Management of Cutaneous Squamous Cell Carcinoma of the Scalp: The Role of Imaging and Therapeutic Approaches
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Cutaneous Squamous Cell Carcinoma
1.2. Staging System in Cutaneous Squamous Cell Carcinoma
2. Cutaneous Squamous Cell Carcinoma of the Scalp
2.1. The Scalp: A Special Location
2.2. Scalp Anatomy
2.3. Clinical Presentation and Diagnosis
2.4. Clinical and Pathological Risk Factors in Scalp cSCCs
3. Surgical Treatment
3.1. Surgery of the Scalp: Some Initial Considerations
3.2. Curettage and Electrodesiccation
3.3. Excision with Postoperative Margin Assessment
3.4. Histological Margins
- Peripheral or deep positive margins. Local and regional recurrences, as well as pathological positivity after re-excisions, are higher in these group of patients [36,68,71]. Thus, most guidelines recommend, when possible, re-excision as the treatment option of choice, commonly yielding clean margins [16,35,51]. If available, MMS should be the treatment of choice, rather than re-excision with postoperative margin assessment, to ensure free histological margins and avoid complications, especially in tumors with high-risk factors [34,39]. When surgery is not feasible, other treatments, such as radiotherapy (RT), might be considered [34,39].
- Free but close histological margins (by consensus, those margins between 0.1–0.9 mm, according to the Royal College of Pathologists and the BAD [39,72]). While there is scarce evidence in the literature regarding the conduct in this scenario [7,10,39,51,52,65], the British and the Scottish guidelines recommend discussing those cSCCs with histological margin <1 mm in a multidisciplinary tumor board to assess the need or not of further adjuvant treatments [39,52,72]. Thus, they consider observation in those pT1 cSCC with <1 mm histological margins in immunocompetent patients [39].
3.5. Mohs Micrographic Surgery or Excision with Complete Circumferential Peripheral and Deep Margin Assessment
3.6. Reconstructive Approaches on the Scalp
4. Management of Locoregional Disease
4.1. Management of Patients with Satellitosis or In-Transit Metastases
4.2. Management of Patients with Clinically Detected Lymph Nodes
4.3. Management of Patients without Clinically Detected Lymph Nodes
5. Non-Surgical Treatment
5.1. Radiation Therapy
5.2. Systemic Treatment
5.2.1. Immunotherapy with Checkpoint Inhibitors
Treatment of Advanced cSCC
Neoadjuvant Therapy
5.2.2. EGFR Inhibitors
5.2.3. Conventional Chemotherapy
6. Imaging Approach
6.1. The Role of Imaging in Diagnosis and Staging
6.2. Follow-Up
7. Algorithms for the Management and Treatment of Primary Scalp cSCC with Localized Disease
7.1. Proposed Algorithm for the Initial Management of Primary cSCC of the Scalp
7.2. Proposed Algorithm for the Management of Histological Margins and Other Histological Features
7.3. Proposed Algorithm for the Follow-Up of cSCC of the Scalp
8. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| cSCC | Cutaneous squamous cell carcinoma |
| PNI | Perineural invasion |
| MMS | Mohs micrographic surgery |
| SLNB | Sentinel lymph node biopsy |
| S-ITM | Satellitosis or in-transit metastasis |
| LNM | Lymph node metastases |
| RT | Radiotherapy |
| CT | Computed tomography |
References
- Kwa, R.E.; Campana, K.; Moy, R.L. Biology of Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 1992, 26, 1–26. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Incidence, Risk Factors, Diagnosis, and Staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Nagarajan, P.; Asgari, M.M.; Green, A.C.; Guhan, S.M.; Arron, S.T.; Proby, C.M.; Rollison, D.E.; Harwood, C.A.; Toland, A.E. Keratinocyte Carcinomas: Current Concepts and Future Research Priorities. Clin. Cancer Res. 2019, 25, 2379–2391. [Google Scholar] [CrossRef]
- Andrade, P.; Vieira, R.; Reis, J.P.; Brites, M.M.; Mariano, A. Epidemiology of Basal Cell Carcinomas and Squamous Cell Carcinomas in a Department of Dermatology–A 5 Year Review. An. Bras. Dermatol. 2012, 87, 212–219. [Google Scholar] [CrossRef]
- Youl, P.H.; Janda, M.; Aitken, J.F.; Del Mar, C.B.; Whiteman, D.C.; Baade, P.D. Body-Site Distribution of Skin Cancer, Pre-Malignant and Common Benign Pigmented Lesions Excised in General Practice. Br. J. Dermatol. 2011, 165, 35–43. [Google Scholar] [CrossRef]
- Weinstock, M.A. Death from Skin Cancer Among the Elderly: Epidemiological Patterns. Arch. Dermatol. 1997, 133, 1207–1209. [Google Scholar] [CrossRef]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bordeaux, J.; Chen, P.-L. National Comprehensive Cancer Network (NCCN). Squamous Cell Skin Cancer Version 1.2024. Natl. Compr. Cancer Netw. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1465 (accessed on 26 December 2023).
- Green, A.C.; Olsen, C.M. Cutaneous Squamous Cell Carcinoma: An Epidemiological Review. Br. J. Dermatol. 2017, 177, 373–381. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 1. Epidemiology, Diagnostics and Prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef]
- Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J.; et al. Guidelines of Care for the Management of Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2019, 78, 560–578. [Google Scholar] [CrossRef]
