Macrophages Promote Subtype Conversion and Endocrine Resistance in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Human Samples
2.2. Cell Lines and Culture
2.3. Western Blotting
2.4. RNA Isolation and RT-qPCR
2.5. Immunohistochemistry Staining
2.6. Mice and Tumor Models
2.7. Macrophage Polarization
2.8. Cell Viability Assay
2.9. Apoptosis Assay
2.10. Statistical Analysis
2.11. Data Availability
3. Results
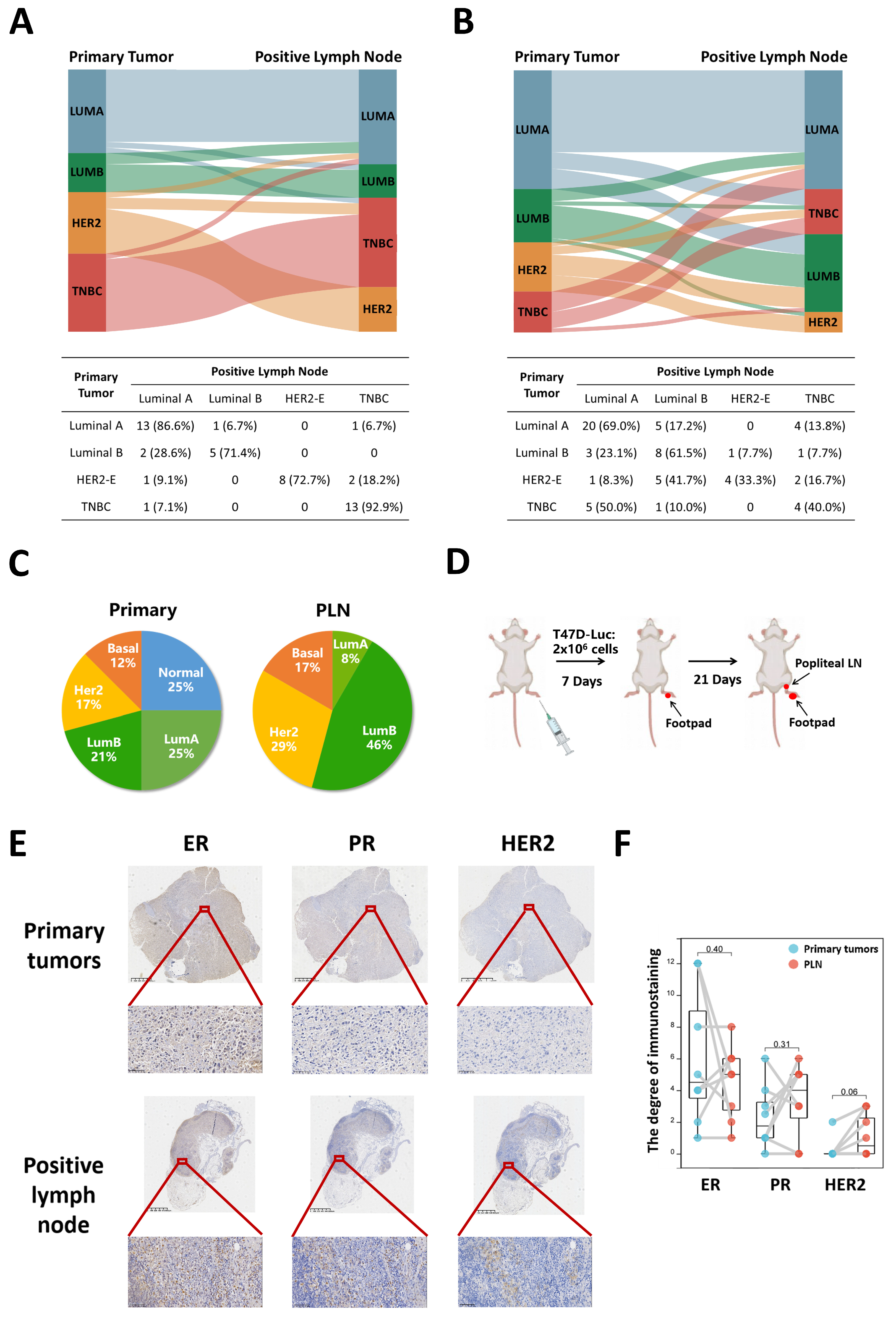
3.1. Subtype Concordance between Primary Tumors and PLN
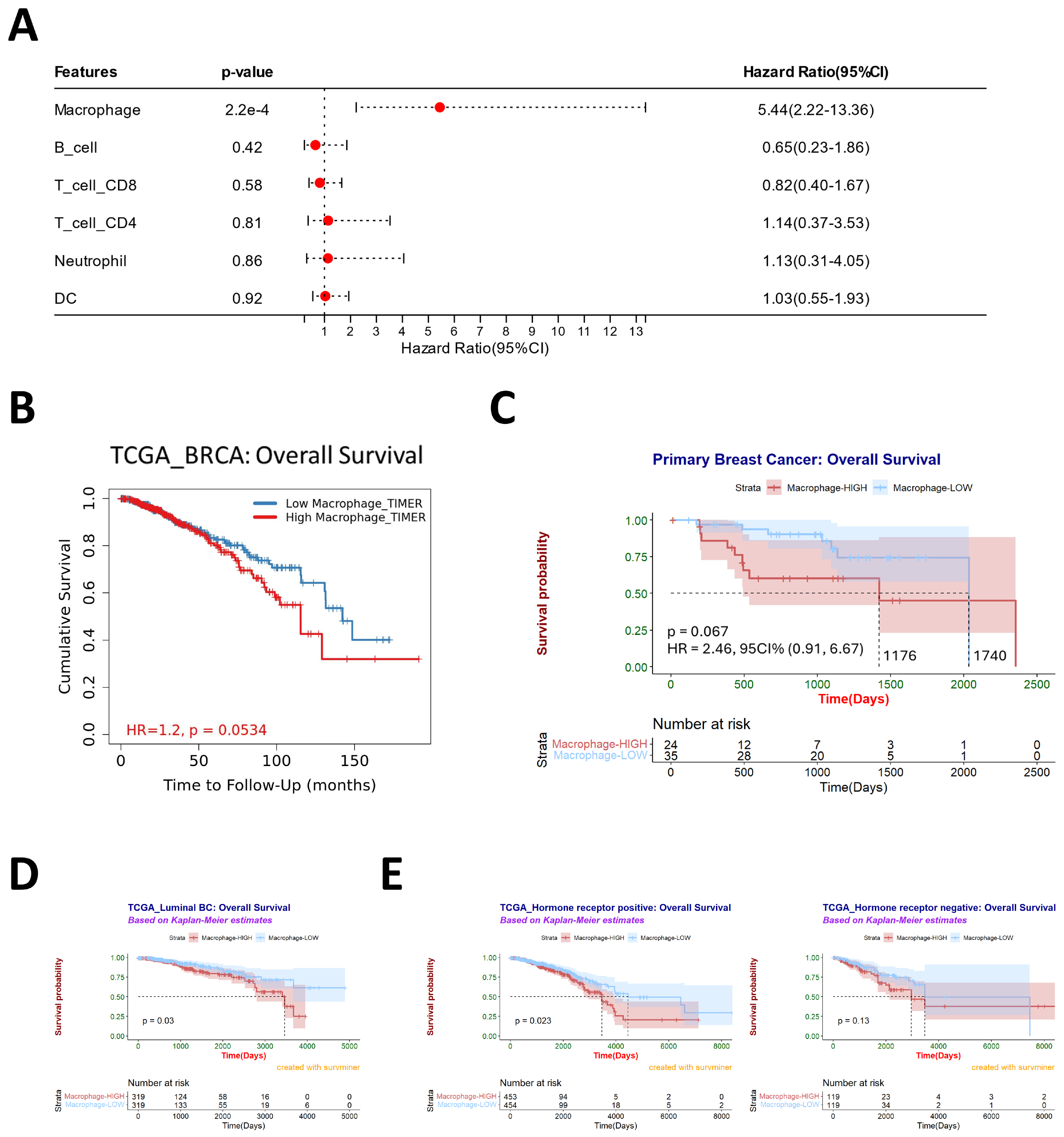
3.2. Macrophages Are Associated with Poor Prognosis and May Contribute to Subtype Conversation
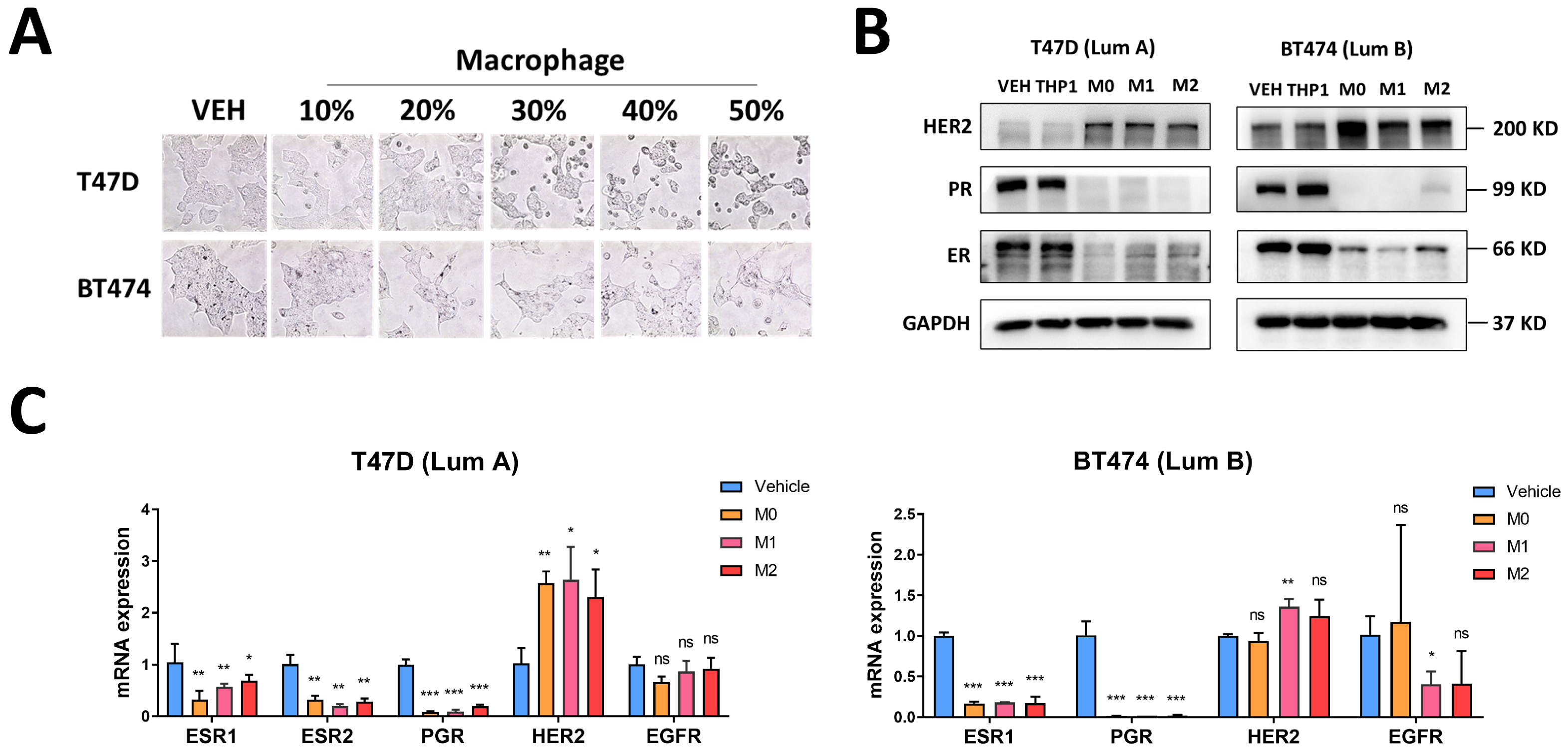
3.3. Macrophages Induce Changes in Luminal Breast Cancer Hormone Receptors and HER2
3.4. Macrophages Induce Tamoxifen Resistance in Breast Cancer
3.5. Macrophages May Regulate the Expression of Hormone Receptors and HER2 through the MNX1 Transcription Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HT | 4-Hydroxytamoxifen |
| BC | Breast cancer |
| ER | Estrogen receptor |
| FBS | Fetal bovine serum |
| HER2-E | HER2-enriched |
| HR | Hormone receptor |
| IHC | Immunohistochemistry |
| PLN | Positive lymph node |
| PMA | Phorbol 12-myristate 13-acetate |
| PR | Progesterone receptor |
| TAM | Tumor-associated macrophage |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Cejalvo, J.M.; Martínez de Dueñas, E.; Galván, P.; García-Recio, S.; Burgués Gasión, O.; Paré, L.; Antolín, S.; Martinello, R.; Blancas, I.; Adamo, B.; et al. Intrinsic Subtypes and Gene Expression Profiles in Primary and Metastatic Breast Cancer. Cancer Res. 2017, 77, 2213–2221. [Google Scholar] [CrossRef]
- Haughian, J.M.; Pinto, M.P.; Harrell, J.C.; Bliesner, B.S.; Joensuu, K.M.; Dye, W.W.; Sartorius, C.A.; Tan, A.C.; Heikkilä, P.; Perou, C.M.; et al. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc. Natl. Acad. Sci. USA 2012, 109, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Karaayvaz, M.; Cristea, S.; Gillespie, S.M.; Patel, A.P.; Mylvaganam, R.; Luo, C.C.; Specht, M.C.; Bernstein, B.E.; Michor, F.; Ellisen, L.W. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 2018, 9, 3588. [Google Scholar] [CrossRef]
