In Vitro Sensitivity of Neuroendocrine Neoplasms to an Armed Oncolytic Measles Vaccine Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. NET/NEC Cell Lines
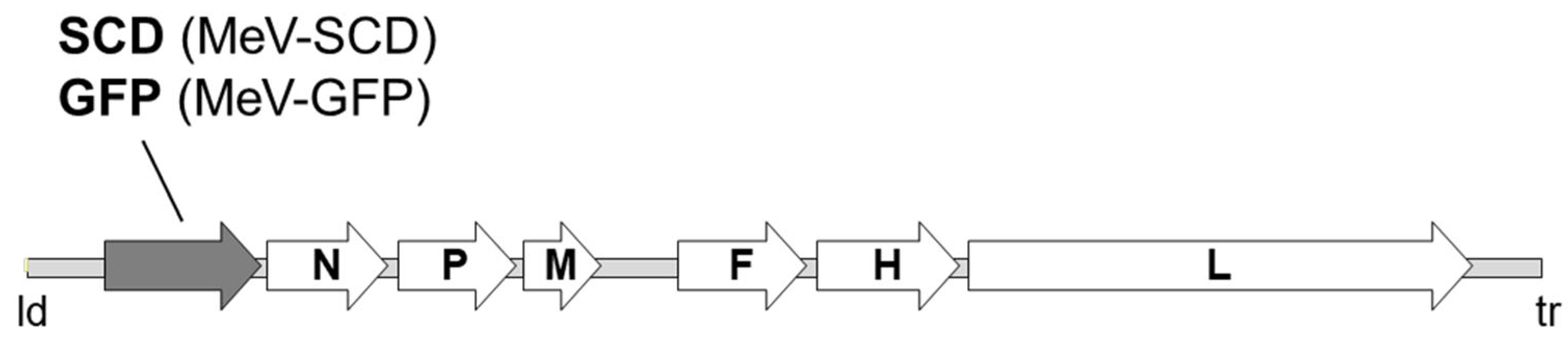
2.2. Measles Vaccine Virus
2.3. Sulforhodamine B (SRB) Viability Assay
2.4. FACS Analysis
2.5. Immunoblot Analysis
2.6. Viral Growth Curve
2.7. Statistical Analysis
3. Results
3.1. Determination of CD46 Receptor Expression on the Surfaces of NET/NEC Cells
3.2. Assessing Primary Infection Efficiency of NET/NEC Cell Lines Using MeV-GFP
3.3. Combating NET/NEC Cells with MeV-SCD
3.3.1. Oncolytic Effect of MeV-SCD Monotherapy
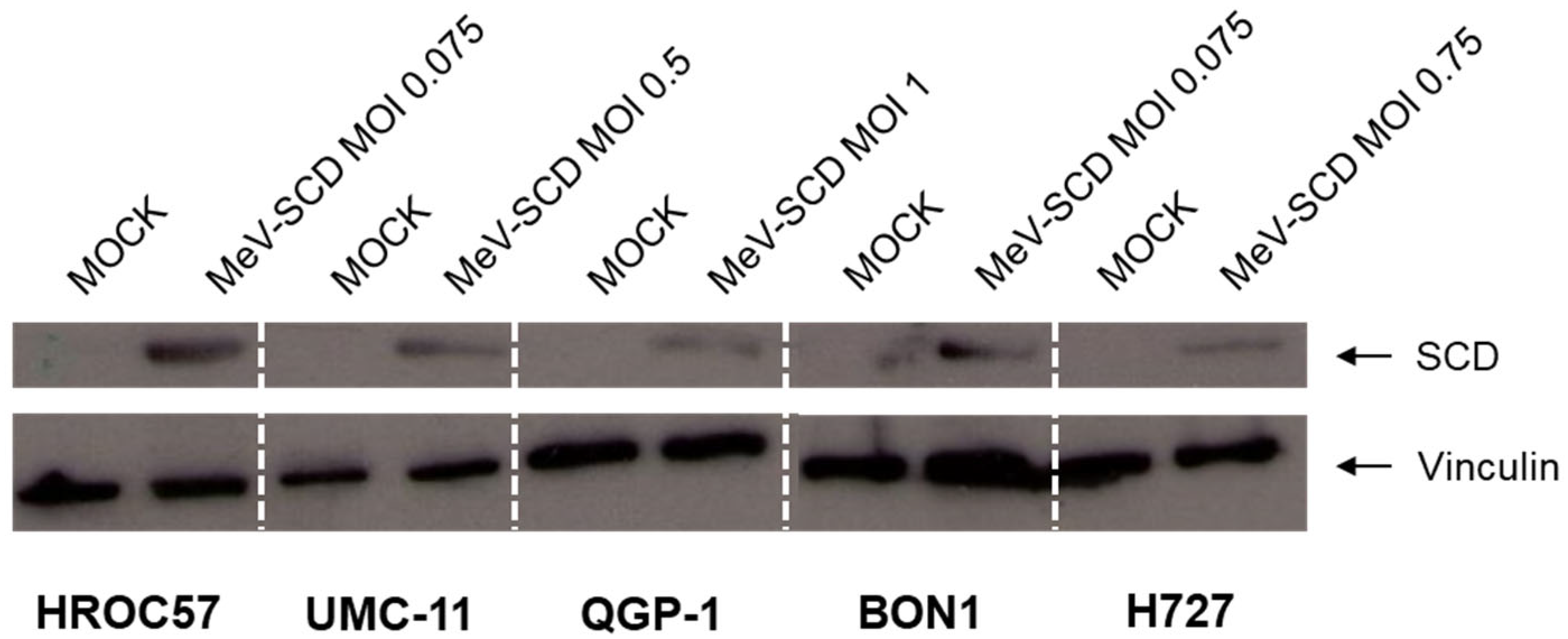
3.3.2. Expression of SCD in MeV-SCD-Infected NET/NEC Cell Lines
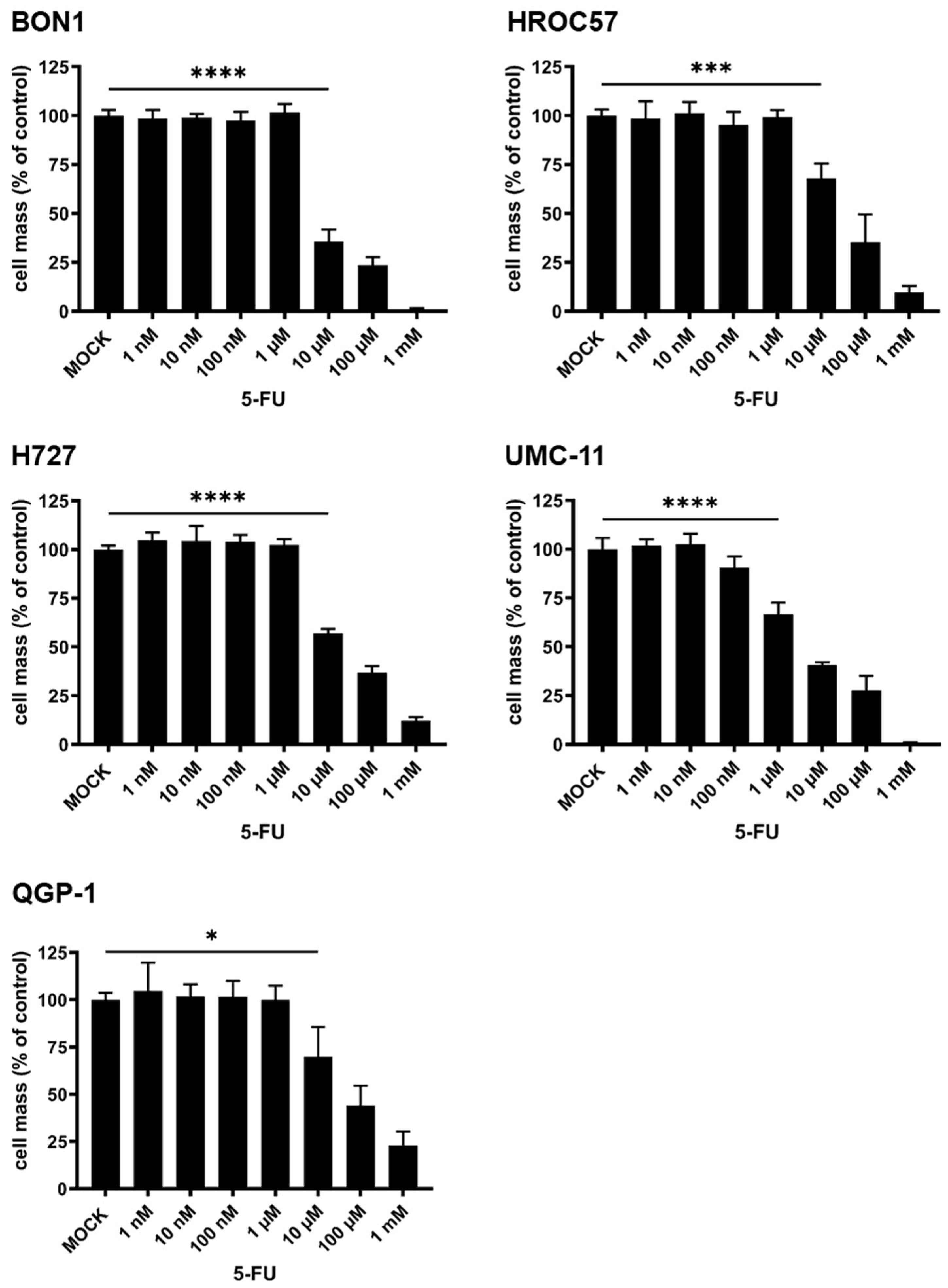
3.3.3. Cytotoxic Effect of 5-Fluorouracil (5-FU) Monotherapy
3.3.4. Exploitation of the SCD Prodrug-Converting System by Addition of 5-Fluorocytosine (5-FC) to MeV-SCD Therapy
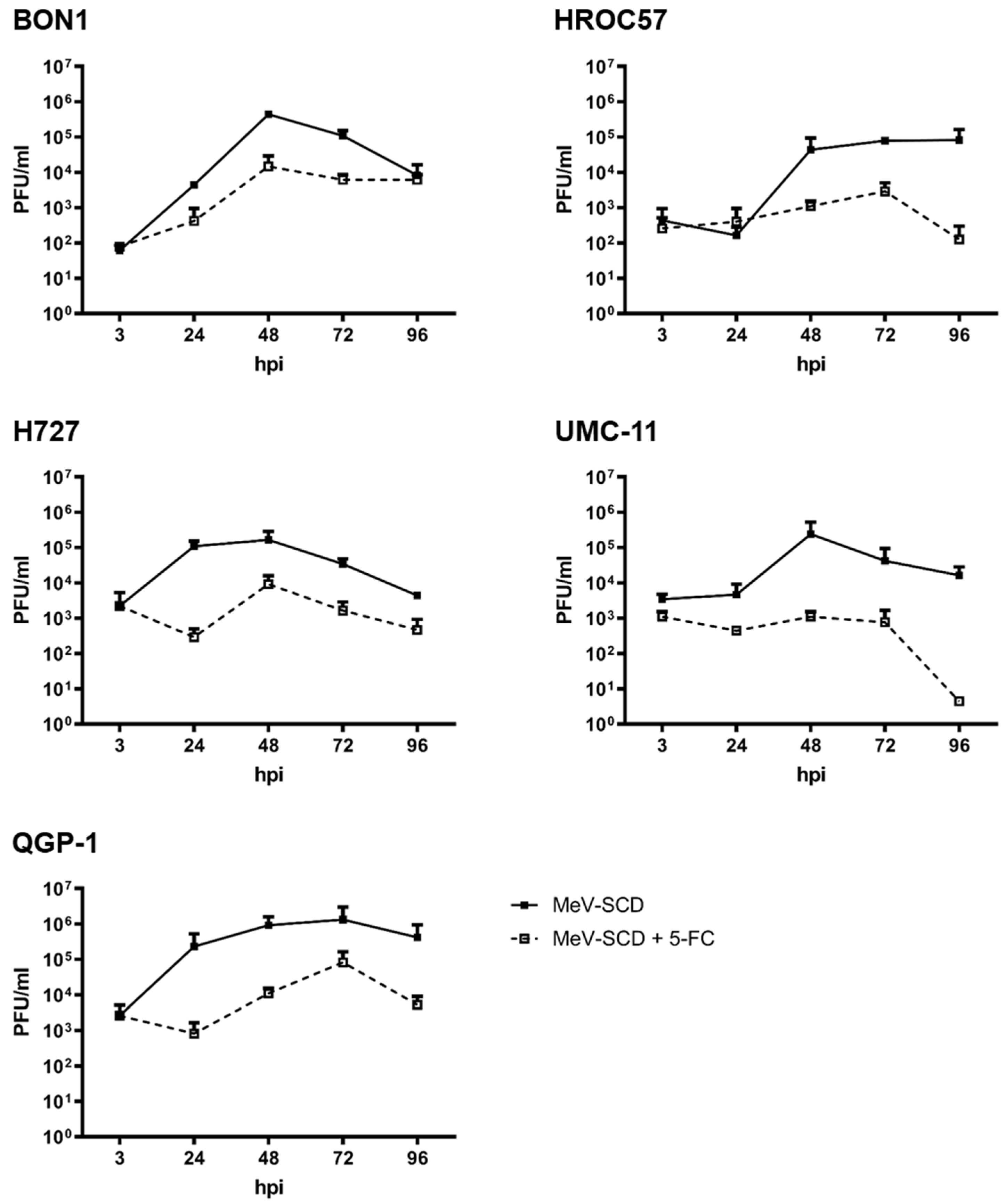
3.3.5. Replication Characteristics of MeV-SCD When Combined with 5-FC
3.4. Principles for Planned Clinical Combination Therapy of MeV-SCD with the mTOR-Inhibitor Everolimus
3.4.1. Cytotoxic Effect of Everolimus Monotherapy
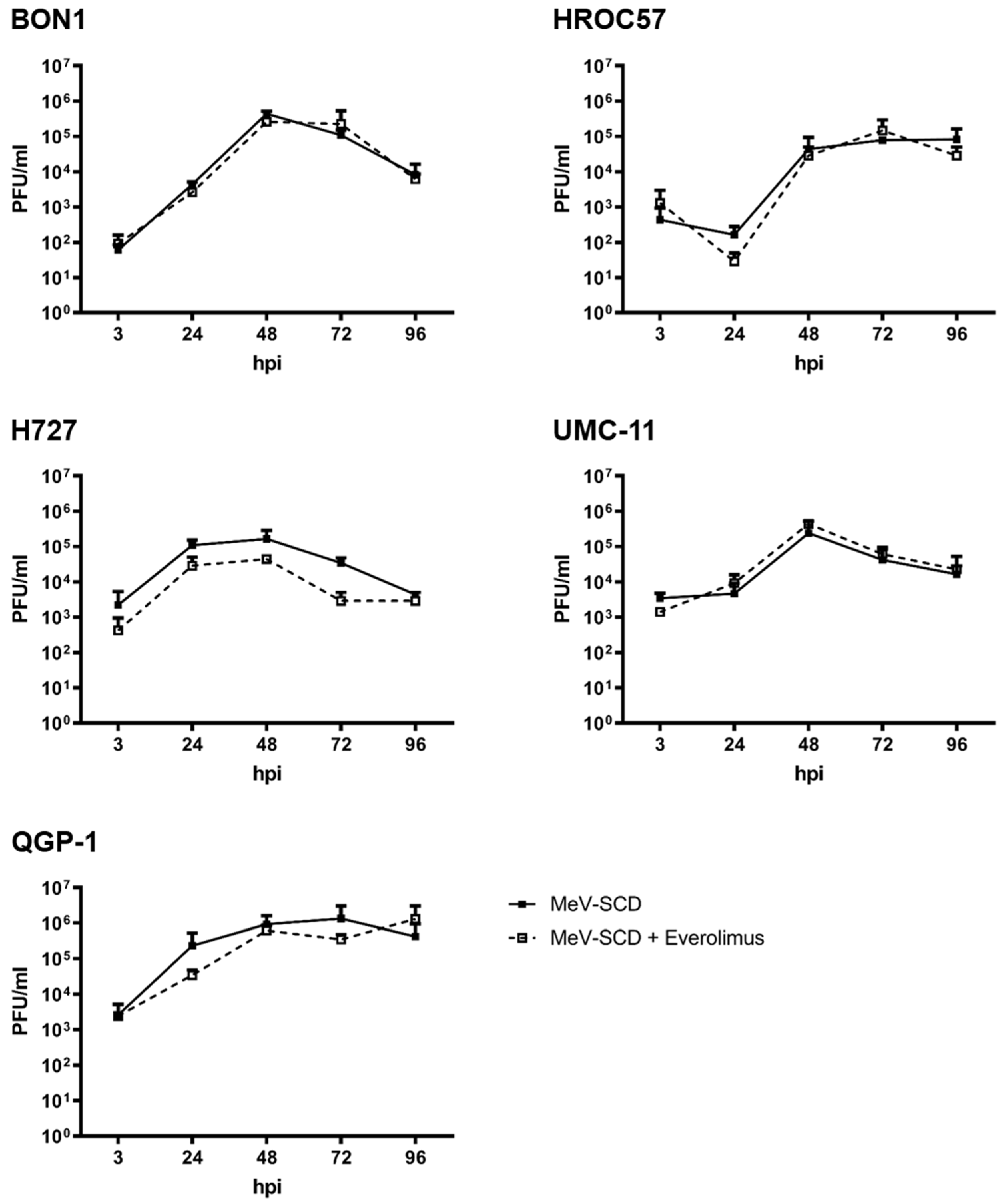
3.4.2. Replication Characteristics of MeV-SCD When Combined with Everolimus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taal, B.G.; Visser, O. Epidemiology of neuroendocrine tumours. Neuroendocrinology 2004, 80 (Suppl. S1), 3–7. [Google Scholar] [CrossRef]
