Changes in Health-Related Quality of Life following Surgery in Patients with High-Grade Extremity Soft-Tissue Sarcoma: A Prospective Longitudinal Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients and Data Collection
2.2. Health-Related Quality of Life
2.3. Statistical Analyses
3. Results
3.1. Patients Characteristics
3.2. Overall HRQoL Mean Scores over Time
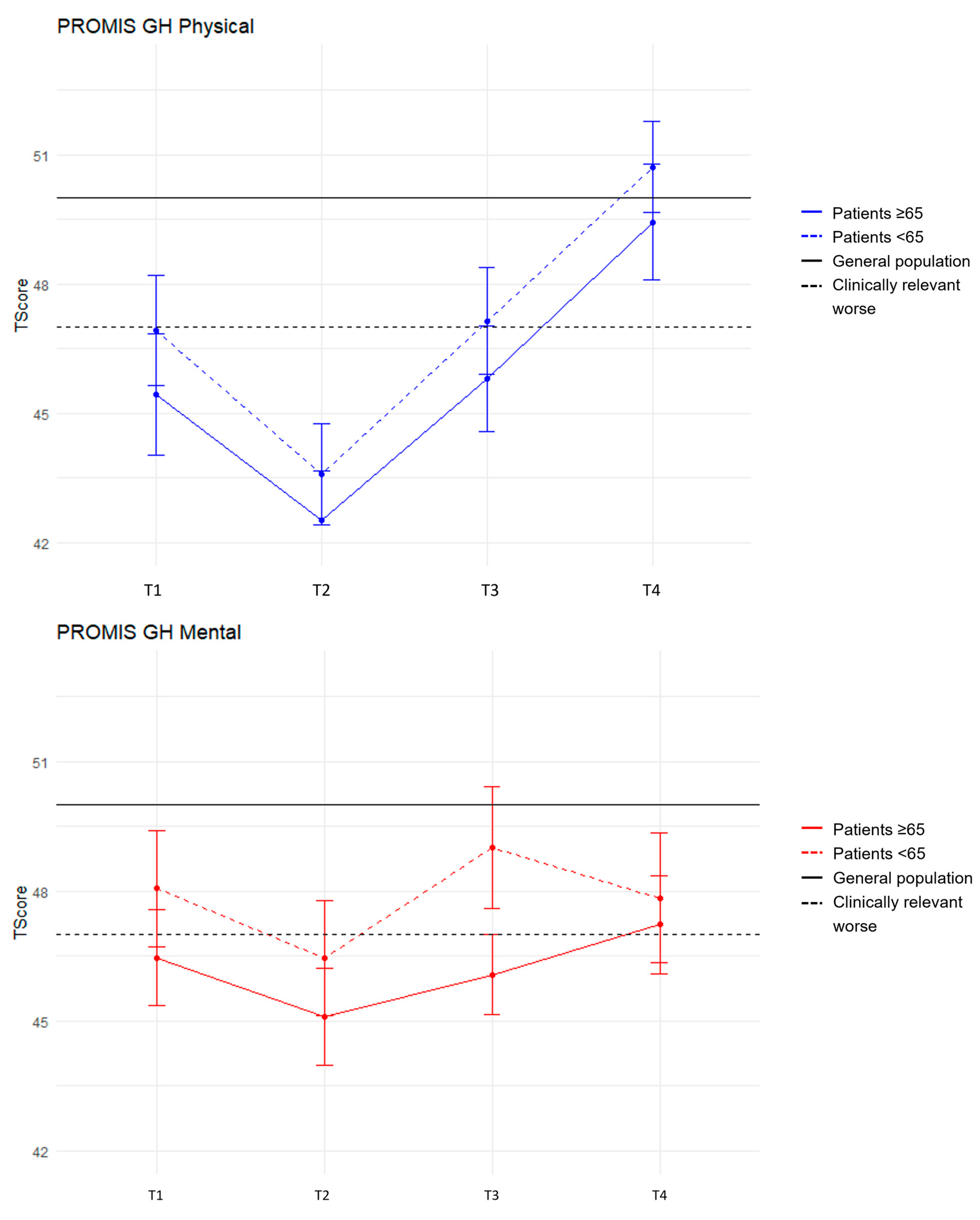
3.3. HRQoL Mean Scores over Time Stratified by Age
4. Discussion
4.1. Results in Context
4.2. Strength and Limitations
4.3. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picci, P. Epidemiology of Soft Tissue Lesions. In Diagnosis of Musculoskeletal Tumors and Tumor-like Conditions: Clinical, Radiological and Histological Correlations—The Rizzoli Case Archive; Picci, P., Manfrini, M., Donati, D.M., Gambarotti, M., Righi, A., Vanel, D., Dei Tos, A.P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 15–18. [Google Scholar]
- Fletcher, C.D.M. WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC Press: Lyon, France, 2013. [Google Scholar]
- Trautmann, F.; Schuler, M.; Schmitt, J. Burden of soft-tissue and bone sarcoma in routine care Estimation of incidence, prevalence and survival for health services research. Cancer Epidemiol. 2015, 39, 440–446. [Google Scholar] [CrossRef]
- Ho, V.; de Heus, E.; de Peuter, R. Sarcomenzorg in Nederland. In Overzicht van de Nederlandse Kankerregistratie over de Periode 2009–2018; IKNL: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Meropol, N.J.; Egleston, B.L.; Buzaglo, J.S.; Benson, A.B.; Cegala, D.J.; Diefenbach, M.A.; Fleisher, L.; Miller, S.M.; Sulmasy, D.R.; Weinfurt, K.P.; et al. Cancer Patient Preferences for Quality and Length of Life. Cancer 2008, 113, 3459–3466. [Google Scholar] [CrossRef]
- Higginson, I.J.; Gomes, B.; Calanzani, N.; Gao, W.; Bausewein, C.; Daveson, B.A.; Deliens, L.; Ferreira, P.L.; Toscani, F.; Gysels, M.; et al. Priorities for treatment, care and information if faced with serious illness: A comparative population-based survey in seven European countries. Palliative Med. 2014, 28, 101–110. [Google Scholar] [CrossRef]
