasinghe, N.B.; Lim, D.; Low, M.; Lim, S.E.; Lim, Y.W.; Leong, Y.H.; et al. Phase I study of low-dose fractionated whole abdominal radiation therapy in combination with weekly paclitaxel for platinum-resistant ovarian cancer (GCGS-01). Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Arians, N.; Kieser, M.; Benner, L.; Rochet, N.; Schroder, L.; Katayama, S.; Herfarth, K.; Schubert, K.; Schneeweiss, A.; Sohn, C.; et al. Adjuvant intensity modulated whole-abdominal radiation therapy for high-risk patients with ovarian cancer FIGO stage III: Final results of a prospective phase 2 study. Radiat. Oncol. 2019, 14, 179. [Google Scholar] [CrossRef]
- Alme, A.K.; Karir, B.S.; Faltas, B.M.; Drake, C.G. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: An overview. Urol. Oncol. 2016, 34, 171–181. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD81 tumor-infiltrating lymphocytes and a high CD81/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Hwang, W.T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Gonzalez-Martin, A.; Sanchez-Lorenzo, L. Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising? Cancer 2019, 125, 4616–4622. [Google Scholar] [CrossRef]
- Zamarin, D.; Burger, R.A.; Sill, M.W.; Powell, D.J., Jr.; Lankes, H.A.; Feldman, M.A.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C.; et al. Randomized Phase II trial of Nivolumab versus Nivolumab and Ipilimumab for recurrent or persistent Ovarian cancer: An NRG Oncology Study. J. Clin. Oncol. 2020, 38, 1814–1823. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, R.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Liu, J.; Gordon, M.; Veneris, J.; Braiteh, F.; Balmanoukian, A.; Eder, J.P.; Oaknin, A.; Hamilton, E.; Wang, Y.; Sarkar, I.; et al. Safety, clinical activity, and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol. Oncol. 2019, 154, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Taylor, M.H.; Kelly, K.; Beck, J.T.; Gordon, M.; Moore, K.M.; Patel, M.R.; Chaves, J.; Park, H.; Mita, A.C.; et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019, 5, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Cimino, M.A.; Peer, C.J.; Zimmer, A.; Lipkowitz, S.; Annunziata, C.M.; Cao, L.; Harrell, M.I.; Swisher, E.M.; Houston, N.; et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor Durvalumab in combination with poly (ADP-Ribose) polymerase inhibitor Olaparib or vascular endothelial growth factor receptor 1-3 inhibitor Cediranib in women’s cancers: A dose-escalation, Phase I Study. J. Clin. Oncol. 2017, 35, 2193–2202. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamade, D.F.; Epperly, M.W.; Fisher, R.; Hou, W.; Shields, D.; van Pijkeren, J.-P.; Leibowitz, B.J.; Coffman, L.G.; Wang, H.; Huq, M.S.; et al. Genetically Engineered Probiotic Limosilactobacillus reuteri Releasing IL-22 (LR-IL-22) Modifies the Tumor Microenvironment, Enabling Irradiation in Ovarian Cancer. Cancers 2024, 16, 474. https://doi.org/10.3390/cancers16030474
Hamade DF, Epperly MW, Fisher R, Hou W, Shields D, van Pijkeren J-P, Leibowitz BJ, Coffman LG, Wang H, Huq MS, et al. Genetically Engineered Probiotic Limosilactobacillus reuteri Releasing IL-22 (LR-IL-22) Modifies the Tumor Microenvironment, Enabling Irradiation in Ovarian Cancer. Cancers. 2024; 16(3):474. https://doi.org/10.3390/cancers16030474
Chicago/Turabian StyleHamade, Diala F., Michael W. Epperly, Renee Fisher, Wen Hou, Donna Shields, Jan-Peter van Pijkeren, Brian J. Leibowitz, Lan G. Coffman, Hong Wang, M. Saiful Huq, and et al. 2024. "Genetically Engineered Probiotic Limosilactobacillus reuteri Releasing IL-22 (LR-IL-22) Modifies the Tumor Microenvironment, Enabling Irradiation in Ovarian Cancer" Cancers 16, no. 3: 474. https://doi.org/10.3390/cancers16030474
APA StyleHamade, D. F., Epperly, M. W., Fisher, R., Hou, W., Shields, D., van Pijkeren, J.-P., Leibowitz, B. J., Coffman, L. G., Wang, H., Huq, M. S., Huang, Z., Rogers, C. J., Vlad, A. M., Greenberger, J. S., & Mukherjee, A. (2024). Genetically Engineered Probiotic Limosilactobacillus reuteri Releasing IL-22 (LR-IL-22) Modifies the Tumor Microenvironment, Enabling Irradiation in Ovarian Cancer. Cancers, 16(3), 474. https://doi.org/10.3390/cancers16030474