- Alwan, H.; La Rosa, S.; Andreas Kopp, P.; Germann, S.; Maspoli-Conconi, M.; Sempoux, C.; Bulliard, J.L. Incidence trends of lung and gastroenteropancreatic neuroendocrine neoplasms in Switzerland. Cancer Med. 2020, 9, 9454–9461. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, N.; Voigtländer, S.; Hakimhashemi, A.; Pape, U.F.; Meyer, M.; Müller-Nordhorn, J. Site-specific trends in gastroenteropancreatic neuroendocrine neoplasms in Bavaria, Germany. Cancer Med. 2023, 12, 19949–19958. [Google Scholar] [CrossRef] [PubMed]
- Gosain, R.; Ball, S.; Rana, N.; Groman, A.; Gage-Bouchard, E.; Dasari, A.; Mukherjee, S. Geographic and demographic features of neuroendocrine tumors in the United States of America: A population-based study. Cancer 2019, 126, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kımıloğlu Şahan, E.; Erdoğan, N.; Ulusoy, İ.; Samet, E.; Akyıldız İğdem, A.; Gönüllü, D. P53, KI-67, CD117 expression in gastrointestinal and pancreatic neuroendocrine tumours and evaluation of their correlation with clinicopathological and prognostic parameters. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2015, 26, 104–111. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Terrivel, M.; Gromicho, C.; Matos, A.M. Oncolytic viruses: What to expect from its use in cancer treatment. Microbiol. Immunol. 2019, 64, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Lauer, U.M.; Beil, J. Oncolytic viruses: Challenges and considerations in an evolving clinical landscape. Future Oncol. 2022, 18, 2713–2732. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Zloza, A.; Kaufman, H.L. Talimogene laherparepvec (T-VEC) as cancer immunotherapy. Drugs Today 2015, 51, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Puzanov, I.; Kelley, M.C. