The Short- and Long-Term Anticipation of Prostate Cancer Incidence in Korea: Based on Social Aging Trends and Prostate-Specific Antigen Testing Rate during the Last Decade
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data sources for Nationwide PCa Incidence and PSA Incidence
2.2. Statistics Estimating the PCa Population in the Future
3. Results
3.1. Current Trend of PCa and PSA Incidence in Each Age Subgroup
3.2. Anticipated Future Trend of PCa in Each Age Subgroup
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H. Urologic cancer in Japan: Role of Japan at the frontier of issues in Asia. Jpn. J. Clin. Oncol. 2016, 46, 23–30. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Center. Annual Report of Cancer Statistics in Korea in 2015; Report No.: 117044; National Cancer Center: Goyang, Republic of Korea, 2017; 221p.
- Zhou, C.K.; Check, D.P.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Ferlay, J.; Bray, F.; Cook, M.B.; Devesa, S.S. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int. J. Cancer 2016, 138, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Kim, B.H. Should Contemporary Western Guidelines Based on Studies Conducted in the 2000s Be Adopted for the Prostate-Specific Antigen Screening Policy for Asian Men in the 2020s? World J. Mens. Health 2022, 40, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Catalon, W.J.; Partin, A.W.; Slawin, K.M.; Brawer, M.K.; Flanigan, R.C.; Patel, A.; Richie, J.P.; DeKernion, J.B.; Walsh, P.C.; Scardino, P.T.; et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: A prospective multicenter clinical trial. JAMA 1998, 279, 1542–1547. [Google Scholar] [CrossRef]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of prostatespecific antigen in serum as a screening test for prostate cancer. N. Engl. J. Med. 1991, 324, 1156–1161. [Google Scholar] [CrossRef]
- Arsov, C.; Albers, P.; Herkommer, K.; Gschwend, J.; Imkamp, F.; Peters, I.; Kuczyk, M.; Hadaschik, B.; Kristiansen, G.; Schimmoeller, L.; et al. A randomized trial of risk-adapted screening for prostate cancer in young men-Results of the first screening round of the PROBASE trial. Int. J. Cancer 2022, 150, 1861–1869. [Google Scholar] [CrossRef]
- Boschheidgen, M.; Albers, P.; Schlemmer, H.P.; Hellms, S.; Bonekamp, D.; Sauter, A.; Hadaschik, B.; Krilaviciute, A.; Radtke, J.P.; Seibold, P.; et al. Multiparametric Magnetic Resonance Imaging in Prostate Cancer Screening at the Age of 45 Years: Results from the First Screening Round of the PROBASE Trial. Eur. Urol. 2024, 85, 105–111. [Google Scholar] [CrossRef]
- Ko, Y.H.; Roh, K.C.; Kim, B.H. The national-wide incidence of prostate-specific antigen testing trend for a decade in Korea by age group. Investig. Clin. Urol. 2022, 63, 184–191. [Google Scholar] [CrossRef]
- National Cancer Center. Updated Report of Cancer Statistics in Korea in 2021; National Cancer Center: Goyang, Republic of Korea, 2023. Available online: https://ncc.re.kr/cancerStatsView.ncc?bbsnum=658&searchKey=total&searchValue=&pageNum=1 (accessed on 5 January 2024).
- Korean Statistical Information System. National Statistical Office, Korea. Available online: http://kosis.nso.go.kr (accessed on 1 December 2023).
- National Cancer Institute. Cancer Stat Facts: Prostate Cancer [Internet]; National Cancer Institute: Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 11 May 2023).