- Pereira, B.; Chin, S.F.; Rueda, O.M.; Vollan, H.K.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.J.; et al. The somatic mutation profiles of 2433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Cao, Y.; Zou, Z.; Xia, J.; Zhao, J. An enzyme-powered microRNA discriminator for the subtype-specific diagnosis of breast cancer. Chem. Sci. 2023, 14, 2097–2106. [Google Scholar] [CrossRef]
- Onkar, S.S.; Carleton, N.M.; Lucas, P.C.; Bruno, T.C.; Lee, A.V.; Vignali, D.A.A.; Oesterreich, S. The Great Immune Escape: Understanding the Divergent Immune Response in Breast Cancer Subtypes. Cancer Discov. 2023, 13, 23–40. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 316. [Google Scholar] [CrossRef]
- Joensuu, H.; Kellokumpu-Lehtinen, P.L.; Huovinen, R.; Jukkola-Vuorinen, A.; Tanner, M.; Kokko, R.; Ahlgren, J.; Auvinen, P.; Lahdenperä, O.; Kosonen, S.; et al. Adjuvant Capecitabine in Combination With Docetaxel, Epirubicin, and Cyclophosphamide for Early Breast Cancer: The Randomized Clinical FinXX Trial. JAMA Oncol. 2017, 3, 793–800. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 1809–1815. [Google Scholar] [CrossRef]
- Roy, M.; Fowler, A.M.; Ulaner, G.A.; Mahajan, A. Molecular Classification of Breast Cancer. PET Clin. 2023, 18, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Lluch, A.; González-Angulo, A.M.; Casadevall, D.; Eterovic, A.K.; Martínez de Dueñas, E.; Zheng, X.; Guerrero-Zotano, Á.; Liu, S.; Pérez, R.; Chen, K.; et al. Dynamic clonal remodelling in breast cancer metastases is associated with subtype conversion. Eur. J. Cancer 2019, 120, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, W.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef]
- Krøigård, A.B.; Larsen, M.J.; Thomassen, M.; Kruse, T.A. Molecular Concordance Between Primary Breast Cancer and Matched Metastases. Breast J. 2016, 22, 420–430. [Google Scholar] [CrossRef]
- Aurilio, G.; Disalvatore, D.; Pruneri, G.; Bagnardi, V.; Viale, G.; Curigliano, G.; Adamoli, L.; Munzone, E.; Sciandivasci, A.; De Vita, F.; et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 2014, 50, 277–289. [Google Scholar] [CrossRef]
- de Dueñas, E.M.; Hernández, A.L.; Zotano, A.G.; Carrión, R.M.; López-Muñiz, J.I.; Novoa, S.A.; Rodríguez, A.L.; Fidalgo, J.A.; Lozano, J.F.; Gasión, O.B.; et al. Prospective evaluation of the conversion rate in the receptor status between primary breast cancer and metastasis: Results from the GEICAM 2009-03 ConvertHER study. Breast Cancer Res. Treat. 2014, 143, 507–515. [Google Scholar] [CrossRef]