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 2015, 7, 611–619. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Ressler, J.M.; Karasek, M.; Koch, L.; Silmbrod, R.; Mangana, J.; Latifyan, S.; Aedo-Lopez, V.; Kehrer, H.; Weihsengruber, F.; Koelblinger, P.; et al. Real-life use of talimogene laherparepvec (T-VEC) in melanoma patients in centers in Austria, Switzerland and Germany. J. Immunother. Cancer 2021, 9, e001701. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.E.; Speranza, M.C.; Cho, C.F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic virus immunotherapy: Future prospects for oncology. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef]
- Engeland, C.E.; Ungerechts, G. Measles Virus as an Oncolytic Immunotherapy. Cancers 2021, 13, 544. [Google Scholar] [CrossRef]
- Essand, M. Virotherapy of neuroendocrine tumors. Neuroendocrinology 2013, 97, 26–34. [Google Scholar] [CrossRef]
- Popa Ilie, I.R.; Georgescu, C.E. Immunotherapy in Gastroenteropancreatic Neuroendocrine Neoplasia. Neuroendocrinology 2023, 113, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Burroughs, K.D.; Hales, L.M.; Ganesh, S.; Jones, B.H.; Idamakanti, N.; Hay, C.; Li, S.S.; Skele, K.L.; Vasko, A.J.; et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J. Natl. Cancer Inst. 2007, 99, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Poirier, J.T.; Senzer, N.N.; Stephenson, J., Jr.; Loesch, D.; Burroughs, K.D.; Reddy, P.S.; Hann, C.L.; Hallenbeck, P.L. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 888–895. [Google Scholar] [CrossRef]