- Soos, G.; Tsakiris, I.; Szanto, J.; Turzo, C.; Haas, P.G.; Dezso, B. The prevalence of prostate carcinoma and its precursor in Hungary: An autopsy study. Eur. Urol. 2005, 48, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Oki, R.; Sekine, Y.; Arai, S.; Miyazawa, Y.; Shibata, Y.; Suzuki, K.; Kurosawa, I. Screening for prostate cancer: History, evidence, controversies and future perspectives toward individualized screening. Int. J. Urol. 2019, 26, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Caire, A.A.; Robertson, C.N.; George, D.J.; Polascik, T.J.; Maloney, K.E.; Walther, P.J.; Stackhouse, D.A.; Lack, B.D.; Albala, D.M.; et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J. Urol. 2009, 182, 2242–2248. [Google Scholar] [CrossRef]
- Brassell, S.A.; Rice, K.R.; Parker, P.M.; Chen, Y.; Farrell, J.S.; Cullen, J.; McLeod, D.G. Prostate cancer in men 70 years old or older, indolent or aggressive: Clinicopathological analysis and outcomes. J. Urol. 2011, 185, 132–137. [Google Scholar] [CrossRef]
- Dahm, P.; Silverstein, A.D.; Weizer, A.Z.; Crisci, A.; Vieweg, J.; Paulson, D.F. When to diagnose and how to treat prostate cancer in the “not too fit” elderly. Crit. Rev. Oncol. Hematol. 2003, 48, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kang, M.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2022. Cancer Res. Treat. 2022, 54, 345–351. [Google Scholar] [CrossRef]
- Pak, S.; Jung, K.W.; Park, E.H.; Ko, Y.H.; Won, Y.J.; Joung, J.Y. Incidence and mortality projections for major cancers among Korean men until 2034, with a focus on prostate cancer. Investig. Clin. Urol. 2022, 63, 175–183. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Pyun, J.H.; Kang, S.H.; Kim, J.Y.; Shin, J.E.; Jeong, I.G.; Kim, J.W.; No, T.I.; Oh, J.J.; Yu, J.H.; Chung, H.S.; et al. Survey results on the perception of prostate-specific antigen and prostate cancer screening among the general public. Korean J. Urol. Oncol. 2020, 18, 40–46. [Google Scholar] [CrossRef]