- Baker, N.J.; Webb, A.A.C.; Macpherson, D. Surgical Management of Cutaneous Squamous Cell Carcinoma of the Head and Neck. Br. J. Oral Maxillofac. Surg. 2001, 39, 87–90. [Google Scholar] [CrossRef]
- Venables, Z.C.; Nijsten, T.; Wong, K.F.; Autier, P.; Broggio, J.; Deas, A.; Harwood, C.A.; Hollestein, L.M.; Langan, S.M.; Morgan, E.; et al. Epidemiology of Basal and Cutaneous Squamous Cell Carcinoma in the U.K. 2013–15: A Cohort Study. Br. J. Dermatol. 2019, 181, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Seretis, K.; Thomaidis, V.; Karpouzis, A.; Tamiolakis, D.; Tsamis, I. Epidemiology of Surgical Treatment of Nonmelanoma Skin Cancer of the Head and Neck in Greece. Dermatol. Surg. 2010, 36, 15–22. [Google Scholar] [CrossRef]
- Martorell-Calatayud, A.; Sanmartín Jimenez, O.; Cruz Mojarrieta, J.; Guillén Barona, C. Carcinoma Epidermoide Cutáneo: Definiendo La Variante de Alto Riesgo. Actas Dermosifiliogr. 2013, 104, 367–379. [Google Scholar] [CrossRef]
- Rowe, D.E.; Carroll, R.J.; Day, C.L. Prognostic Factors for Local Recurrence, Metastasis, and Survival Rates in Squamous Cell Carcinoma of the Skin, Ear, and Lip. Implications for Treatment Modality Selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.W.; Suvarna, S.K.; Feeley, K. Audit of Clinical and Histological Prognostic Factors in Primary Invasive Squamous Cell Carcinoma of the Skin: Assessment in a Minimum 5 Year Follow-up Study after Conventional Excisional Surgery. Br. J. Plast. Surg. 2002, 55, 287–292. [Google Scholar] [CrossRef]
- Brougham, N.D.L.S.; Dennett, E.R.; Cameron, R.; Tan, S.T. The Incidence of Metastasis from Cutaneous Squamous Cell Carcinoma and the Impact of Its Risk Factors. J. Surg. Oncol. 2012, 106, 811–815. [Google Scholar] [CrossRef]
- Møller, R.; Reymann, F.; Hou-Jensen, K. Metastases in Dermatological Patients with Squamous Cell Carcinoma. Arch. Dermatol. 1979, 115, 703–705. [Google Scholar] [CrossRef]
- Epstein, E.; Epstein, N.N.; Bragg, K.; Linden, G. Metastases From Squamous Cell Carcinomas of the Skin. Arch. Dermatol. 1968, 97, 245–251. [Google Scholar] [CrossRef]
- Karia, P.S.; Han, J.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Estimated Incidence of Disease, Nodal Metastasis, and Deaths from Disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of Risk Factors Determining Prognosis of Cutaneous Squamous-Cell Carcinoma: A Prospective Study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Eigentler, T.K.; Leiter, U.; Häfner, H.M.; Garbe, C.; Röcken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors Predictive of Recurrence and Death From Cutaneous Squamous Cell Carcinoma: A 10-Year, Single-Institution Cohort Study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef]
- Jambusaria-Pahlajani, A.; Kanetsky, P.A.; Karia, P.S.; Hwang, W.T.; Gelfand, J.M.; Whalen, F.M.; Elenitsas, R.; Xu, X.; Schmults, C.D. Evaluation of AJCC Tumor Staging for Cutaneous Squamous Cell Carcinoma and a Proposed Alternative Tumor Staging System. JAMA Dermatol. 2013, 149, 402–410. [Google Scholar] [CrossRef]
- Cañueto, J.; Román-Curto, C. Los Nuevos Sistemas de Estadificación Del AJCC Incorporan Novedades En El Cáncer Cutáneo. Actas Dermosifiliogr. 2017, 108, 818–826. [Google Scholar] [CrossRef]
- Cañueto, J.; Burguillo, J.; Moyano-Bueno, D.; Viñolas-Cuadros, A.; Conde-Ferreirós, A.; Corchete-Sánchez, L.A.; Pérez-Losada, J.; Román-Curto, C. Comparing the Eighth and the Seventh Editions of the American Joint Committee on Cancer Staging System and the Brigham and Women’s Hospital Alternative Staging System for Cutaneous Squamous Cell Carcinoma: Implications for Clinical Practice. J. Am. Acad. Dermatol. 2019, 80, 106–113.e2. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Karia, P.S.; Besaw, R.; Schmults, C.D. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019, 155, 819–825. [Google Scholar] [CrossRef]
- Karia, P.S.; Jambusaria-Pahlajani, A.; Harrington, D.P.; Murphy, G.F.; Qureshi, A.A.; Schmults, C.D. Evaluation of American Joint Committee on Cancer, International Union against Cancer, and Brigham and Women’s Hospital Tumor Staging for Cutaneous Squamous Cell Carcinoma. J. Clin. Oncol. 2014, 32, 327. [Google Scholar] [CrossRef]
- Puebla-Tornero, L.; Corchete-Sánchez, L.A.; Conde-Ferreirós, A.; García-Sancha, N.; Corchado-Cobos, R.; Román-Curto, C.; Cañueto, J. Performance of Salamanca Refinement of the T3-AJCC8 versus the Brigham and Women’s Hospital and Tübingen Alternative Staging Systems for High-Risk Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2021, 84, 938–945. [Google Scholar] [CrossRef]
- Jung, G.W.; Metelitsa, A.I.; Dover, D.C.; Salopek, T.G. Trends in Incidence of Nonmelanoma Skin Cancers in Alberta, Canada, 1988–2007. Br. J. Dermatol. 2010, 163, 146–154. [Google Scholar] [CrossRef]
- Prodinger, C.M.; Koller, J.; Laimer, M. Scalp Tumors. J. Der Dtsch. Dermatol. Ges. 2018, 16, 730–753. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.; Smith, A.B.; Kanatas, A.N.; Houghton, D.R.; Telfer, M.R. Anatomical Restrictions in the Surgical Excision of Scalp Squamous Cell Carcinomas: Does This Affect Local Recurrence and Regional Nodal Metastases? Int. J. Oral Maxillofac. Surg. 2014, 43, 142–146. [Google Scholar] [CrossRef]