- Burke, M.J.; Ahern, C.; Weigel, B.J.; Poirier, J.T.; Rudin, C.M.; Chen, Y.; Cripe, T.P.; Bernhardt, M.B.; Blaney, S.M. Phase I trial of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors: A report of the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J. Oncolytic Seneca Valley Virus: Past perspectives and future directions. Oncolytic Virotherapy 2016, 5, 81–89. [Google Scholar] [CrossRef]
- Yu, D.; Leja-Jarblad, J.; Loskog, A.; Hellman, P.; Giandomenico, V.; Oberg, K.; Essand, M. Preclinical Evaluation of AdVince, an Oncolytic Adenovirus Adapted for Treatment of Liver Metastases from Neuroendocrine Cancer. Neuroendocrinology 2017, 105, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Leja, J.; Nilsson, B.; Yu, D.; Gustafson, E.; Akerstrom, G.; Oberg, K.; Giandomenico, V.; Essand, M. Double-detargeted oncolytic adenovirus shows replication arrest in liver cells and retains neuroendocrine cell killing ability. PLoS ONE 2010, 5, e8916. [Google Scholar] [CrossRef]
- Kloker, L.D.; Berchtold, S.; Smirnow, I.; Schaller, M.; Fehrenbacher, B.; Krieg, A.; Sipos, B.; Lauer, U.M. The Oncolytic Herpes Simplex Virus Talimogene Laherparepvec Shows Promising Efficacy in Neuroendocrine Cancer Cell Lines. Neuroendocrinology 2019, 109, 346–361. [Google Scholar] [CrossRef]
- Kloker, L.D.; Berchtold, S.; Smirnow, I.; Beil, J.; Krieg, A.; Sipos, B.; Lauer, U.M. Oncolytic vaccinia virus GLV-1h68 exhibits profound antitumoral activities in cell lines originating from neuroendocrine neoplasms. BMC Cancer 2020, 20, 628. [Google Scholar] [CrossRef]