- Kluetz, P.G.; Papadopoulos, E.J.; Johnson, L.L.; Donoghue, M.; Kwitkowski, V.E.; Chen, W.H.; Sridhara, R.; Farrell, A.T.; Keegan, P.; Kim, G.; et al. Focusing on Core Patient-Reported Outcomes in Cancer Clinical Trials-Response. Clin. Cancer Res. 2016, 22, 5618. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; Jakob, J.; Singer, S.; Grutzmann, R.; et al. The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany-Cross-Sectional Results of a Nationwide Observational Study (PROSa). Cancers 2020, 12, 3590. [Google Scholar] [CrossRef]
- Drabbe, C.; Van der Graaf, W.T.A.; De Rooij, B.H.; Grunhagen, D.J.; Soomers, V.L.M.N.; Van de Sande, M.A.J.; Been, L.B.; Keymeulen, K.B.M.I.; van der Geest, I.C.M.; Van Houdt, W.J.; et al. The age-related impact of surviving sarcoma on health-related quality of life: Data from the SURVSARC study. Esmo Open 2021, 6, 100047. [Google Scholar] [CrossRef]
- van Eck, I.; den Hollander, D.; Desar, I.M.E.; Soomers, V.L.M.N.; van de Sande, M.A.J.; de Haan, J.J.; Verhoef, C.; Vriens, I.J.H.; Bonenkamp, J.J.; van der Graaf, W.T.A.; et al. Unraveling the Heterogeneity of Sarcoma Survivors’ Health-Related Quality of Life Regarding Primary Sarcoma Location: Results from the SURVSARC Study. Cancers 2020, 12, 3083. [Google Scholar] [CrossRef] [PubMed]
- Paredes, T.; Pereira, M.; Moreira, H.; Simoes, M.R.; Canavarro, M.C. Quality of life of sarcoma patients from diagnosis to treatments: Predictors and longitudinal trajectories. Eur. J. Oncol. Nurs. 2011, 15, 492–499. [Google Scholar] [CrossRef]
- Eichler, M.; Hentschel, L.; Singer, S.; Hornemann, B.; Richter, S.; Hofbauer, C.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; et al. Health related Quality of Life over time in German sarcoma patients. An analysis of associated factors—Results of the PROSa study. Front. Endocrinol. 2023, 14, 1166838. [Google Scholar] [CrossRef] [PubMed]
- Younger, E.; Husson, O.; Bennister, L.; Whelan, J.; Wilson, R.; Roast, A.; Jones, R.L.; van der Graaf, W.T.A. Age-related sarcoma patient experience: Results from a national survey in England. BMC Cancer 2018, 18, 991. [Google Scholar] [CrossRef]
- Zebrack, B.J. Psychological, Social, and Behavioral Issues for Young Adults with Cancer. Cancer 2011, 117, 2289–2294. [Google Scholar] [CrossRef]
- Younger, E.; Litière, S.; Le Cesne, A.; Mir, O.; Gelderblom, H.; Italiano, A.; Marreaud, S.; Jones, R.L.; Gronchi, A.; van der Graaf, W.T.A. Outcomes of Elderly Patients with Advanced Soft Tissue Sarcoma Treated with First-Line Chemotherapy: A Pooled Analysis of 12 EORTC Soft Tissue and Bone Sarcoma Group Trials. Oncologist 2018, 23, 1250–1259. [Google Scholar] [CrossRef]