- Mo, J.; Miller, C.J.; Karakousis, G.; Keele, L.; Cohen, J.; Krouse, R.S. The Scalp Is a High-Risk Site for Cutaneous Squamous Cell Carcinoma Metastasis. J. Am. Acad. Dermatol. 2021, 84, 1742–1744. [Google Scholar] [CrossRef]
- Yu, W.Y.; Salmon, P.; Thuener, J.; Bordeaux, J.S. Mohs Surgery for Advanced Tumors of the Scalp. Dermatol. Surg. 2019, 45, S110–S117. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.F.; Chawla, R.; Patel, A.J.K. Galea vs Periosteum: Impact of Excision Depth on Outcomes for Cutaneous Squamous Cell Carcinoma of the Scalp. J. Plast. Surg. Hand Surg. 2022, 57, 253–256. [Google Scholar] [CrossRef]
- Khan, K.; Mykula, R.; Kerstein, R.; Rabey, N.; Bragg, T.; Crick, A.; Heppell, S.; Budny, P.; Potter, M. A 5-Year Follow-up Study of 633 Cutaneous SCC Excisions: Rates of Local Recurrence and Lymph Node Metastasis. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Alvi, S.; Jenzer, A.C. Scalp Reconstruction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gurney, B.; Newlands, C. Management of Regional Metastatic Disease in Head and Neck Cutaneous Malignancy. 1. Cutaneous Squamous Cell Carcinoma. Br. J. Oral Maxillofac. Surg. 2014, 52, 294–300. [Google Scholar] [CrossRef]
- Keohane, S.G.; Botting, J.; Budny, P.G.; Dolan, O.M.; Fife, K.; Harwood, C.A.; Mallipeddi, R.; Marsden, J.R.; Motley, R.J.; Newlands, C.; et al. British Association of Dermatologists Guidelines for the Management of People with Cutaneous Squamous Cell Carcinoma 2020. Br. J. Dermatol. 2021, 184, 401–414. [Google Scholar] [CrossRef]
- Combalia, A.; Carrera, C. Squamous Cell Carcinoma: An Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020066. [Google Scholar] [CrossRef]
- Vauterin, T.J.; Veness, M.J.; Morgan, G.J.; Poulsen, M.G.; O’Brien, C.J. Patterns of Lymph Node Spread of Cutaneous Squamous Cell Carcinoma of the Head and Neck. Head Neck 2006, 28, 785–791. [Google Scholar] [CrossRef]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Outcomes: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2016, 152, 419. [Google Scholar] [CrossRef]
- Quaedvlieg, P.J.F.; Creytens, D.H.K.V.; Epping, G.G.; Peutz-Kootstra, C.J.; Nieman, F.H.M.; Thissen, M.R.T.M.; Krekels, G.A. Histopathological Characteristics of Metastasizing Squamous Cell Carcinoma of the Skin and Lips. Histopathology 2006, 49, 256. [Google Scholar] [CrossRef]
- Rabinovics, N.; Mizrachi, A.; Hadar, T.; Ad-El, D.; Feinmesser, R.; Guttman, D.; Shpitzer, T.; Bachar, G. Cancer of the Head and Neck Region in Solid Organ Transplant Recipients. Head Neck 2014, 36, 181–186. [Google Scholar] [CrossRef]
- Manyam, B.V.; Garsa, A.A.; Chin, R.I.; Reddy, C.A.; Gastman, B.; Thorstad, W.; Yom, S.S.; Nussenbaum, B.; Wang, S.J.; Vidimos, A.T.; et al. A Multi-Institutional Comparison of Outcomes of Immunosuppressed and Immunocompetent Patients Treated with Surgery and Radiation Therapy for Cutaneous Squamous Cell Carcinoma of the Head and Neck. Cancer 2017, 123, 2054–2060. [Google Scholar] [CrossRef]
- Lam, J.K.S.; Sundaresan, P.; Gebski, V.; Veness, M.J. Immunocompromised Patients with Metastatic Cutaneous Nodal Squamous Cell Carcinoma of the Head and Neck: Poor Outcome Unrelated to the Index Lesion. Head Neck 2018, 40, 985–992. [Google Scholar] [CrossRef]
- Mehrany, K.; Weenig, R.H.; Lee, K.K.; Pittelkow, M.R.; Otley, C.C. Increased Metastasis and Mortality from Cutaneous Squamous Cell Carcinoma in Patients with Chronic Lymphocytic Leukemia. J. Am. Acad. Dermatol. 2005, 53, 1067–1071. [Google Scholar] [CrossRef]
- Heredia, L.; Vargas-Mora, P.; Jahr, C.; Herranz, J.; Ferrer-Rosende, P. Tumour Budding in Cutaneous Squamous Cell Carcinoma: A Novel Prognosis Risk Factor. Australas. J. Dermatol. 2023, 64, e340–e347. [Google Scholar] [CrossRef]
- Fujimoto, M.; Yamamoto, Y.; Takai, T.; Fujimoto, N.; Ogawa, K.; Yoshikawa, T.; Matsuzaki, I.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; et al. Tumor Budding Is an Objective High-Risk Factor Associated With Metastasis and Poor Clinical Prognosis in Cutaneous Squamous Cell Carcinoma Sized <4 Cm. Am. J. Surg. Pathol. 2019, 43, 975–983. [Google Scholar] [CrossRef]
- Farah, M.; Milton, D.R.; Gross, N.D.; Nagarajan, P.; Gu, J.; Curry, J.L.; Ivan, D.; Torres-Cabala, C.A.; Myers, J.N.; Prieto, V.G.; et al. Histopathologic Features Predictive of Metastasis and Survival in 230 Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck and Non-Head and Neck Locations: A Single-Center Retrospective Study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; van Akkooi, A.; Bataille, V.; Bastholt, L.; Dreno, B.; Dummer, R.; Fargnoli, M.C.; et al. European Consensus-Based Interdisciplinary Guideline for Invasive Cutaneous Squamous Cell Carcinoma: Part 2. Treatment–Update 2023. Eur. J. Cancer 2023, 193, 113252. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network. SIGN 140 • Management of Primary Cutaneous Squamous Cell Carcinoma; Scottish Intercollegiate Guidelines Network: Edinburgh, UK, 2014. [Google Scholar]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Hill, D.; Richards, S.; Paver, R. Cutaneous Squamous Cell Carcinoma Treated with Mohs Micrographic Surgery in Australia I. Experience over 10 Years. J. Am. Acad. Dermatol. 2005, 53, 253–260. [Google Scholar] [CrossRef]
