A Meta-Analysis and Review of Radiation Dose Escalation in Definitive Radiation Therapy between Squamous Cell Carcinoma and Adenocarcinoma of Esophageal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Selection
2.2. Endpoints of Interest
2.3. Calculation of the Overall Survival Rate
2.4. Limitations
3. Result
3.1. Study Characteristics
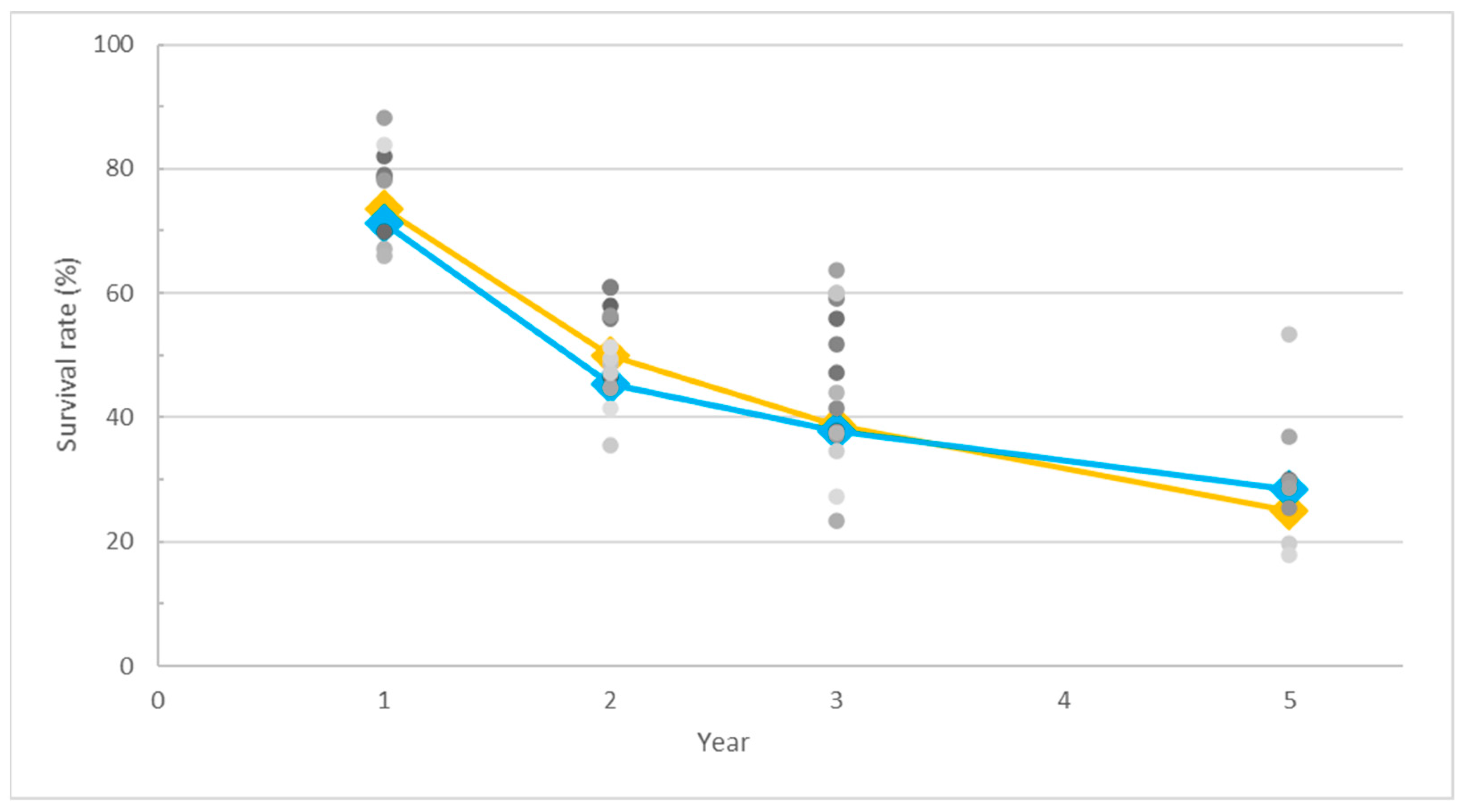
3.2. Survival Rate Trends in SCC and AC
3.3. Impact of Radiation Dose on Overall Survival in SCC and AC
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.Q.; Ma, Y.L.; Qin, Q.; Wang, P.H.; Luo, Y.; Xu, P.F.; Cui, Y. Epidemiology of Esophageal Cancer in 2020 and Projections to 2030 and 2040. Thorac. Cancer 2023, 14, 3. [Google Scholar] [CrossRef]
- Cooper, J.S.; Guo, M.D.; Herskovic, A.; Macdonald, J.S.; James, A.; Martenson, J.; Al-Sarraf, M.; Byhardt, R.; Russell, A.H.; Beitler, J.J.; et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer: Long-Term Follow-up of a Prospective Randomized Trial (RTOG 85-01). JAMA 1999, 281, 1623–1627. [Google Scholar] [CrossRef]
- al-Sarraf, M.; Martz, K.; Herskovic, A.; Leichman, L.; Brindle, J.S.; Vaitkevicius, V.K.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Progress Report of Combined Chemoradiotherapy versus Radiotherapy Alone in Patients with Esophageal Cancer: An Intergroup Study. J. Clin. Oncol. 1997, 15, 277–284. [Google Scholar] [CrossRef]
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal Cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal Carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef]
- Kong, F.-M.; Ten Haken, R.K.; Schipper, M.J.; Sullivan, M.A.; Chen, M.; Lopez, C.; Kalemkerian, G.P.; Hayman, J.A. High-Dose Radiation Improved Local Tumor Control and Overall Survival in Patients with Inoperable/Unresectable Non–Small-Cell Lung Cancer: Long-Term Results of a Radiation Dose Escalation Study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 324–333. [Google Scholar] [CrossRef]
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A.; Kelsen, D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, M.C.C.M.; Geijsen, E.D.; Rozema, T.; Oppedijk, V.; Buijsen, J.; Neelis, K.J.; Nuyttens, J.J.M.E.; van der Sangen, M.J.C.; Jeene, P.M.; Reinders, J.G.; et al. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients with Locally Advanced Esophageal Cancer (ARTDECO Study). J. Clin. Oncol. 2021, 39, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658. [Google Scholar] [CrossRef]