- Aref, S.; Bailey, K.; Fielding, A. Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses 2016, 8, 294. [Google Scholar] [CrossRef]
- Russell, S.J.; Federspiel, M.J.; Peng, K.W.; Tong, C.; Dingli, D.; Morice, W.G.; Lowe, V.; O’Connor, M.K.; Kyle, R.A.; Leung, N.; et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin. Proc. 2014, 89, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Berchtold, S.; Lampe, J.; Malek, N.P.; Bitzer, M.; Lauer, U.M. Primary resistance phenomena to oncolytic measles vaccine viruses. Int. J. Oncol. 2013, 43, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.; Salih, H.R.; Smirnow, I.; Lauer, U.M.; Berchtold, S. Suicide genearmed measles vaccine virus for the treatment of AML. Int. J. Oncol. 2019, 55, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, S.; Lampe, J.; Weiland, T.; Smirnow, I.; Schleicher, S.; Handgretinger, R.; Kopp, H.G.; Reiser, J.; Stubenrauch, F.; Mayer, N.; et al. Innate immune defense defines susceptibility of sarcoma cells to measles vaccine virus-based oncolysis. J. Virol. 2013, 87, 3484–3501. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Battey, J.; Gazdar, A.F.; Oie, H.; Draoui, M.; Moody, T.W. Neuromedin B is present in lung cancer cell lines. Cancer Res. 1992, 52, 2732s–2736s. [Google Scholar] [PubMed]
- Linnebacher, M.; Maletzki, C.; Ostwald, C.; Klier, U.; Krohn, M.; Klar, E.; Prall, F. Cryopreservation of human colorectal carcinomas prior to xenografting. BMC Cancer 2010, 10, 362. [Google Scholar] [CrossRef]