- van Lee, C.B.; Roorda, B.M.; Wakkee, M.; Voorham, Q.; Mooyaart, A.L.; de Vijlder, H.C.; Nijsten, T.; van den Bos, R.R. Recurrence Rates of Cutaneous Squamous Cell Carcinoma of the Head and Neck after Mohs Micrographic Surgery vs. Standard Excision: A Retrospective Cohort Study. Br. J. Dermatol. 2019, 181, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.D.; Beal, B.T.; Varra, V.; Rodriguez, M.; Cundall, H.; Woody, N.M.; Vidimos, A.T.; Koyfman, S.A.; Knackstedt, T.J. Outcomes in Intermediate-Risk Squamous Cell Carcinomas Treated with Mohs Micrographic Surgery Compared with Wide Local Excision. J. Am. Acad. Dermatol. 2020, 82, 1195–1204. [Google Scholar] [CrossRef]
- Udkoff, J.; Beal, B.T.; Brodland, D.G.; Knackstedt, T. Cost Effectiveness of Intermediate-Risk Squamous Cell Carcinoma Treated with Mohs Micrographic Surgery Compared with Wide Local Excision. J. Am. Acad. Dermatol. 2022, 86, 303–311. [Google Scholar] [CrossRef]
- Leibovitch, I.; Huilgol, S.C.; Richards, S.; Paver, R.; Selva, D. Scalp Tumors Treated with Mohs Micrographic Surgery: Clinical Features and Surgical Outcome. Dermatol. Surg. 2006, 32, 1369–1374. [Google Scholar] [CrossRef]
- Mohs, F.E.; Zitelli, J.A. Microscopically Controlled Surgery in the Treatment of Carcinoma of the Scalp. Arch. Dermatol. 1981, 117, 764–769. [Google Scholar] [CrossRef]
- Lederhandler, M.; Stokar, E.; Meehan, S.A.; Geronemus, R.G. Deep Initial Mohs Stage for Scalp Cutaneous Squamous Cell Carcinoma to Avoid Occult Tumor. J. Am. Acad. Dermatol. 2020, 82, e129–e130. [Google Scholar] [CrossRef]
- Redondo Bellón, P. Atlas Práctico de Cirugía Dermatológica: Fundamentos de Cirugía Dermatológica, Cirugía Topográfica Reconstructiva, 2nd ed.; Aula Médica: Solna, Sweden, 2014; ISBN 9788478855803. [Google Scholar]
- Uttamani, R.; Venkataram, A.; Venkataram, J.; Mysore, V. Tumescent Anesthesia for Dermatosurgical Procedures Other Than Liposuction. J. Cutan. Aesthet. Surg. 2020, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.G.; Ashton, R.E. Low Incidence of Metastasis and Recurrence from Cutaneous Squamous Cell Carcinoma Found in a UK Population: Do We Need to Adjust Our Thinking on This Rare but Potentially Fatal Event? J. Surg. Oncol. 2017, 116, 783–788. [Google Scholar] [CrossRef]
- Brodland, D.G.; Zitelli, J.A. Surgical Margins for Excision of Primary Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 1992, 27, 241–248. [Google Scholar] [CrossRef]
- Schell, A.E.; Russell, M.A.; Park, S.S. Suggested Excisional Margins for Cutaneous Malignant Lesions Based on Mohs Micrographic Surgery. JAMA Facial Plast. Surg. 2013, 15, 337–343. [Google Scholar] [CrossRef]
- Paoli, J.; Larkö, O. Actinic Keratosis, Squamous Cell Carcinoma and Basal Cell Carcinoma Clinical Guidelines, Sweden. Forum Nord. Derm.-Venerol. 2009, 14, 71–77. [Google Scholar]
- Thiem, D.G.E.; Scharr, K.; Pabst, A.M.; Saka, B.; Kämmerer, P.W. Facial Cutaneous Squamous Cell Carcinoma—Microscopic Safety Margins and Their Impact on Developing Local Recurrences. J. Cranio-Maxillofac. Surg. 2020, 48, 49–55. [Google Scholar] [CrossRef]
- Sepehripour, S.; Dawood, O.; Hatter, S.; Williams, L.; Zahd, Z.; Liebmann, R.; Dheansa, B. An Assessment of Histological Margins and Recurrence of Completely Excised Cutaneous SCC. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 899–903. [Google Scholar] [CrossRef]
- Mourouzis, C.; Boynton, A.; Grant, J.; Umar, T.; Wilson, A.; Macpheson, D.; Pratt, C. Cutaneous Head and Neck SCCs and Risk of Nodal Metastasis–UK Experience. J. Cranio-Maxillofac. Surg. 2009, 37, 443–447. [Google Scholar] [CrossRef]
- Khan, A.A.; Potter, M.; Cubitt, J.J.; Khoda, B.J.; Smith, J.; Wright, E.H.; Scerri, G.; Crick, A.; Cassell, O.C.; Budny, P.G. Guidelines for the Excision of Cutaneous Squamous Cell Cancers in the United Kingdom: The Best Cut Is the Deepest. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 467–471. [Google Scholar] [CrossRef]
- Van Lee, C.B.; Kouloubis, N.; Wakkee, M.; Kelleners-Smeets, N.W.J.; Nellen, R.G.L.; Van Rengen, A.; De Vijlder, H.C.; Wijne, L.C.C.; Nijsten, T.; Van Den Bos, R.R. Rate and Characteristics of Incompletely Excised Cutaneous Squamous Cell Carcinoma: A Dermatological Daily Practice Multicenter Prospective Cohort Study. Dermatol. Surg. 2022, 48, 1269–1273. [Google Scholar] [CrossRef]
- Bovill, E.S.; Cullen, K.W.; Barrett, W.; Banwell, P.E. Clinical and Histological Findings in Re-Excision of Incompletely Excised Cutaneous Squamous Cell Carcinoma. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 457–461. [Google Scholar] [CrossRef]
- Slater, D.; Barrett, P. Standards and Datasets for Reporting Cancers Dataset for the Histological Reporting of Primary Invasive Cutaneous Squamous Cell Carcinoma and Regional Lymph Nodes February 2019; The Royal College of Pathologists: London, UK, 2019; pp. 1–36. [Google Scholar]
- Levy, R.M.; Hanke, C.W. Mohs Micrographic Surgery: Facts and Controversies. Clin. Dermatol. 2010, 28, 269–274. [Google Scholar] [CrossRef]
- Marrazzo, G.; Zitelli, J.A.; Brodland, D. Clinical Outcomes in High-Risk Squamous Cell Carcinoma Patients Treated with Mohs Micrographic Surgery Alone. J. Am. Acad. Dermatol. 2019, 80, 633–638. [Google Scholar] [CrossRef]