- Hu, K.; Wang, W.; Liu, X.; Meng, Q.; Zhang, F. Comparison of Treatment Outcomes between Squamous Cell Carcinoma and Adenocarcinoma of Cervix after Definitive Radiotherapy or Concurrent Chemoradiotherapy. Radiat. Oncol. 2018, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Tamaki, Y.; Kitamoto, Y.; Takahashi, T.; Ishikawa, H.; Nonaka, T.; Murata, K.; Satoh, Y.; Higuchi, K.; Nakano, T. Comparison of Chemoradiotherapy with Radiotherapy Alone Inpatients with Esophageal Adenocarcinoma. J. Radiat. Res. 2011, 52, 264–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, C.; Wang, X.; Wang, L.; Chen, J.; Zhang, W.; Pang, Q.; Zhao, Y.; Sun, X.; Zhang, K.; Li, G.; et al. Clinical Practice and Outcome of Radiotherapy for Advanced Esophageal Squamous Cell Carcinoma between 2002 and 2018 in China: The Multi-Center 3JECROG Survey. Acta Oncol. 2021, 60, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F.; Nicolay, N.H.; Nguyen, T.; Saleh-Ebrahimi, L.; Askoxylakis, V.; Bostel, T.; Zwicker, F.; Debus, J.; Timke, C.; Huber, P.E. Intensity Modulated Radiotherapy (IMRT) with Concurrent Chemotherapy as Definitive Treatment of Locally Advanced Esophageal Cancer. Radiat. Oncol. 2014, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yang, X.; Lu, X.; Wen, W.; Zhen, F.; Ye, H.; Zhu, H.; Cao, Y.; Zhang, S.; Cheng, H.; et al. Long-Term Clinical Outcome of Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Squamous Cell Carcinoma. Tumori 2015, 101, 168–173. [Google Scholar] [CrossRef]
- La, T.H.; Minn, A.Y.; Su, Z.; Fisher, G.A.; Ford, J.M.; Kunz, P.; Goodman, K.A.; Koong, A.C.; Chang, D.T. Multimodality Treatment with Intensity Modulated Radiation Therapy for Esophageal Cancer. Dis. Esophagus 2010, 23, 300–308. [Google Scholar] [CrossRef]
- Tu, L.; Sun, L.; Xu, Y.; Wang, Y.; Zhou, L.; Liu, Y.; Zhu, J.; Peng, F.; Wei, Y.; Gong, Y. Paclitaxel and Cisplatin Combined with Intensity-Modulated Radiotherapy for Upper Esophageal Carcinoma. Radiat. Oncol. 2013, 8, 75. [Google Scholar] [CrossRef]
- Gerber, N.; Ilson, D.H.; Wu, A.J.; Janjigian, Y.Y.; Kelsen, D.P.; Zheng, J.; Zhang, Z.; Bains, M.S.; Rizk, N.; Rusch, V.W.; et al. Outcomes of Induction Chemotherapy Followed by Chemoradiation Using Intensity-Modulated Radiation Therapy for Esophageal Adenocarcinoma. Dis. Esophagus 2014, 27, 235–241. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Liu, G.; Zheng, X.; Wang, Y.; Feng, W.; Lai, X.; Zhou, X.; Li, P.; Ma, H.; et al. The Efficacy and Safety of Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Esophageal Squamous Cell Carcinoma in Chinese Population: A Single Institution Experience. J. Cancer Res. Ther. 2016, 12, C82–C88. [Google Scholar] [CrossRef]
- Lin, S.H.; Komaki, R.; Liao, Z.; Wei, C.; Myles, B.; Guo, X.; Palmer, M.; Mohan, R.; Swisher, S.G.; Hofstetter, W.L.; et al. Proton Beam Therapy and Concurrent Chemotherapy for Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e345–e351. [Google Scholar] [CrossRef]