- Takahashi, T.; Nau, M.M.; Chiba, I.; Birrer, M.J.; Rosenberg, R.K.; Vinocour, M.; Levitt, M.; Pass, H.; Gazdar, A.F.; Minna, J.D. p53: A frequent target for genetic abnormalities in lung cancer. Science 1989, 246, 491–494. [Google Scholar] [CrossRef]
- Evers, B.M.; Ishizuka, J.; Townsend, C.M., Jr.; Thompson, J.C. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann. N. Y. Acad. Sci. 1994, 733, 393–406. [Google Scholar] [CrossRef]
- Kaku, M.; Nishiyama, T.; Yagawa, K.; Abe, M. Establishment of a carcinoembryonic antigen-producing cell line from human pancreatic carcinoma. Gan 1980, 71, 596–601. [Google Scholar]
- Scheubeck, G.; Berchtold, S.; Smirnow, I.; Schenk, A.; Beil, J.; Lauer, U.M. Starvation-Induced Differential Virotherapy Using an Oncolytic Measles Vaccine Virus. Viruses 2019, 11, 614. [Google Scholar] [CrossRef]
- Lampe, J.; Bossow, S.; Weiland, T.; Smirnow, I.; Lehmann, R.; Neubert, W.; Bitzer, M.; Lauer, U.M. An armed oncolytic measles vaccine virus eliminates human hepatoma cells independently of apoptosis. Gene Ther. 2013, 20, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Graepler, F.; Lemken, M.L.; Wybranietz, W.A.; Schmidt, U.; Smirnow, I.; Gross, C.D.; Spiegel, M.; Schenk, A.; Graf, H.; Lauer, U.A.; et al. Bifunctional chimeric SuperCD suicide gene -YCD: YUPRT fusion is highly effective in a rat hepatoma model. World J. Gastroenterol. 2005, 11, 6910–6919. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch. Für Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Spearman, C. The method of right and wrong cases (constant stimuli) without Gauss’s formulae. Br. J. Psychol. 1908, 2, 227. [Google Scholar] [CrossRef]