- Berkowitz, Z.; Li, J.; Richards, T.B.; Marcus, P.M. Patterns of Prostate-Specific Antigen Test Use in the U.S., 2005–2015. Am. J. Prev. Med. 2017, 53, 909–913. [Google Scholar] [CrossRef] [PubMed]
- U.S. Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2008, 149, 185–191. [Google Scholar]
- Seetharam Bhat, K.R.; Moschovas, M.C.; Onol, F.F.; Sandri, M.; Rogers, T.; Roof, S.; Rocco, B.; Patel, V.R. Trends in clinical and oncological outcomes of robot-assisted radical prostatectomy before and after the 2012 US Preventive Services Task Force recommendation against PSA screening: A decade of experience. BJU Int. 2020, 125, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Leapman, M.S.; Wang, R.; Park, H.; Yu, J.B.; Sprenkle, P.C.; Cooperberg, M.R.; Gross, C.P.; Ma, X. Changes in Prostate-Specific Antigen Testing Relative to the Revised US Preventive Services Task Force Recommendation on Prostate Cancer Screening. JAMA Oncol. 2022, 8, 41–47. [Google Scholar] [CrossRef]
- Alkhatib, K.; Labban, M.; Briggs, L.; Nguyen, D.D.; Herzog, P.; Cole, A.P.; Haag, A.; Trinh, Q.D. Does Veteran Status Mitigate Racial Disparities in Prostate Cancer Screening? Analysis of Prostate Specific Antigen Screening Patterns in the 2018 Behavioral Risk Factor Surveillance System Data. J. Urol. 2022, 207, 993–1000. [Google Scholar] [CrossRef]
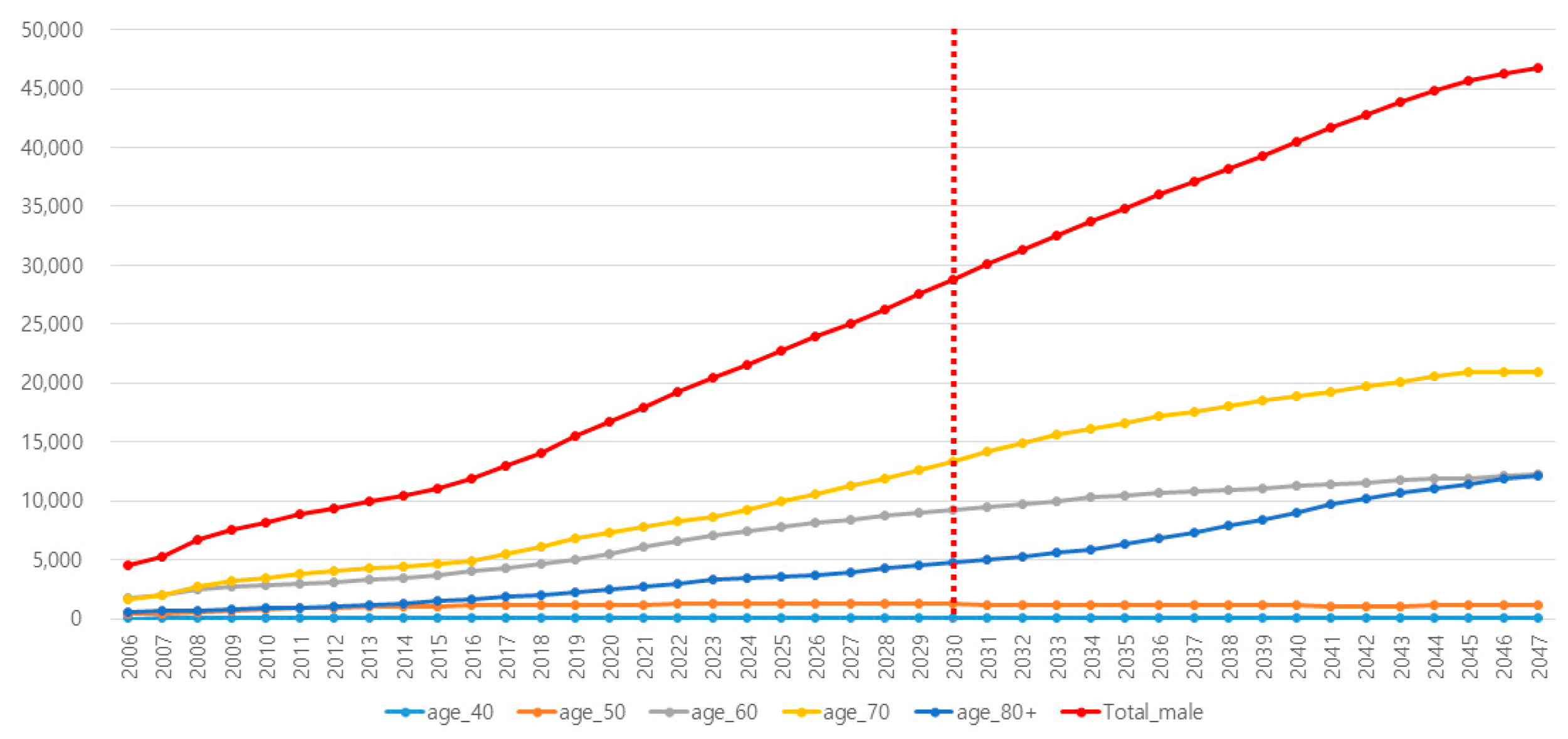
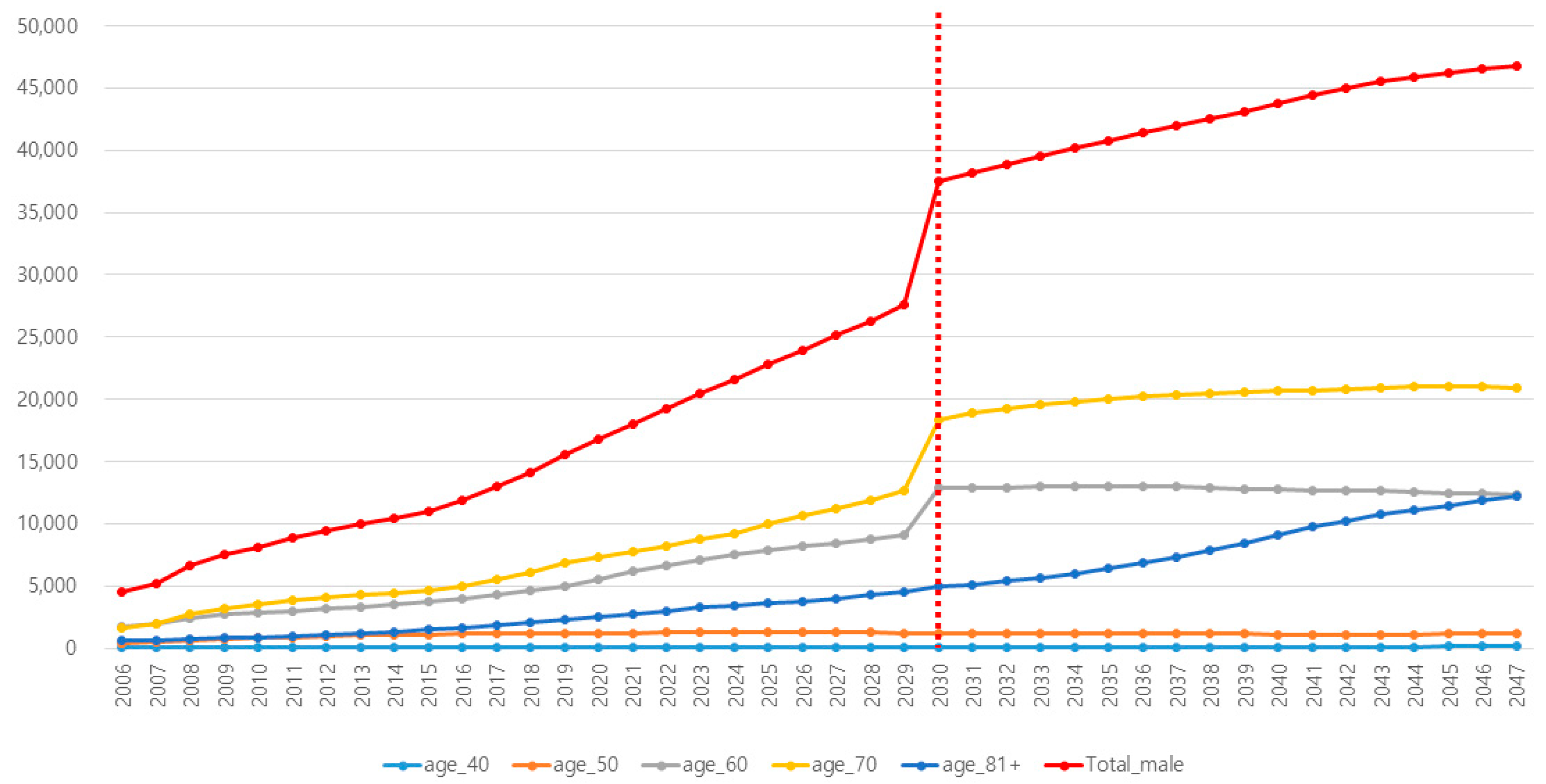
| Reflected Incidence of PCa in Korea | Reflected Incidence of PSA Testing in Korea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Total | 40s | 50s | 60s | 70s | Over 80s | Total | 40s | 50s | 60s | 70s | Over 80s |
| 2021 | 17,997 | 95 | 1235 | 6163 | 7767 | 2737 | 6.4 | 2.0 | 5.8 | 16.8 | 32.1 | 35.4 |
| 2022 | 19,227 | 97 | 1257 | 6606 | 8240 | 3026 | 7.1 | 2.1 | 5.9 | 18.4 | 34.1 | 38.2 |
| 2023 | 20,486 | 100 | 1262 | 7114 | 8713 | 3297 | 7.9 | 2.1 | 6.1 | 20.1 | 36.0 | 41.0 |
| 2024 | 21,606 | 102 | 1290 | 7496 | 9258 | 3461 | 8.7 | 2.2 | 6.2 | 21.7 | 38.0 | 43.8 |
| 2025 | 22,793 | 104 | 1290 | 7827 | 9937 | 3635 | 9.6 | 2.3 | 6.4 | 23.3 | 40.0 | 46.6 |