- Takada, A.; Nakamura, T.; Takayama, K.; Makita, C.; Suzuki, M.; Azami, Y.; Kato, T.; Tsukiyama, I.; Hareyama, M.; Kikuchi, Y.; et al. Preliminary Treatment Results of Proton Beam Therapy with Chemoradiotherapy for Stage I-III Esophageal Cancer. Cancer Med. 2016, 5, 506–515. [Google Scholar] [CrossRef]
- Freilich, J.; Hoffe, S.E.; Almhanna, K.; Dinwoodie, W.; Yue, B.; Fulp, W.; Meredith, K.L.; Shridhar, R. Comparative Outcomes for Three-Dimensional Conformal versus Intensity-Modulated Radiation Therapy for Esophageal Cancer. Dis. Esophagus 2015, 28, 352–357. [Google Scholar] [CrossRef]
- He, L.; Chapple, A.; Liao, Z.; Komaki, R.; Thall, P.F.; Lin, S.H. Bayesian Regression Analyses of Radiation Modality Effects on Pericardial and Pleural Effusion and Survival in Esophageal Cancer. Radiother. Oncol. 2016, 121, 70–74. [Google Scholar] [CrossRef]
- Yang, H.; Feng, C.; Cai, B.N.; Yang, J.; Liu, H.X.; Ma, L. Comparison of Three-Dimensional Conformal Radiation Therapy, Intensity-Modulated Radiation Therapy, and Volumetric-Modulated Arc Therapy in the Treatment of Cervical Esophageal Carcinoma. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Iwase, H.; Shimada, M.; Tsuzuki, T.; Hirashima, N.; Okeya, M.; Hibino, Y.; Ryuge, N.; Yokoi, M.; Kida, Y.; Kuno, T.; et al. Concurrent Chemoradiotherapy with a Novel Fluoropyrimidine, S-1, and Cisplatin for Locally Advanced Esophageal Cancer: Long-Term Results of a Phase II Trial. Oncol. 2013, 84, 342–349. [Google Scholar] [CrossRef]
- Kato, K.; Nakajima, T.E.; Ito, Y.; Katada, C.; Ishiyama, H.; Tokunaga, S.Y.; Tanaka, M.; Hironaka, S.; Hashimoto, T.; Ura, T.; et al. Phase II Study of Concurrent Chemoradiotherapy at the Dose of 50.4 Gy with Elective Nodal Irradiation for Stage II-III Esophageal Carcinoma. Jpn. J. Clin. Oncol. 2013, 43, 608–615. [Google Scholar] [CrossRef]
- Nishimura, Y.; Hiraoka, M.; Koike, R.; Nakamatsu, K.; Itasaka, S.; Kawamura, M.; Negoro, Y.; Araki, N.; Ishikawa, H.; Fujii, T.; et al. Long-Term Follow-up of a Randomized Phase II Study of Cisplatin/5-FU Concurrent Chemoradiotherapy for Esophageal Cancer (KROSG0101/JROSG021). Jpn. J. Clin. Oncol. 2012, 42, 807–812. [Google Scholar] [CrossRef][Green Version]
- Kato, K.; Muro, K.; Minashi, K.; Ohtsu, A.; Ishikura, S.; Boku, N.; Takiuchi, H.; Komatsu, Y.; Miyata, Y.; Fukuda, H. Phase II Study of Chemoradiotherapy with 5-Fluorouracil and Cisplatin for Stage II-III Esophageal Squamous Cell Carcinoma: JCOG Trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011, 81, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Galais, M.P.; Raoul, J.L.; Bouché, O.; Gourgou-Bourgade, S.; Douillard, J.Y.; Etienne, P.L.; Boige, V.; Martel-Lafay, I.; Michel, P.; et al. Definitive Chemoradiotherapy with FOLFOX versus Fluorouracil and Cisplatin in Patients with Oesophageal Cancer (PRODIGE5/ACCORD17): Final Results of a Randomised, Phase 2/3 Trial. Lancet Oncol. 