- Anderson, B.D.; Nakamura, T.; Russell, S.J.; Peng, K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004, 64, 4919–4926. [Google Scholar] [CrossRef]
- Pusceddu, S.; De Braud, F.; Lo Russo, G.; Concas, L.; Femia, D.; Vernieri, C.; Indini, A.; Formisano, B.; Buzzoni, R. How do the results of the RADIANT trials impact on the management of NET patients? A systematic review of published studies. Oncotarget 2016, 7, 44841–44847. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Munk Jensen, M.; Haagen Nielsen, C.; Fisker Hag, A.M.; Knigge, U.; Kjaer, A. 18F-FDG and 18F-FLT-PET imaging for monitoring everolimus effect on tumor-growth in neuroendocrine tumors: Studies in human tumor xenografts in mice. PLoS ONE 2014, 9, e91387. [Google Scholar] [CrossRef]
- Lun, X.Q.; Jang, J.H.; Tang, N.; Deng, H.; Head, R.; Bell, J.C.; Stojdl, D.F.; Nutt, C.L.; Senger, D.L.; Forsyth, P.A.; et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2777–2788. [Google Scholar] [CrossRef]
- Lun, X.; Chan, J.; Zhou, H.; Sun, B.; Kelly, J.J.; Stechishin, O.O.; Bell, J.C.; Parato, K.; Hu, K.; Vaillant, D.; et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 1927–1936. [Google Scholar] [CrossRef]
- Lun, X.; Alain, T.; Zemp, F.J.; Zhou, H.; Rahman, M.M.; Hamilton, M.G.; McFadden, G.; Bell, J.; Senger, D.L.; Forsyth, P.A. Myxoma virus virotherapy for glioma in immunocompetent animal models: Optimizing administration routes and synergy with rapamycin. Cancer Res. 2010, 70, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.M.; Dijkstra, K.K.; Fanchi, L.F.; Kelderman, S.; Kaing, S.; van Rooij, N.; van den Brink, S.; Schumacher, T.N.; Voest, E.E. Tumor organoid-T-cell coculture systems. Nat. Protoc. 