- Turner, N.H.; Di Leo, A. HER2 discordance between primary and metastatic breast cancer: Assessing the clinical impact. Cancer Treat. Rev. 2013, 39, 947–957. [Google Scholar] [CrossRef]
- Houssami, N.; Macaskill, P.; Balleine, R.L.; Bilous, M.; Pegram, M.D. HER2 discordance between primary breast cancer and its paired metastasis: Tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res. Treat. 2011, 129, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Monfardini, L.; Rizzo, S.; Sciandivasci, A.; Preda, L.; Bagnardi, V.; Disalvatore, D.; Pruneri, G.; Munzone, E.; Della Vigna, P.; et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. 2013, 52, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Priedigkeit, N.; Hartmaier, R.J.; Chen, Y.; Vareslija, D.; Basudan, A.; Watters, R.J.; Thomas, R.; Leone, J.P.; Lucas, P.C.; Bhargava, R.; et al. Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol. 2017, 3, 666–671. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Thennavan, A.; East, M.P.; Parker, J.S.; Cejalvo, J.M.; Garay, J.P.; Hollern, D.P.; He, X.; Mott, K.R.; Galván, P.; et al. FGFR4 regulates tumor subtype differentiation in luminal breast cancer and metastatic disease. J. Clin. Investig. 2020, 130, 4871–4887. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Stossi, F.; Madak-Erdoğan, Z.; Katzenellenbogen, B.S. Macrophage-elicited loss of estrogen receptor-α in breast cancer cells via involvement of MAPK and c-Jun at the ESR1 genomic locus. Oncogene 2012, 31, 1825–1834. [Google Scholar] [CrossRef]
- Gunnarsdóttir, F.B.; Hagerling, C.; Bergenfelz, C.; Mehmeti, M.; Källberg, E.; Allaoui, R.; Mohlin, S.; Påhlman, S.; Larsson, C.; Jirström, K.; et al. Inflammatory macrophage derived TNFα downregulates estrogen receptor α via FOXO3a inactivation in human breast cancer cells. Exp. Cell Res. 2020, 390, 111932. [Google Scholar] [CrossRef]