2014, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Yataghène, Y.; Etienne, P.L.; Michel, P.; Senellart, H.; Raoul, J.L.; Mineur, L.; Rives, M.; Mirabel, X.; Lamezec, B.; et al. Phase II Randomised Trial of Chemoradiotherapy with FOLFOX4 or Cisplatin plus Fluorouracil in Oesophageal Cancer. Br. J. Cancer 2010, 103, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Komori, S.; Tanabe, S.; Katada, C.; Azuma, M.; Ishiyama, H.; Sasaki, T.; Ishido, K.; Katada, N.; Hayakawa, K.; et al. Definitive Chemoradiation Therapy with Docetaxel, Cisplatin, and 5-Fluorouracil (DCF-R) in Advanced Esophageal Cancer: A Phase 2 Trial (KDOG 0501-P2). Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ryu, J.; Gandara, D.; Bold, R.J.; Urayama, S.; Tanaka, M.; Goldberg, Z.; Follette, D.; Narayan, S.; Lau, D. A Phase II Study of Paclitaxel, Carboplatin, and Radiation with or without Surgery for Esophageal Cancer. J. Thorac. Oncol. 2007, 2, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, J.; Sun, X.; Wang, L.; Ye, M.; Feng, P.; Zhu, G.; Lu, Y.; Han, C.; Zhu, S.; et al. Cetuximab in Combination with Chemoradiotherapy in Chinese Patients with Non-Resectable, Locally Advanced Esophageal Squamous Cell Carcinoma: A Prospective, Multicenter Phase II Trail. Radiother. Oncol. 2013, 109, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Crosby, T.; Hurt, C.N.; Falk, S.; Gollins, S.; Mukherjee, S.; Staffurth, J.; Ray, R.; Bashir, N.; Bridgewater, J.A.; Geh, J.I.; et al. Chemoradiotherapy with or without Cetuximab in Patients with Oesophageal Cancer (SCOPE1): A Multicentre, Phase 2/3 Randomised Trial. Lancet Oncol. 2013, 14, 627–637. [Google Scholar] [CrossRef]
- Ishida, Y.; Sakanaka, K.; Fujii, K.; Itasaka, S.; Mizowaki, T. Intensity-Modulated Radiotherapy for Cervical Esophageal Squamous Cell Carcinoma without Hypopharyngeal Invasion: Dose Distribution and Clinical Outcome. J. Radiat. Res. 2019, 60, 517–526. [Google Scholar] [CrossRef]
- Clavier, J.B.; Antoni, D.; Atlani, D.; Ben Abdelghani, M.; Schumacher, C.; Salze, P.; Noël, G. Chimioradiothérapie Exclusive Pour Cancer de l’œsophage: Comparaison Entre 66Gy et 50Gy, Une Étude Rétrospective. Cancer/Radiothérapie 2013, 17, 221–228. [Google Scholar] [CrossRef]
- Suh, Y.G.; Lee, I.J.; Koom, W.S.; Cha, J.; Lee, J.Y.; Kim, S.K.; Lee, C.G. High-Dose versus Standard-Dose Radiotherapy with Concurrent Chemotherapy in Stages II-III Esophageal Cancer. Jpn. J. Clin. Oncol. 2014, 44, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Li, C.C.; Chien, C.R. Does Higher Radiation Dose Lead to Better Outcome for Non-Operated Localized Esophageal Squamous Cell Carcinoma Patients Who Received Concurrent Chemoradiotherapy? A Population Based Propensity-Score Matched Analysis. Radiother. Oncol. 2016, 120, 136–139. [Google Scholar] [CrossRef]