2020, 15, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, S.; Beil, J.; Raff, C.; Smirnow, I.; Schell, M.; D’Alvise, J.; Gross, S.; Lauer, U.M. Assessing and Overcoming Resistance Phenomena against a Genetically Modified Vaccinia Virus in Selected Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 7618. [Google Scholar] [CrossRef] [PubMed]
- Kloker, L.D.; Yurttas, C.; Lauer, U.M. Three-dimensional tumor cell cultures employed in virotherapy research. Oncolytic Virotherapy 2018, 7, 79–93. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheicher, N.V.; Berchtold, S.; Beil, J.; Smirnow, I.; Schenk, A.; Lauer, U.M. In Vitro Sensitivity of Neuroendocrine Neoplasms to an Armed Oncolytic Measles Vaccine Virus. Cancers 2024, 16, 488. https://doi.org/10.3390/cancers16030488
Scheicher NV, Berchtold S, Beil J, Smirnow I, Schenk A, Lauer UM. In Vitro Sensitivity of Neuroendocrine Neoplasms to an Armed Oncolytic Measles Vaccine Virus. Cancers. 2024; 16(3):488. https://doi.org/10.3390/cancers16030488
Chicago/Turabian StyleScheicher, Nikolai V., Susanne Berchtold, Julia Beil, Irina Smirnow, Andrea Schenk, and Ulrich M. Lauer. 2024. "In Vitro Sensitivity of Neuroendocrine Neoplasms to an Armed Oncolytic Measles Vaccine Virus" Cancers 16, no. 3: 488. https://doi.org/10.3390/cancers16030488
APA StyleScheicher, N. V., Berchtold, S., Beil, J., Smirnow, I., Schenk, A., & Lauer, U. M. (2024). In Vitro Sensitivity of Neuroendocrine Neoplasms to an Armed Oncolytic Measles Vaccine Virus. Cancers, 16(3), 488. https://doi.org/10.3390/cancers16030488