| 2026 | 23,921 | 106 | 1283 | 8167 | 10,632 | 3733 | 10.4 | 2.4 | 6.6 | 25.0 | 41.9 | 49.4 |
| 2027 | 25,108 | 109 | 1276 | 8462 | 11,256 | 4005 | 11.4 | 2.5 | 6.7 | 26.6 | 43.9 | 52.3 |
| 2028 | 26,310 | 111 | 1268 | 8727 | 11,925 | 4279 | 12.3 | 2.5 | 6.9 | 28.3 | 45.9 | 55.1 |
| 2029 | 27,563 | 113 | 1243 | 9050 | 12,625 | 4531 | 13.4 | 2.6 | 7.0 | 29.9 | 47.8 | 57.9 |
| 2030 | 28,822 | 115 | 1241 | 9283 | 13,383 | 4799 | 14.5 | 2.7 | 7.2 | 31.5 | 49.8 | 60.7 |
| 2031 | 30,076 | 118 | 1234 | 9507 | 14,237 | 4979 | 15.7 | 2.8 | 7.4 | 33.2 | 51.7 | 63.5 |
| 2032 | 31,317 | 120 | 1233 | 9792 | 14,876 | 5297 | 16.8 | 2.9 | 7.5 | 34.8 | 53.7 | 66.3 |
| 2033 | 32,543 | 122 | 1222 | 10,007 | 15,592 | 5600 | 18.0 | 2.9 | 7.7 | 36.5 | 55.7 | 69.1 |
| 2034 | 33,692 | 124 | 1202 | 10,309 | 16,143 | 5915 | 19.2 | 3.0 | 7.8 | 38.1 | 57.6 | 71.9 |
| 2035 | 34,871 | 125 | 1185 | 10,499 | 16,647 | 6415 | 20.5 | 3.1 | 8.0 | 39.7 | 59.6 | 74.7 |
| 2036 | 35,991 | 127 | 1177 | 10,659 | 17,157 | 6871 | 21.7 | 3.2 | 8.2 | 41.4 | 61.5 | 77.5 |
| 2037 | 37,090 | 129 | 1167 | 10,823 | 17,609 | 7362 | 23.0 | 3.3 | 8.3 | 43.0 | 63.5 | 80.0 |
| 2038 | 38,179 | 131 | 1155 | 10,978 | 18,029 | 7887 | 24.1 | 3.3 | 8.5 | 44.7 | 65.5 | 80.0 |
| 2039 | 39,300 | 133 | 1145 | 11,066 | 18,528 | 8429 | 25.2 | 3.4 | 8.6 | 46.3 | 67.4 | 80.0 |
| 2040 | 40,478 | 135 | 1125 | 11,250 | 18,917 | 9051 | 26.4 | 3.5 | 8.8 | 47.9 | 69.4 | 80.0 |
| 2041 | 41,679 | 137 | 1104 | 11,412 | 19,303 | 9723 | 27.6 | 3.6 | 9.0 | 49.6 | 71.4 | 80.0 |
| 2042 | 42,781 | 140 | 1096 | 11,597 | 19,754 | 10,195 | 28.8 | 3.7 | 9.1 | 51.2 | 73.3 | 80.0 |
| 2043 | 43,864 | 143 | 1103 | 11,744 | 20,122 | 10,753 | 30.0 | 3.7 | 9.3 | 52.9 | 75.3 | 80.0 |
| 2044 | 44,781 | 145 | 1120 | 11,851 | 20,587 | 11,078 | 31.1 | 3.8 | 9.4 | 54.5 | 77.2 | 80.0 |
| 2045 | 45,654 | 148 | 1146 | 11,967 | 20,910 | 11,483 | 32.1 | 3.9 | 9.6 | 56.1 | 79.2 | 80.0 |
| 2046 | 46,294 | 151 | 1167 | 12,119 | 21,002 | 11,854 | 32.9 | 4.0 | 9.8 | 57.8 | 80.0 | 80.0 |