- Pugliano-Mauro, M.; Goldman, G. Mohs Surgery Is Effective for High-Risk Cutaneous Squamous Cell Carcinoma. Dermatol. Surg. 2010, 36, 1544–1553. [Google Scholar] [CrossRef]
- Tschetter, A.J.; Campoli, M.R.; Zitelli, J.A.; Brodland, D.G. Long-Term Clinical Outcomes of Patients with Invasive Cutaneous Squamous Cell Carcinoma Treated with Mohs Micrographic Surgery: A 5-Year, Multicenter, Prospective Cohort Study. J. Am. Acad. Dermatol. 2020, 82, 139–148. [Google Scholar] [CrossRef]
- Hunt, W.T.N.; Earp, E.; Brown, A.C.; Veitch, D.; Wernham, A.G.H. A Review of Mohs Micrographic Surgery for Skin Cancer. Part 3: Squamous Cell Carcinoma. Clin. Exp. Dermatol. 2022, 47, 1765–1773. [Google Scholar] [CrossRef]
- Mohs, F.E. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer—Past, Present and Future. J. Dermatol. Surg. Oncol. 1978, 4, 41–54. [Google Scholar] [CrossRef]
- Tomás-Velázquez, A.; Sanmartin-Jiménez, O.; Garcés, J.R.; Rodríguez-Prieto, M.A.; Ruiz-Salas, V.; De Eusebio-Murillo, E.; Miñano-Medrano, R.; Escutia-Muñoz, B.; Flórez-Menéndez, Á.; Artola-Igarza, J.L.; et al. Risk Factors and Rate of Recurrence after Mohs Surgery in Basal Cell and Squamous Cell Carcinomas: A Nationwide Prospective Cohort (REGESMOHS, Spanish Registry of Mohs Surgery). Acta Derm. Venereol. 2021, 101, adv00602. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.M.; Baker, D.R.; Coldiron, B.M.; Fazio, M.J.; Storrs, P.A.; Vidimos, A.T.; Zalla, M.J.; Brewer, J.D.; Smith Begolka, W.; Berger, T.G.; et al. AAD/ACMS/ASDSA/ASMS 2012 Appropriate Use Criteria for Mohs Micrographic Surgery: A Report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Su. J. Am. Acad. Dermatol. 2012, 67, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Hill, D.; Richards, S.; Paver, R. Cutaneous Squamous Cell Carcinoma Treated with Mohs Micrographic Surgery in Australia II. Perineural Invasion. J. Am. Acad. Dermatol. 2005, 53, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, R. Split-Thickness Skin Grafts. In Essence of Anesthesia Practice; Elsevier: Amsterdam, The Netherlands, 2011; Volume 532. [Google Scholar] [CrossRef]
- Marti-Marti, I.; Podlipnik, S.; Cañueto, J.; Ferrándiz-Pulido, C.; Deza, G.; Sanmartín, O.; Jaka, A.; Beà-Ardèbol, S.; Botella-Estrada, R.; Redondo, P.; et al. Prognostic Factors for Satellitosis or In-Transit Metastasis in Cutaneous Squamous Cell Carcinoma: A Multicentric Cohort Study. J. Am. Acad. Dermatol. 2023, 89, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.H.Y.; Wu, A.; Veness, M.; Estall, V.; Hong, A.; Borg, M.; James, C.; Ibbetson, J.; Ooi, C.; Weightman, W.; et al. In-Transit Metastasis From Squamous Cell Carcinoma. Dermatol. Surg. 2016, 42, 1285–1292. [Google Scholar] [CrossRef]
- Xu, M.J.; Lazar, A.A.; Garsa, A.A.; Arron, S.T.; Ryan, W.R.; El-Sayed, I.H.; George, J.R.; Algazi, A.P.; Heaton, C.M.; Ha, P.K.; et al. Major Prognostic Factors for Recurrence and Survival Independent of the American Joint Committee on Cancer Eighth Edition Staging System in Patients with Cutaneous Squamous Cell Carcinoma Treated with Multimodality Therapy. Head Neck 2018, 40, 1406–1414. [Google Scholar] [CrossRef]
- Smile, T.D.; Ruiz, E.S.; Kus, K.J.B.; Murad, F.; Wei, W.; Xiong, D.D.; Vidimos, A.T.; Schmults, C.D.; Koyfman, S.A. Implications of Satellitosis or In-Transit Metastasis in Cutaneous Squamous Cell Carcinoma: A Prognostic Omission in Cancer Staging Systems. JAMA Dermatol. 2022, 158, 390. [Google Scholar] [CrossRef]
- Smile, T.D.; Xiong, D.X.; Varra, V.; Winter, I.W.; Beal, B.T.; Gastman, B.R.; Geiger, J.L.; Adelstein, D.J.; Bergfeld, W.F.; Piliang, M.P.; et al. Disease Progression in Cutaneous Squamous Cell Carcinoma Patients With Satellitosis and In-Transit Metastasis. Anticancer. Res. 2021, 41, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Palme, C.E.; Wang, A.Y.; Morgan, G.J.; Gebski, V.; Veness, M.J. In Patients with Metastatic Cutaneous Head and Neck Squamous Cell Carcinoma to Cervical Lymph Nodes, the Extent of Neck Dissection Does Not Influence Outcome. J. Laryngol. Otol. 2013, 127 (Suppl. 1), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.P.; Grilli, G.; Shah, J.P.; Medina, J.E.; Robbins, K.T.; Takes, R.P.; Hamoir, M.; Kowalski, L.P.; Suárez, C.; López, F.; et al. Selective Neck Dissection in Surgically Treated Head and Neck Squamous Cell Carcinoma Patients with a Clinically Positive Neck: Systematic Review. Eur. J. Surg. Oncol. 2018, 44, 395–403. [Google Scholar] [CrossRef]
- Rotman, A.; Kerr, S.J.; Giddings, C.E.B. Elective Neck Dissection in Metastatic Cutaneous Squamous Cell Carcinoma to the Parotid Gland: A Systematic Review and Meta-Analysis. Head Neck 2019, 41, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Porceddu, S.V.; Bressel, M.; Poulsen, M.G.; Stoneley, A.; Veness, M.J.; Kenny, L.M.; Wratten, C.; Corry, J.; Cooper, S.; Fogarty, G.B.; et al. Postoperative Concurrent Chemoradiotherapy Versus Postoperative Radiotherapy in High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck: The Randomized Phase III TROG 05.01 Trial. J. Clin. Oncol. 2018, 36, 1275–1283. [Google Scholar] [CrossRef]