- Kruiswijk, A.A.; van de Sande, M.A.J.; Haas, R.L.; van den Akker-van Marle, E.M.; Engelhardt, E.G.; Marang-van de Mheen, P.; van Bodegom-Vos, L.; VALUE-PERSARC Research Group. (Cost-)effectiveness of an individualised risk prediction tool (PERSARC) on patient’s knowledge and decisional conflict among soft-tissue sarcomas patients: Protocol for a parallel cluster randomised trial (the VALUE-PERSARC study). BMJ Open 2023, 13, e074853. [Google Scholar] [CrossRef]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; Demascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-Tissue Sarcomas of Adults—Study of Pathological Prognostic Variables and Definition of a Histopathological Grading System. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Terwee, C.B.; Roorda, L.D.; de Vet, H.C.W.; Dekker, J.; Westhovens, R.; van Leeuwen, J.; Cella, D.; Correia, H.; Arnold, B.; Perez, B.; et al. Dutch-Flemish translation of 17 item banks from the Patient-Reported Outcomes Measurement Information System (PROMIS). Qual. Life Res. 2014, 23, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Bjorner, J.B.; Revicki, D.A.; Spritzer, K.L.; Cella, D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual. Life Res. 2009, 18, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Hanmer, J.; Jensen, R.E.; Rothrock, N.; Team, H. A reporting checklist for HealthMeasures’ patient-reported outcomes: ASCQ-Me, Neuro-QoL, NIH Toolbox, and PROMIS. J. Patient-Rep. Outcom. 2020, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Garin, O.; Dima, A.L.; Pont, A.; Martí Pastor, M.; Alonso, J.; Van Ganse, E.; Laforest, L.; de Bruin, M.; Mayoral, K.; et al. EuroQol (EQ-5D-5L) Validity in Assessing the Quality of Life in Adults with Asthma: Cross-Sectional Study. J. Med. Internet Res. 2019, 21, e10178. [Google Scholar] [CrossRef] [PubMed]
- Versteegh, M.M.; Vermeulen, K.M.; Evers, S.M.A.A.; de Wit, G.A.; Prenger, R.; Stolk, E.A. Dutch Tariff for the Five-Level Version of EQ-5D. Value Health 2016, 19, 343–352. [Google Scholar] [CrossRef]
- Szende, A.; Janssen, B.; Cabases, J. Self-Reported Population Health: An International Perspective Based on EQ-5D; Dordrecht, N.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Terwee, C.B. Scoringsmanual Dutch-Flemish PROMIS Instrumenten. 2022. Available online: http://www.dutchflemishpromis.nl/ (accessed on 16 August 2022).