- Deng, Y.; Bian, C.; Tao, H.; Zhang, H. Improved Survival with Higher Radiation Dose for Esophageal Squamous Cell Carcinoma Patients Treated with Definitive Chemoradiotherapy. Oncotarget 2017, 8, 79662. [Google Scholar] [CrossRef]
- Chang, C.L.; Tsai, H.C.; Lin, W.C.; Chang, J.H.; Hsu, H.L.; Chow, J.M.; Yuan, K.S.P.; Wu, A.T.H.; Wu, S.Y. Dose Escalation Intensity-Modulated Radiotherapy–Based Concurrent Chemoradiotherapy Is Effective for Advanced-Stage Thoracic Esophageal Squamous Cell Carcinoma. Radiother. Oncol. 2017, 125, 73–79. [Google Scholar] [CrossRef]
- Murray, G.F.; Wilcox, B.R.; Starek, P.J.K. The Assessment of Operability of Esophageal Carcinoma. Ann. Thorac. Surg. 1977, 23, 393–399. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef]
- Grass, G.D.; Alfonso, J.C.L.; Welsh, E.; Ahmed, K.A.; Teer, J.K.; Pilon-Thomas, S.; Harrison, L.B.; Cleveland, J.L.; Mulé, J.J.; Eschrich, S.A.; et al. The Radiosensitivity Index Gene Signature Identifies Distinct Tumor Immune Microenvironment Characteristics Associated with Susceptibility to Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 635–647. [Google Scholar] [CrossRef]
- Crehange, G.; M’vondo, C.; Bertaut, A.; Pereira, R.; Rio, E.; Peiffert, D.; Gnep, K.; Benezery, K.; Ronchin, P.; Noel, G.; et al. Exclusive Chemoradiotherapy with or Without Radiation Dose Escalation in Esophageal Cancer: Multicenter Phase 2/3 Randomized Trial CONCORDE (PRODIGE-26). Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, S5. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.S.; Tang, L.; Gomez, D.R.; Xu, T.; Luo, Y.; Huo, J.; Mouhayar, E.; Liao, Z. Incidence and Predictors of Pericardial Effusion After Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 70–79. [Google Scholar] [CrossRef]
- Wiedemann, J.; Coppes, R.P.; van Luijk, P. Radiation-Induced Cardiac Side-Effects: The Lung as Target for Interacting Damage and Intervention. Front. Oncol. 2022, 12, 931023. [Google Scholar] [CrossRef]
- Apte, N.; Dherange, P.; Mustafa, U.; Ya’qoub, L.; Dawson, D.; Higginbotham, K.; Boerma, M.; Morin, D.P.; Gupta, D.; McLarty, J.; et al. Cancer Radiation Therapy May Be Associated with Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 610915. [Google Scholar] [CrossRef]