- Castellaro, A.M.; Rodriguez-Baili, M.C.; Di Tada, C.E.; Gil, G.A. Tumor-Associated Macrophages Induce Endocrine Therapy Resistance in ER+ Breast Cancer Cells. Cancers 2019, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Q.J.; Wang, J.X.; Nanding, A.; Wang, Z.P.; Liu, H.; Lian, X.; Zhang, Q.Y. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol. Oncol. Res. POR 2014, 20, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos Gil, E.M. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat. Rev. 2014, 40, 862–871. [Google Scholar] [CrossRef]
- Skolariki, A.; D’Costa, J.; Little, M.; Lord, S. Role of PI3K/Akt/mTOR pathway in mediating endocrine resistance: Concept to clinic. Explor. Target. Anti-Tumor Ther. 2022, 3, 172–199. [Google Scholar] [CrossRef]
- Niu, X.; Ma, J.; Li, J.; Gu, Y.; Yin, L.; Wang, Y.; Zhou, X.; Wang, J.; Ji, H.; Zhang, Q. Sodium/glucose cotransporter 1-dependent metabolic alterations induce tamoxifen resistance in breast cancer by promoting macrophage M2 polarization. Cell Death Dis. 2021, 12, 509. [Google Scholar] [CrossRef]
- Qin, Q.; Ji, H.; Li, D.; Zhang, H.; Zhang, Z.; Zhang, Q. Tumor-associated macrophages increase COX-2 expression promoting endocrine resistance in breast cancer via the PI3K/Akt/mTOR pathway. Neoplasma 2021, 68, 938–946. [Google Scholar] [CrossRef]
- Li, D.; Ji, H.; Niu, X.; Yin, L.; Wang, Y.; Gu, Y.; Wang, J.; Zhou, X.; Zhang, H.; Zhang, Q. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020, 111, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cascinelli, N.; Morabito, A.; Santinami, M.; MacKie, R.M.; Belli, F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: A randomised trial. WHO Melanoma Programme. Lancet 1998, 351, 793–796. [Google Scholar] [CrossRef]
- de Boer, M.; van Deurzen, C.H.; van Dijck, J.A.; Borm, G.F.; van Diest, P.J.; Adang, E.M.; Nortier, J.W.; Rutgers, E.J.; Seynaeve, C.; Menke-Pluymers, M.B.; et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N. Engl. J. Med. 2009, 361, 653–663. [Google Scholar] [CrossRef]
- Ulmer, A.; Dietz, K.; Hodak, I.; Polzer, B.; Scheitler, S.; Yildiz, M.; Czyz, Z.; Lehnert, P.; Fehm, T.; Hafner, C.; et al. Quantitative measurement of melanoma spread in sentinel lymph nodes and survival. PLoS Med. 2014, 11, e1001604. [Google Scholar] [CrossRef]
- Xie, J.; Xia, L.; Xiang, W.; He, W.; Yin, H.; Wang, F.; Gao, T.; Qi, W.; Yang, Z.; Yang, X.; et al. Metformin selectively inhibits metastatic colorectal cancer with the KRAS mutation by intracellular accumulation through silencing MATE1. Proc. Natl. Acad. Sci. USA 2020, 117, 13012–13022. [Google Scholar] [CrossRef]