- Yost, K.J.; Eton, D.T.; Garcia, S.F.; Cella, D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J. Clin. Epidemiol. 2011, 64, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.E.; Potosky, A.L.; Moinpour, C.M.; Lobo, T.; Cella, D.; Hahn, E.A.; Thissen, D.; Smith, A.W.; Ahn, J.; Luta, G.; et al. United States Population-Based Estimates of Patient-Reported Outcomes Measurement Information System Symptom and Functional Status Reference Values for Individuals with Cancer. J. Clin. Oncol. 2017, 35, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.S.; Neary, M.P.; Cella, D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual. Life Outcomes 2007, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2009. Available online: http://www.R-project.org (accessed on 10 October 2023).
- Kruiswijk, A.A.; Dorleijn, D.M.J.; Marang-van de Mheen, P.J.; van de Sande, M.A.J.; van Bodegom-Vos, L. Health-Related Quality of Life of Bone and Soft-Tissue Tumor Patients around the Time of Diagnosis. Cancers 2023, 15, 2804. [Google Scholar] [CrossRef]
- Naughton, M.J.; Weaver, K.E. Physical and mental health among cancer survivors: Considerations for long-term care and quality of life. N. C. Med. J. 2014, 75, 283–286. [Google Scholar] [CrossRef]
- Stanton, A.L. What happens now? Psychosocial care for cancer survivors after medical treatment completion. J. Clin. Oncol. 2012, 30, 1215–1220. [Google Scholar] [CrossRef]
- Adler, N.E.; Page, A.E.K. (Eds.) Institute of Medicine Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. In The National Academies Collection: Reports Funded by National Institutes of Health, in Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs; National Academies Press (US), National Academy of Sciences: Washington, DC, USA, 2008. [Google Scholar]


| Characteristics | N = 97 |
|---|---|
| Median age y | |
| IQR | 64 (50–72) |
| Sex | |
| Female | 49 (50%) |
| Male | 48 (50%) |
| Histological subtype | |
| Myxofibrosarcoma | 26 (27%) |
| (myxoid) liposarcoma | 22 (23%) |
| MFH/UPS and NOS | 21 (22%) |
| Dedifferentiated/pleomorphic liposarcoma | 6 (6%) |
| Others | 6 (6%) |
| Leiomyosarcoma | 6 (6%) |
| MPNST | 4 (4%) |
| Synovial sarcoma | 3 (3%) |
| Spindle cell sarcoma | 3 (3%) |
| Tumor size cm | |
| IQR | 9 (5–12) |
| Tumor depth | |
| Superficial | 32 (33%) |
| Deep | 65 (67%) |
| Grade | |
| 2 | 55 (57%) |
| 3 | 42 (43%) |
| Location | |
| Upper extremity | 19 (20%) |
| Lower extremity | 78 (80%) |
| ASA score | |
| 0 | 80 (83%) |
| 1 | 13 (13%) |
| ≥2 | 4 (4%) |
| Surgical margin | |
| R0 | 55 (57%) |
| R1 | 26 (26%) |
| R2 | - |
| Amputation | 1 (1%) |
| No surgery (metastasis/dead) | 7 (7%) |
| Unknown * | 8 (9%) |
| Radiotherapy | |
| Pre- | 85 (88%) |
| Post- | 2 (2%) |
| noRT | 7 (7%) |
| No radiotherapy (metastasis/dead) | 3 (3%) |
| Surgical complications | |
| Wound infection | 10 (10%) |
| Nerve impairment | 2 (2%) |
| Other | 1 (1%) |
| Disease recurrence | |
| LR | - |
| DM | 11 (11%) |