- Price, G.M.G.; Sarigul, N. The Effect of Voxelization in Monte Carlo Simulation to Validate Bragg Peak Characteristics for a Pencil Proton Beam. Rep. Pr. Oncol. Radiother. 2023, 28, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Liu, C.M.; Wang, Y.M.; Hsu, H.C.; Huang, E.Y.; Huang, E.Y.; Huang, T.T.; Lee, C.H.; Hung, S.P.; Huang, B.S.; et al. Proton versus Photon Radiotherapy for Primary Hepatocellular Carcinoma: A Propensity-Matched Analysis. Radiat. Oncol. 2020, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.L.; Lin, C.F.; Lee, Y.Y.; Lin, K.H.; Chang, F.C.; Lin, S.C.; Lee, J.C.; Chou, F.I.; Peir, J.J.; Liu, H.M.; et al. Advances in Boron Neutron Capture Therapy (BNCT) for Recurrent Intracranial Meningioma. Int. J. Mol. Sci. 2023, 24, 4978. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.L.; Chou, F.I.; Lin, K.H.; Pan, P.S.; Lee, J.C.; Huang, W.S.; Liu, Y.M.; Chao, Y.; Chen, Y.W. Using Salvage Boron Neutron Capture Therapy (BNCT) for Recurrent Malignant Brain Tumors in Taiwan. Appl. Radiat. Isot. 2020, 160, 109105. [Google Scholar] [CrossRef] [PubMed]

| Author | SCC Number | AC Number | Study Population | Median Dose (Gy) (Range) | Overall Survival (y: Year) | AE ≥ 3: Total (%) (GI %, Hematology %) |
|---|---|---|---|---|---|---|
| Shirai K, 2011 [12] | 0 | 20 | Japan | 59 (N/A) | 2 y 41% | N/A |
| Li C, 2021 [13] | 3977 | 0 | China | 60 (40–76) | 1 y 69.8% 2 y 46.6% 3 y 37.9% 5 y 30.1% | N/A |
| Roeder F, 2014 [14] | 22 | 3 | German | 56 (19.2–62) | 1 y 82% 2 y 61% 3 y 56% | 52 (Dysphagia 19, Leukopenia 28) |
| Ge X, 2015 [15] | 112 | 0 | China | N/A (60–66) | 1 y 78.57% 3 y 47.32% | N/A (Esophagitis 25, Leukopenia 32.14%) |
| La T, 2010 [16] | 6 | 12 | USA | 50.4 (34.2–58.8) | 1 y 79% 2 y 56% | 40 (Esophagitis/Dysphagia 46, Hema 7) |
| Tu L, 2013 [17] | 36 | 0 | China | 60 (52–70) | 1 y 83.3% 2 y 42.8% | N/A (GI 11.1, Neutropenia 13.9) |
| Gerber N, 2014 [18] | 0 | 41 | USA | N/A (50.4–56) | 2 y 61% | N/A (Esophagitis 12.2, Hema 17.07) |
| Xu Y, 2016 [19] | 69 | 0 | China | N/A (50.4–56) | 1 y 73.8% 2 y 57.4% 3 y 41.0% | N/A (Esophagitis 14.5, Leukopenia 14.6) |
| Lin S, 2012 [20] | 7 | 25 | USA (Caucasians 95.2%) | 50.4 (N/A) | 3 y 51.7% | N/A (Esophagitis 9.7%, N/A) |
| Takada A, 2016 [21] | 46 | 1 | Japan | 73.4 (64.6–80) | 3 y 59.2% | N/A (Esophagitis 10.6%, Leukopenia 55.3) |
| Freilich J, 2015 [22] | 48 | 184 | USA | N/A (40–60) | 3 y 41.53% | 29.7 (N/A) |
| He L, 2016 [23] | 53 | 317 | USA (white 84.7%) | 50.4 (N/A) | 3 y 37.04% 5 y 25.49% | N/A (N/A) |
| Yang H, 2017 [24] | 78 | 0 | China | N/A (60–70) | 2 y 56.2% | 15.6 (Dysphagia 12.8, hema 10.24) |
| Iwase H, 2013 [25] | 116 | 0 | Japan | 60 (N/A) | 1 y 78.2% 5 y 29.8% | N/A (Nausea 3.4, Neutropenia 37.9) |
| Kato K, 2013 [26] | 50 | 1 | Japan | 50.4 (N/A) | 1 y 88.2% 3 y 63.8% | N/A (Esophagitis 35, Leukopenia 82.3) |
| Nishimura Y, 2012 [27] | 90 | 1 | Japan | 60 (N/A) | 2 y 45% 5 y 28.57% | 16.48 (Esophagitis 2.1, N/A) |