- Morita, Y.; Zhang, R.; Leslie, M.; Adhikari, S.; Hasan, N.; Chervoneva, I.; Rui, H.; Tanaka, T. Pathologic evaluation of tumor-associated macrophage density and vessel inflammation in invasive breast carcinomas. Oncol. Lett. 2017, 14, 2111–2118. [Google Scholar] [CrossRef]
- Lee, C.K.; Jeong, S.H.; Jang, C.; Bae, H.; Kim, Y.H.; Park, I.; Kim, S.K.; Koh, G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019, 363, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta 2022, 1, e36. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Van Poznak, C.; Somerfield, M.R.; Bast, R.C.; Cristofanilli, M.; Goetz, M.P.; Gonzalez-Angulo, A.M.; Hicks, D.G.; Hill, E.G.; Liu, M.C.; Lucas, W.; et al. Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Lower, E.E.; Glass, E.; Blau, R.; Harman, S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res. Treat. 2009, 113, 301–306. [Google Scholar] [CrossRef]
- Pusztai, L.; Viale, G.; Kelly, C.M.; Hudis, C.A. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. The oncologist 2010, 15, 1164–1168. [Google Scholar] [CrossRef]
- Gulati, G.S.; Sikandar, S.S.; Wesche, D.J.; Manjunath, A.; Bharadwaj, A.; Berger, M.J.; Ilagan, F.; Kuo, A.H.; Hsieh, R.W.; Cai, S.; et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 2020, 367, 405–411. [Google Scholar] [CrossRef]
- Idirisinghe, P.K.; Thike, A.A.; Cheok, P.Y.; Tse, G.M.; Lui, P.C.; Fook-Chong, S.; Wong, N.S.; Tan, P.H. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am. J. Clin. Pathol. 2010, 133, 416–429. [Google Scholar] [CrossRef]
- Jensen, J.D.; Knoop, A.; Ewertz, M.; Laenkholm, A.V. ER, HER2, and TOP2A expression in primary tumor, synchronous axillary nodes, and asynchronous metastases in breast cancer. Breast Cancer Res. Treat. 2012, 132, 511–521. [Google Scholar] [CrossRef]
- Nakamura, R.; Yamamoto, N.; Onai, Y.; Watanabe, Y.; Kawana, H.; Miyazaki, M. Importance of confirming HER2 overexpression of recurrence lesion in breast cancer patients. Breast Cancer 2013, 20, 336–341. [Google Scholar] [CrossRef]