- Sahovaler, A.; Krishnan, R.J.; Yeh, D.H.; Zhou, Q.; Palma, D.; Fung, K.; Yoo, J.; Nichols, A.; Macneil, S.D. Outcomes of Cutaneous Squamous Cell Carcinoma in the Head and Neck Region With Regional Lymph Node Metastasis: A Systematic Review and Meta-Analysis. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 352–360. [Google Scholar] [CrossRef]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 387, 1557–1568. [Google Scholar] [CrossRef]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab and Surgery for Stage II-IV Cutaneous Squamous-Cell Carcinoma: Follow-up and Survival Outcomes of a Single-Arm, Multicentre, Phase 2 Study. Lancet. Oncol. 2023, 24, 1196–1205. [Google Scholar] [CrossRef]
- Tejera-Vaquerizo, A.; García-Doval, I.; Llombart, B.; Cañueto, J.; Martorell-Calatayud, A.; Descalzo-Gallego, M.A.; Sanmartín, O. Systematic Review of the Prevalence of Nodal Metastases and the Prognostic Utility of Sentinel Lymph Node Biopsy in Cutaneous Squamous Cell Carcinoma. J. Dermatol. 2018, 45, 781–790. [Google Scholar] [CrossRef]
- Schmitt, A.R.; Brewer, J.D.; Bordeaux, J.S.; Baum, C.L. Staging for Cutaneous Squamous Cell Carcinoma as a Predictor of Sentinel Lymph Node Biopsy Results: Meta-Analysis of American Joint Committee on Cancer Criteria and a Proposed Alternative System. JAMA Dermatol. 2014, 150, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Vaquerizo, A.; Cañueto, J.; Llombart, B.; Martorell-Calatayud, A.; Sanmartín, O. Predictive Value of Sentinel Lymph Node Biopsy in Cutaneous Squamous Cell Carcinoma Based on the AJCC-8 and Brigham and Women’s Hospital Staging Criteria. Dermatol. Surg. 2020, 46, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.M.; Shaw, D.; Martin, R.C.W.; Kelder, W.; Roth, K.; Uren, R.; Gao, K.; Davies, S.; Ashford, B.G.; Ngo, Q.; et al. Prospective Study of Sentinel Node Biopsy for High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck. Head Neck 2016, 38, E884–E889. [Google Scholar] [CrossRef]
- Takahashi, A.; Imafuku, S.; Nakayama, J.; Nakaura, J.; Ito, K.; Shibayama, Y. Sentinel Node Biopsy for High-Risk Cutaneous Squamous Cell Carcinoma. Eur. J. Surg. Oncol. 2014, 40, 1256–1262. [Google Scholar] [CrossRef]
- Walker, T.D.; Cusick, A.; Shahwan, K.T.; Carr, D.R. Timing of Sentinel Lymph Node Biopsy in Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2023, 88, 249–251. [Google Scholar] [CrossRef]
- Lansbury, L.; Bath-Hextall, F.; Perkins, W.; Stanton, W.; Leonardi-Bee, J. Interventions for Non-Metastatic Squamous Cell Carcinoma of the Skin: Systematic Review and Pooled Analysis of Observational Studies. BMJ 2013, 347, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Parsons, J.T.; Mendenhall, N.P.; Million, R.R. T2-T4 Carcinoma of the Skin of the Head and Neck Treated with Radical Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Mendenhall, W.M.; Parsons, J.T.; Million, R.R. Radical Radiotherapy for T4 Carcinoma of the Skin of the Head and Neck: A Multivariate Analysis. Head Neck 1993, 15, 320–324. [Google Scholar] [CrossRef]
- Gruber, I.; Koelbl, O. Dramatic Radiotherapy Response of a Giant T4 Cutaneous Squamous Cell Carcinoma of the Scalp with Extensive Bone Destruction: A Case Report. J. Med. Case Rep. 2021, 15, 610. [Google Scholar] [CrossRef]
- Revelles-Peñas, L.; Revilla-Nebreda, D.; Becerril, S.; Corchete, L.A.; Domínguez-Rullán, I.; Martins-Lopes, M.; Arias-Rodríguez, P.; Rodríguez-Guitiérrez, A.; Pérez-Romansanta, L.A.; Román-Curto, C.; et al. Outcome of Cutaneous Squamous Cell Carcinoma with Microscopic Residual Disease after Surgery and Usefulness of Postoperative Radiotherapy: A Retrospective Cohort Study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 846–854. [Google Scholar] [CrossRef]
- Herman, M.P.; Amdur, R.J.; Werning, J.W.; Dziegielewski, P.; Morris, C.G.; Mendenhall, W.M. Elective Neck Management for Squamous Cell Carcinoma Metastatic to the Parotid Area Lymph Nodes. Eur. Arch. Otorhinolaryngol. 2016, 273, 3875–3879. [Google Scholar] [CrossRef]
- Cañueto, J.; Jaka, A.; Corchete, L.A.; González-Pérez, A.M.; García-Castro, R.; Fuente, M.J.; Membrive, I.; March, Á.; Mañes, A.; Posada, R.; et al. Postoperative Radiotherapy Provides Better Local Control and Long-Term Outcome in Selective Cases of Cutaneous Squamous Cell Carcinoma with Perineural Invasion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Sapir, E.; Tolpadi, A.; McHugh, J.; Samuels, S.E.; Elalfy, E.; Spector, M.; Shuman, A.G.; Malloy, K.M.; Prince, M.E.; Bradford, C.R.; et al. Skin Cancer of the Head and Neck with Gross or Microscopic Perineural Involvement: Patterns of Failure. Radiother. Oncol. 2016, 120, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lehrer, E.J.; Wirth, P.J.; Khesroh, E.A.; Brewer, J.D.; Billingsley, E.M.; Zaorsky, N.G.; Lam, C. Adjuvant Radiotherapy May Not Significantly Change Outcomes in High-Risk Cutaneous Squamous Cell Carcinomas with Clear Surgical Margins: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2022, 86, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Pêtre, A.; Pommier, P.; Brahmi, T.; Chabaud, S.; King, S.; Fayette, J.; Neidhart, E.M.; Amini-Adle, M. Benefit from Adjuvant Radiotherapy According to the Number of Risk Factors in Cutaneous Squamous Cell Carcinoma. Radiother. Oncol. 