| Kato K, 2011 [28] | 76 | 0 | Japan | 60 (N/A) | 3 y 44.7% 5 y 36.8% | N/A (Esophagitis 17, Leukocytosis 43) |
| Conroy T, 2014 [29] | 229 | 37 | France | 50 (N/A) | 3 y 23.41% | N/A (Dysphagia 26.64, Neutropenia 28.96) |
| Conroy T, 2010 [30] | 80 | 17 | France | 50 (N/A) | 1 y 67.18% 3 y 37.64% | 73.68 (Dysphagia 17.89, Hema 73.68) |
| Higuchi K, 2014 [31] | 42 | 0 | Japan | 61.2 (12), 50.4 (30) | 1 y 66.1% 3 y 43.9% | N/A (Esophagitis 28.6, Leukopenia 71.4) |
| Wang H, 2007 [32] | 8 | 16 | USA | 50.4 (N/A) | 3 y 60% | N/A (Nausea 19, Neutropenia 4) |
| Meng X, 2013 [33] | 55 | 0 | China | 59.4 (N/A) | 1 y 92.55% 2 y 75.20% | N/A (Mucositis 12.7, Neutropenia 32.7) |
| Crosby T, 2013 [34] | 188 | 65 | UK | 50 (N/A) | 2 y 48.65% | N/A (GI 43.5, Hema 24.5) |
| Ishida Y, 2019 [35] | 21 | 0 | Japan | 60 | 3 y 60% 5 y 53.4% | N/A (Esophagitis 14.29, Neutropenia 28.57) |
| Minsky B, 2002 [8] | 187 | 31 | USA (white 69%, black 24%) | 64.8 vs. 50.4 | 2 y 31% vs. 40% | 76% vs. 71% (N/A) |
| Clavier J, 2011 [36] | 113 | 30 | France | 66 vs. 50 | 2 y 44.7% vs. 50.8% 3 y 36.8% vs. 31.6% 5 y 19.1% vs. 20.7% | 8.43 vs. 6.67 (N/A) |
| Suh Y, 2014 [37] | 117 | 9 | Republic of Korea | >60 (60–75.6) vs. <60 (45–59.4) | 2 y 52.4% vs. 45.2% | N/A (10.4% vs. 8.2%, N/A) |
| Chen C, 2016 [38] | 648 | 0 | China | >60 vs. 50.4 | 5 y 22% vs. 14% | N/A |
| Deng Y, 2017 [39] | 139 | 0 | China | >59.4 vs. 50.4 | 1 y 89% vs. 78% 2 y 61.0% vs. 39.0% 3 y 30% vs. 24% | N/A (10.5% vs. 2.2%, N/A) |
| Chang C, 2017 [40] | 2061 | 0 | Taiwan | >60 (60–72) vs. <60 (45–59.4) | 2 y 35.47% vs. 26.74% | >2: 22.56% (23.52% vs. 21.78%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, Y.; Lan, T.-L.; Lan, C.-C. A Meta-Analysis and Review of Radiation Dose Escalation in Definitive Radiation Therapy between Squamous Cell Carcinoma and Adenocarcinoma of Esophageal Cancer. Cancers 2024, 16, 658. https://doi.org/10.3390/cancers16030658
Liou Y, Lan T-L, Lan C-C. A Meta-Analysis and Review of Radiation Dose Escalation in Definitive Radiation Therapy between Squamous Cell Carcinoma and Adenocarcinoma of Esophageal Cancer. Cancers. 2024; 16(3):658. https://doi.org/10.3390/cancers16030658
Chicago/Turabian StyleLiou, Yu, Tien-Li Lan, and Chin-Chun Lan. 2024. "A Meta-Analysis and Review of Radiation Dose Escalation in Definitive Radiation Therapy between Squamous Cell Carcinoma and Adenocarcinoma of Esophageal Cancer" Cancers 16, no. 3: 658. https://doi.org/10.3390/cancers16030658
APA StyleLiou, Y., Lan, T.-L., & Lan, C.-C. (2024). A Meta-Analysis and Review of Radiation Dose Escalation in Definitive Radiation Therapy between Squamous Cell Carcinoma and Adenocarcinoma of Esophageal Cancer. Cancers, 16(3), 658. https://doi.org/10.3390/cancers16030658