- Bogina, G.; Bortesi, L.; Marconi, M.; Venturini, M.; Lunardi, G.; Coati, F.; Massocco, A.; Manfrin, E.; Pegoraro, C.; Zamboni, G. Comparison of hormonal receptor and HER-2 status between breast primary tumours and relapsing tumours: Clinical implications of progesterone receptor loss. Virchows Arch. An. Int. J. Pathol. 2011, 459, 1–10. [Google Scholar] [CrossRef]
- Thompson, A.M.; Jordan, L.B.; Quinlan, P.; Anderson, E.; Skene, A.; Dewar, J.A.; Purdie, C.A. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: The Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. BCR 2010, 12, R92. [Google Scholar] [CrossRef]
- Fabi, A.; Di Benedetto, A.; Metro, G.; Perracchio, L.; Nisticò, C.; Di Filippo, F.; Ercolani, C.; Ferretti, G.; Melucci, E.; Buglioni, S.; et al. HER2 protein and gene variation between primary and metastatic breast cancer: Significance and impact on patient care. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2055–2064. [Google Scholar] [CrossRef]
- Bi, M.; Zhang, Z.; Jiang, Y.Z.; Xue, P.; Wang, H.; Lai, Z.; Fu, X.; De Angelis, C.; Gong, Y.; Gao, Z.; et al. Enhancer reprogramming driven by high-order assemblies of transcription factors promotes phenotypic plasticity and breast cancer endocrine resistance. Nat. Cell Biol. 2020, 22, 701–715. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Kabos, P.; Haughian, J.M.; Wang, X.; Dye, W.W.; Finlayson, C.; Elias, A.; Horwitz, K.B.; Sartorius, C.A. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res. Treat. 2011, 128, 45–55. [Google Scholar] [CrossRef]
- Chung, W.; Eum, H.H.; Lee, H.O.; Lee, K.M.; Lee, H.B.; Kim, K.T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8, 15081. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.Y.; Black, A.; Qian, B.Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef] [PubMed]
- von Bergh, A.R.; van Drunen, E.; van Wering, E.R.; van Zutven, L.J.; Hainmann, I.; Lönnerholm, G.; Meijerink, J.P.; Pieters, R.; Beverloo, H.B. High incidence of t(7;12)(q36;p13) in infant AML but not in infant ALL, with a dismal outcome and ectopic expression of HLXB9. Genes Chromosom. Cancer 2006, 45, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Das, M. MNX1: A novel prostate cancer oncogene. Lancet. Oncol. 2016, 17, e521. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, Y.; Zhang, Y.; Castro, P.; Shao, L.; Sreekumar, A.; Putluri, N.; Guha, N.; Deepak, S.; et al. MNX1 Is Oncogenically Upregulated in African-American Prostate Cancer. Cancer Res. 2016, 76, 6290–6298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wu, Y.; Luo, J.; Zhang, Q.; Huang, J.; Li, Q.; Xu, L.; Lu, E.; Ren, B. MNX1 Promotes Malignant Progression of Cervical Cancer via Repressing the Transcription of p21(cip1). Front. Oncol. 2020, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, M.; Zhu, Y.; Zhu, W.; Yang, T.; Li, H.; Lin, S.; Dai, C.; Deng, Y.; Song, D.; et al. Expression, Clinical Significance, and Functional Prediction of MNX1 in Breast Cancer. Mol. Therapy. Nucleic Acids 2018, 13, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pan, Q.; Lu, Y.; Jiang, X.; Zhang, S.; Wu, J. MNX1 promotes cell proliferation and activates Wnt/β-catenin signaling in colorectal cancer. Cell Biol. Int. 2019, 43, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Yang, S.X.; Polley, E.; Lipkowitz, S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat. Rev. 2016, 45, 87–96. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Q.; Zhang, F.; Meng, F.; Liu, S.; Zhou, R.; Wu, Q.; Li, X.; Shen, L.; Huang, J.; et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat. Cell Biol. 2019, 21, 1027–1040. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, F.; Huang, Z.; Liu, X.; Xia, G.; Huang, J.; Yang, Y.; Li, J.; Huang, J.; Liu, Y.; et al. Macrophages Promote Subtype Conversion and Endocrine Resistance in Breast Cancer. Cancers 2024, 16, 678. https://doi.org/10.3390/cancers16030678
Zhang X, Yang F, Huang Z, Liu X, Xia G, Huang J, Yang Y, Li J, Huang J, Liu Y, et al. Macrophages Promote Subtype Conversion and Endocrine Resistance in Breast Cancer. Cancers. 2024; 16(3):678. https://doi.org/10.3390/cancers16030678
Chicago/Turabian StyleZhang, Xiaoyan, Fengyu Yang, Zhijian Huang, Xiaojun Liu, Gan Xia, Jieye Huang, Yang Yang, Junchen Li, Jin Huang, Yuxin Liu, and et al. 2024. "Macrophages Promote Subtype Conversion and Endocrine Resistance in Breast Cancer" Cancers 16, no. 3: 678. https://doi.org/10.3390/cancers16030678
APA StyleZhang, X., Yang, F., Huang, Z., Liu, X., Xia, G., Huang, J., Yang, Y., Li, J., Huang, J., Liu, Y., Zhou, T., Qi, W., Gao, G., & Yang, X. (2024). Macrophages Promote Subtype Conversion and Endocrine Resistance in Breast Cancer. Cancers, 16(3), 678. https://doi.org/10.3390/cancers16030678