2022, 168, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.S.; Kus, K.J.B.; Smile, T.D.; Murad, F.; Zhou, G.; Ilori, E.O.; Schoenfeld, J.D.; Margalit, D.N.; Tishler, R.B.; Vidimos, A.T.; et al. Adjuvant Radiation Following Clear Margin Resection of High T-Stage Cutaneous Squamous Cell Carcinoma Halves the Risk of Local and Locoregional Recurrence: A Dual-Center Retrospective Study. J. Am. Acad. Dermatol. 2022, 87, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Porceddu, S.V.; Daniels, C.; Yom, S.S.; Liu, H.; Waldron, J.; Gregoire, V.; Moore, A.; Veness, M.; Yao, M.; Johansen, J.; et al. Head and Neck Cancer International Group (HNCIG) Consensus Guidelines for the Delivery of Postoperative Radiation Therapy in Complex Cutaneous Squamous Cell Carcinoma of the Head and Neck (CSCCHN). Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Likhacheva, A.; Awan, M.; Barker, C.A.; Bhatnagar, A.; Bradfield, L.; Brady, M.S.; Buzurovic, I.; Geiger, J.L.; Parvathaneni, U.; Zaky, S.; et al. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ratushny, V.; Ridky, T.W.; Seykora, J.T.; Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From Keratinocyte to Cancer: The Pathogenesis and Modeling of Cutaneous Squamous Cell Carcinoma Find the Latest Version: Review Series From Keratinocyte to Cancer: The Pathogenesis and Modeling of Cutaneous Squamous Cell Carcinoma. J. Clin. Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 Study of Cemiplimab in Patients with Metastatic Cutaneous Squamous Cell Carcinoma: Primary Analysis of Fixed-Dosing, Long-Term Outcome of Weight-Based Dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in Locally Advanced Cutaneous Squamous Cell Carcinoma: Results from an Open-Label, Phase 2, Single-Arm Trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Munoz-Couselo, E.; Mortier, L.; Bratland, Å.; Gutzmer, R.; Roshdy, O.; González Mendoza, R.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for Locally Advanced and Recurrent/Metastatic Cutaneous Squamous Cell Carcinoma (KEYNOTE-629 Study): An Open-Label, Nonrandomized, Multicenter, Phase II Trial. Ann. Oncol. 2021, 32, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Fargnoli, M.C.; Fantini, F.; Gattoni, M.; Gualdi, G.; Pastore, F.; Pellacani, G.; Quaglino, P.; Queirolo, P.; Troiani, T. Identifying Candidates for Immunotherapy with Cemiplimab to Treat Advanced Cutaneous Squamous Cell Carcinoma: An Expert Opinion. Ther. Adv. Med. Oncol. 2022, 14, 17588359211066272. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Petrow, P.; Scheer-Senyarich, I.; Duvillard, P.; Lacroix, L.; Gelly, J.; Certain, A.; Duval, X.; Crickx, B.; Buffard, V.; et al. Phase II Study of Cetuximab as First-Line Single-Drug Therapy in Patients with Unresectable Squamous Cell Carcinoma of the Skin. J. Clin. Oncol. 2011, 29, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Cañueto, J.; Cardeñoso, E.; García, J.L.; Santos-Briz; Castellanos-Martín, A.; Fernández-López, E.; Blanco Gómez, A.; Pérez-Losada, J.; Román-Curto, C. Epidermal Growth Factor Receptor Expression Is Associated with Poor Outcome in Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2017, 176, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Trodello, C.; Pepper, J.P.; Wong, M.; Wysong, A. Cisplatin and Cetuximab Treatment for Metastatic Cutaneous Squamous Cell Carcinoma: A Systematic Review. Dermatol. Surg. 2017, 43, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Management of Advanced and High-Stage Tumors. J. Am. Acad. Dermatol. 2018, 78, 249–261. [Google Scholar] [CrossRef]
- Fox, M.; Brown, M.; Golda, N.; Goldberg, D.; Miller, C.; Pugliano-Mauro, M.; Schmults, C.; Shin, T.; Stasko, T.; Xu, Y.G.; et al. Nodal Staging of High-Risk Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2019, 81, 548–557. [Google Scholar] [CrossRef]
- Tokez, S.; Koekelkoren, F.H.J.; Baatenburg De Jong, R.J.; Grünhagen, D.J.; Mooyaart, A.L.; Nijsten, T.; Van Der Lugt, A.; Wakkee, M. Assessment of the Diagnostic Accuracy of Baseline Clinical Examination and Ultrasonographic Imaging for the Detection of Lymph Node Metastasis in Patients with High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Dermatol. 2022, 158, 151–159. [Google Scholar] [CrossRef]
- Veness, M.J.; Morgan, G.J.; Palme, C.E.; Gebski, V. Surgery and Adjuvant Radiotherapy in Patients with Cutaneous Head and Neck Squamous Cell Carcinoma Metastatic to Lymph Nodes: Combined Treatment Should Be Considered Best Practice. Laryngoscope 2005, 115, 870–875. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Clark, J.R.; Lorincz, B.B.; Milross, C.G.; Veness, M.J. Metastatic Head and Neck Cutaneous Squamous Cell Carcinoma: Defining a Low-Risk Patient. Head Neck 2012, 34, 365–370. [Google Scholar] [CrossRef]