| T1 (Baseline) n = 97 | T2 (3 Months) n = 81 | T3 (6 Months) n = 66 | T4 (12 Months) n = 39 | |
|---|---|---|---|---|
| PROMIS PF | 46.8 + 1.1 | 41.2 + 1.1 | 45.5 + 0.9 | 49.9 + 0.9 |
PROMIS GH
| 47.3 + 0.9 46.2 + 1.0 | 45.8 + 0.9 43.1 + 0.8 | 47.6 + 0.9 46.5 + 0.9 | 47.5 + 0.9 50.1 + 0.8 |
| EQ-5D-5L | 0.76 + 0.02 | 0.68 + 0.03 | 0.81 + 0.02 | 0.84 + 0.03 |
| EQ-VAS | 72.6 + 2.0 | 70.1 + 2.1 | 77.6 + 1.7 | 81.5 + 2.4 |
| T1 (Baseline) | T2 (3 Months) | T3 (6 Months) | T4 (12 Months) | |||||
|---|---|---|---|---|---|---|---|---|
| <65 Years n = 49 | ≥65 Years n = 48 | <65 Years n = 42 | ≥65 Years n = 39 | <65 Years n = 35 | ≥65 Years n = 31 | <65 Years n = 19 | ≥65 Years n = 20 | |
| PROMIS PF | 47.6 +1.6 | 45.9 +1.5 | 41.4 +1.6 | 40.9 +1.4 | 46.1 +1.2 | 44.8 +1.2 | 53.0 +1.1 | 47.4 +1.3 |
PROMIS GH
| 48.1 + 1.4 46.9 + 1.3 | 46.5 + 1.1 45.4 + 1.4 | 46.5 + 1.3 43.6 + 1.2 | 45.1 + 1.1 42.5 + 1.1 | 49.0 + 1.4 47.1 + 1.2 | 46.1 + 0.9 45.8 + 1.2 | 47.9 + 1.5 50.7 + 1.1 | 47.2 + 1.1 49.4 + 1.3 |
| EQ-5D-5L | 0.76 + 0.04 | 0.75 + 0.04 | 0.66 + 0.04 | 0.71 + 0.04 | 0.78 + 0.03 | 0.84 + 0.03 | 0.83 + 0.05 | 0.82 + 0.03 |
| VAS | 70.5 + 3.1 | 74.8. + 2.5 | 67.4 + 3.1 | 73.2 + 2.5 | 78.2 + 2.5 | 77.0 + 2.2 | 82.8 + 2.2 | 80.2 + 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruiswijk, A.A.; van de Sande, M.A.J.; Verhoef, C.; Schrage, Y.M.; Haas, R.L.; Bemelmans, M.H.A.; van Ginkel, R.J.; Bonenkamp, J.J.; Witkamp, A.J.; van den Akker-van Marle, M.E.; et al. Changes in Health-Related Quality of Life following Surgery in Patients with High-Grade Extremity Soft-Tissue Sarcoma: A Prospective Longitudinal Study. Cancers 2024, 16, 547. https://doi.org/10.3390/cancers16030547
Kruiswijk AA, van de Sande MAJ, Verhoef C, Schrage YM, Haas RL, Bemelmans MHA, van Ginkel RJ, Bonenkamp JJ, Witkamp AJ, van den Akker-van Marle ME, et al. Changes in Health-Related Quality of Life following Surgery in Patients with High-Grade Extremity Soft-Tissue Sarcoma: A Prospective Longitudinal Study. Cancers. 2024; 16(3):547. https://doi.org/10.3390/cancers16030547
Chicago/Turabian StyleKruiswijk, Anouk A., Michiel A. J. van de Sande, Cornelis Verhoef, Yvonne M. Schrage, Rick L. Haas, Marc H. A. Bemelmans, Robert J. van Ginkel, Johannes J. Bonenkamp, Arjen J. Witkamp, M. Elske van den Akker-van Marle, and et al. 2024. "Changes in Health-Related Quality of Life following Surgery in Patients with High-Grade Extremity Soft-Tissue Sarcoma: A Prospective Longitudinal Study" Cancers 16, no. 3: 547. https://doi.org/10.3390/cancers16030547
APA StyleKruiswijk, A. A., van de Sande, M. A. J., Verhoef, C., Schrage, Y. M., Haas, R. L., Bemelmans, M. H. A., van Ginkel, R. J., Bonenkamp, J. J., Witkamp, A. J., van den Akker-van Marle, M. E., Marang-van de Mheen, P. J., & van Bodegom-Vos, L. (2024). Changes in Health-Related Quality of Life following Surgery in Patients with High-Grade Extremity Soft-Tissue Sarcoma: A Prospective Longitudinal Study. Cancers, 16(3), 547. https://doi.org/10.3390/cancers16030547