| 2047 | 46,737 | 154 | 1185 | 12,265 | 20,963 | 12,171 | 33.5 | 4.1 | 9.9 | 59.4 | 80.0 | 80.0 |
| Source | Publication Date | Data Source | Estimated Incidence in 2022 | Estimated Incidence in 2030 | Estimated Incidence in 2034 | Estimated Incidence in 2040 | Statistical Method | Variables Reflected |
|---|---|---|---|---|---|---|---|---|
| Jung et al. [21]. | 2022 | National Cancer Incidence database (1999–2019) | 22,391 | - | - | - | Linear regression model | Age, period |
| Pak et al. [22]. | 2022 | National Cancer Incidence database (1999–2016) | About 17,000 | About 25,000 | 29,339 | - | Age–period–cohort method | Age, period, cohort |
| International Agency for Research on Cancer (IARC) [23] | Last updated in December 2020 | Global Cancer Observatory database (from National Cancer Registry, 2018) | 13,873 (In 2020) | 20,900 | 24,241 (In 2035) | 26,828 | Multiplying age-specific incidence | Age |
| Present study | 2023 | Korean Statistical Information Service Database (2006–2020) | 19,227 | 28,822 | 33,692 | 40,478 | Linear regression model | Age, period, PSA testing incidence (2006–2016) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyun, J.H.; Ko, Y.H.; Kim, S.W.; Son, N.-H. The Short- and Long-Term Anticipation of Prostate Cancer Incidence in Korea: Based on Social Aging Trends and Prostate-Specific Antigen Testing Rate during the Last Decade. Cancers 2024, 16, 503. https://doi.org/10.3390/cancers16030503
Pyun JH, Ko YH, Kim SW, Son N-H. The Short- and Long-Term Anticipation of Prostate Cancer Incidence in Korea: Based on Social Aging Trends and Prostate-Specific Antigen Testing Rate during the Last Decade. Cancers. 2024; 16(3):503. https://doi.org/10.3390/cancers16030503
Chicago/Turabian StylePyun, Jong Hyun, Young Hwii Ko, Sang Won Kim, and Nak-Hoon Son. 2024. "The Short- and Long-Term Anticipation of Prostate Cancer Incidence in Korea: Based on Social Aging Trends and Prostate-Specific Antigen Testing Rate during the Last Decade" Cancers 16, no. 3: 503. https://doi.org/10.3390/cancers16030503