- Eggermont, C.; Nené, L.E.H.; Koekelkoren, F.H.J.; van der Toorn, Y.R.; Snetselaar, L.D.; Kroah-Hartman, M.; Genders, R.E.; Kelleners-Smeets, N.W.J.; Hollestein, L.M.; van Kester, M.S.; et al. The Impact of Routine Ultrasonography on Nodal Metastasis in Head and Neck Cutaneous Squamous Cell Carcinoma: A Retrospective Multicentre Cohort Study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1136–e1140. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Karia, P.S.; Morgan, F.C.; Schmults, C.D. The Positive Impact of Radiologic Imaging on High-Stage Cutaneous Squamous Cell Carcinoma Management. J. Am. Acad. Dermatol. 2017, 76, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.M.; Schmults, C.D.; Murad, F.; Karia, P.S.; Benson, C.B.; Ruiz, E.S. Detection of Subclinical Disease with Baseline and Surveillance Imaging in High-Risk Cutaneous Squamous Cell Carcinomas. J. Am. Acad. Dermatol. 2020, 82, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Gibson, F.T.; Murad, F.; Granger, E.; Schmults, C.D.; Ruiz, E.S. Perioperative Imaging for High-Stage Cutaneous Squamous Cell Carcinoma Helps Guide Management in Nearly a Third of Cases: A Single-Institution Retrospective Cohort. J. Am. Acad. Dermatol. 2023, 88, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, T.R.; Shah, K.; Wysong, A.; Lexa, F.; MacFarlane, D. The Role of Imaging in the Management of Patients with Nonmelanoma Skin Cancer: When Is Imaging Necessary? J. Am. Acad. Dermatol. 2017, 76, 591–607. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.; Shah, K.; Wysong, A.; Wortsman, X.; Humphreys, T.R. The Role of Imaging in the Management of Patients with Nonmelanoma Skin Cancer: Diagnostic Modalities and Applications. J. Am. Acad. Dermatol. 2017, 76, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.S.; Mancuso, A.A.; Mendenhall, W.M. Perineural Spread of Cutaneous Squamous and Basal Cell Carcinoma: CT and MR Detection and Its Impact on Patient Management and Prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1061–1069. [Google Scholar] [CrossRef]
- Stefanovic, N.; Fitzmaurice, C.J.; Ormond, P.; Irvine, A.D.; Barry, R.B. Risk Factors for Distant Metastasis in Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2022, 187, 435–436. [Google Scholar] [CrossRef]

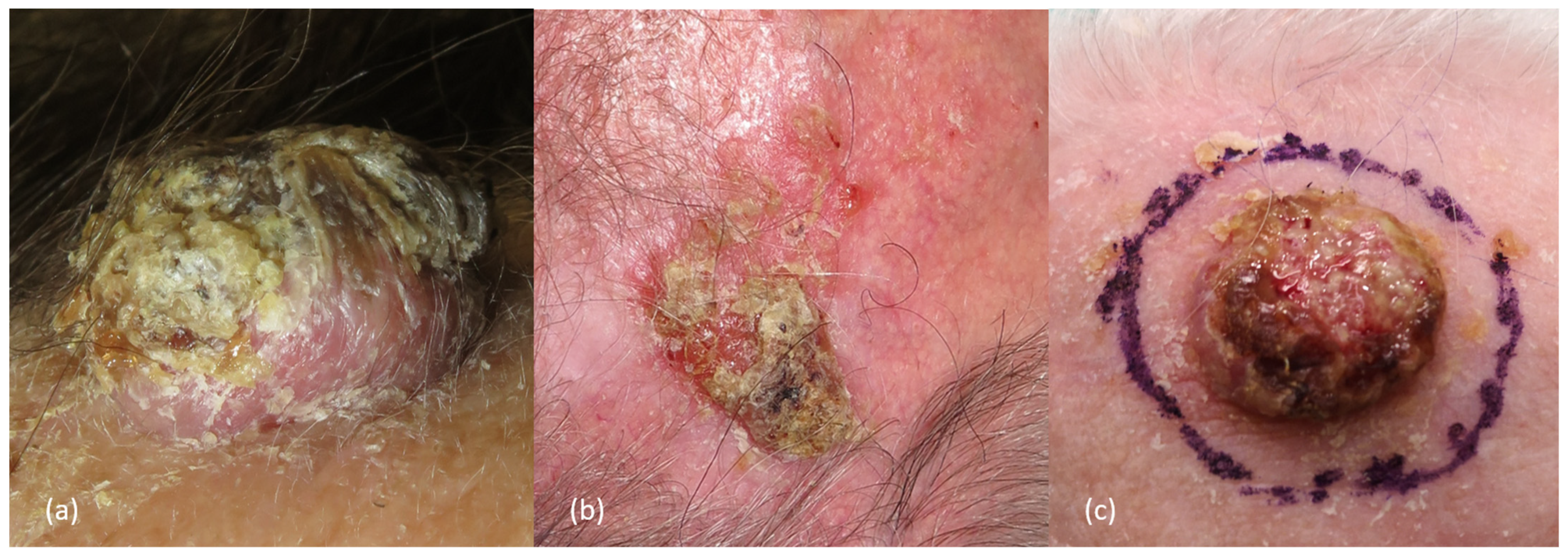



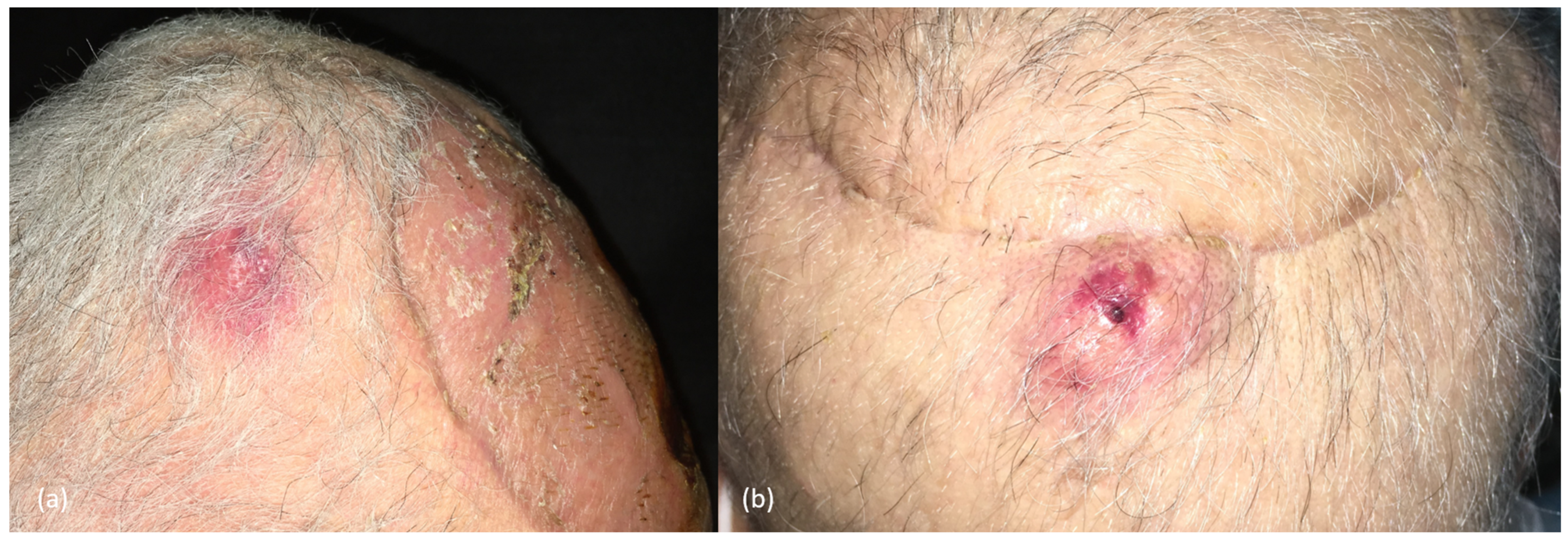

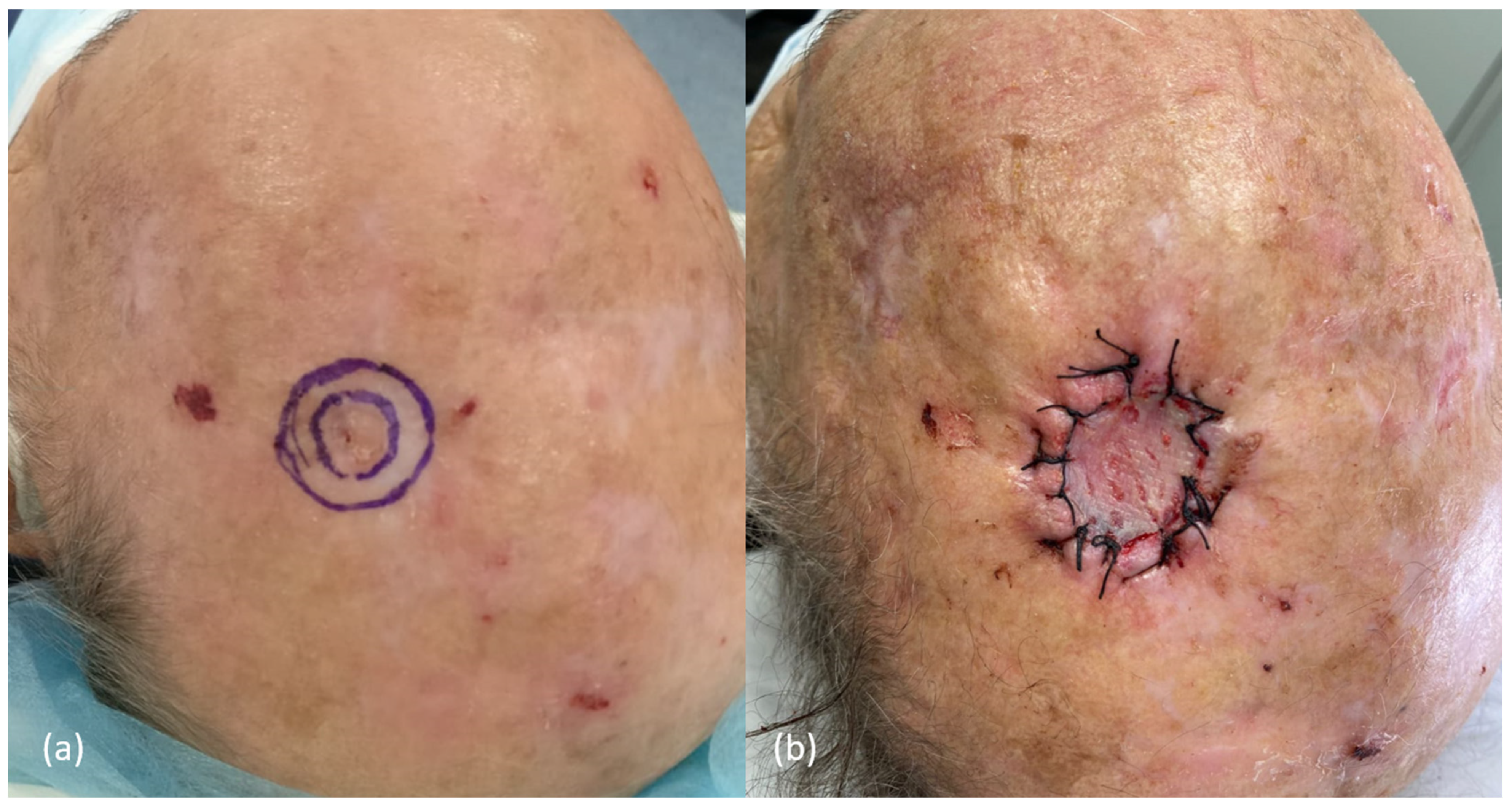


| Factors | Low-Risk | High-Risk | References |
|---|---|---|---|
| Clinical | [2,7,9,14,15,18,21,22,23,39] | ||
| Immune status | Immunocompetent | Immunosuppressed | |
| Primary vs. recurrent | Primary | Recurrent, metastatic | |
| Site of prior radiation therapy | No | Yes | |
| Site of chronic inflammation | No | Yes | |
| Rate of growth | Slow | Rapid | |
| Tumor dimensions (including peripheral rim of erythema) |
|
| |
| Tumor circumscription | Well-defined borders | Poorly defined borders | |
| Neurologic symptoms | Absent | Present | |
| Pathologic | [2,7,9,14,15,16,21,22,23,24,32,39,42,43,48,49,50] | ||
| Tumor dimensions | Size/diameter: <2 cm | Size/diameter: >2 cm | |
| Histologic grade | Well or moderately differentiated (G1-2) | Poorly differentiated (G3) | |
| Histologic type/Growth pattern | Subtype not otherwise specified | Acantholytic (adenoid), adenosquamous (or mucin-producing), desmoplastic, spindled, metaplastic/sarcomatoid | |
| Perineural invasion | Absent | Present, diameter of involved nerve ≥0.1 mm, multifocality, involvement of deep dermal nerves, or named nerves | |
| Lymphovascular invasion | Absent’ | Present | |
| Tumor depth |
|
| |
| Extension into osseus structures | Absent | Present | |
| Lymph node metastasis | Absent | Present, size of metastasis >3.0 cm, presence of extranidal extension, involvement of contralateral lymph nodes | |
| Positive margins | Absent | Present | |
| Tumor budding * | Grade 1: 0 to 4 buds | Grade 2: 5 to 9 buds, Grade 3: ≥10 buds | |
| Deep histological margin ** |
|
|
| Surgical Modalities | Recurrence Rates | Surgical Margins Recommendations | References | |
|---|---|---|---|---|
| Curettage and electrodesiccation | Not recommended. | [7,10] | ||
| Wide local excision |
| Peripheral surgical margins |
| [7,32,33,35,39,51] |
| Deep surgical plane recommended |
| [35,39,51,52] | ||
| Mohs Micrographic Surgery |
| First stage of MMS should include the subcutaneous tissue and run into the subgaleal plane. | [53,54,55,56,57,58,59] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdaguer-Faja, J.; Toll, A.; Boada, A.; Guerra-Amor, Á.; Ferrándiz-Pulido, C.; Jaka, A. Management of Cutaneous Squamous Cell Carcinoma of the Scalp: The Role of Imaging and Therapeutic Approaches. Cancers 2024, 16, 664. https://doi.org/10.3390/cancers16030664
Verdaguer-Faja J, Toll A, Boada A, Guerra-Amor Á, Ferrándiz-Pulido C, Jaka A. Management of Cutaneous Squamous Cell Carcinoma of the Scalp: The Role of Imaging and Therapeutic Approaches. Cancers. 2024; 16(3):664. https://doi.org/10.3390/cancers16030664
Chicago/Turabian StyleVerdaguer-Faja, Júlia, Agustí Toll, Aram Boada, Álvaro Guerra-Amor, Carla Ferrándiz-Pulido, and Ane Jaka. 2024. "Management of Cutaneous Squamous Cell Carcinoma of the Scalp: The Role of Imaging and Therapeutic Approaches" Cancers 16, no. 3: 664. https://doi.org/10.3390/cancers16030664
APA StyleVerdaguer-Faja, J., Toll, A., Boada, A., Guerra-Amor, Á., Ferrándiz-Pulido, C., & Jaka, A. (2024). Management of Cutaneous Squamous Cell Carcinoma of the Scalp: The Role of Imaging and Therapeutic Approaches. Cancers, 16(3), 664. https://doi.org/10.3390/cancers16030664






