Hepatoblastoma Relapse—Findings from the German HB99 Trial and the German Liver Tumor Registry
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Relapse Rate and Initial Characteristics and Treatment of Relapse Patients
3.2. Details of Relapse
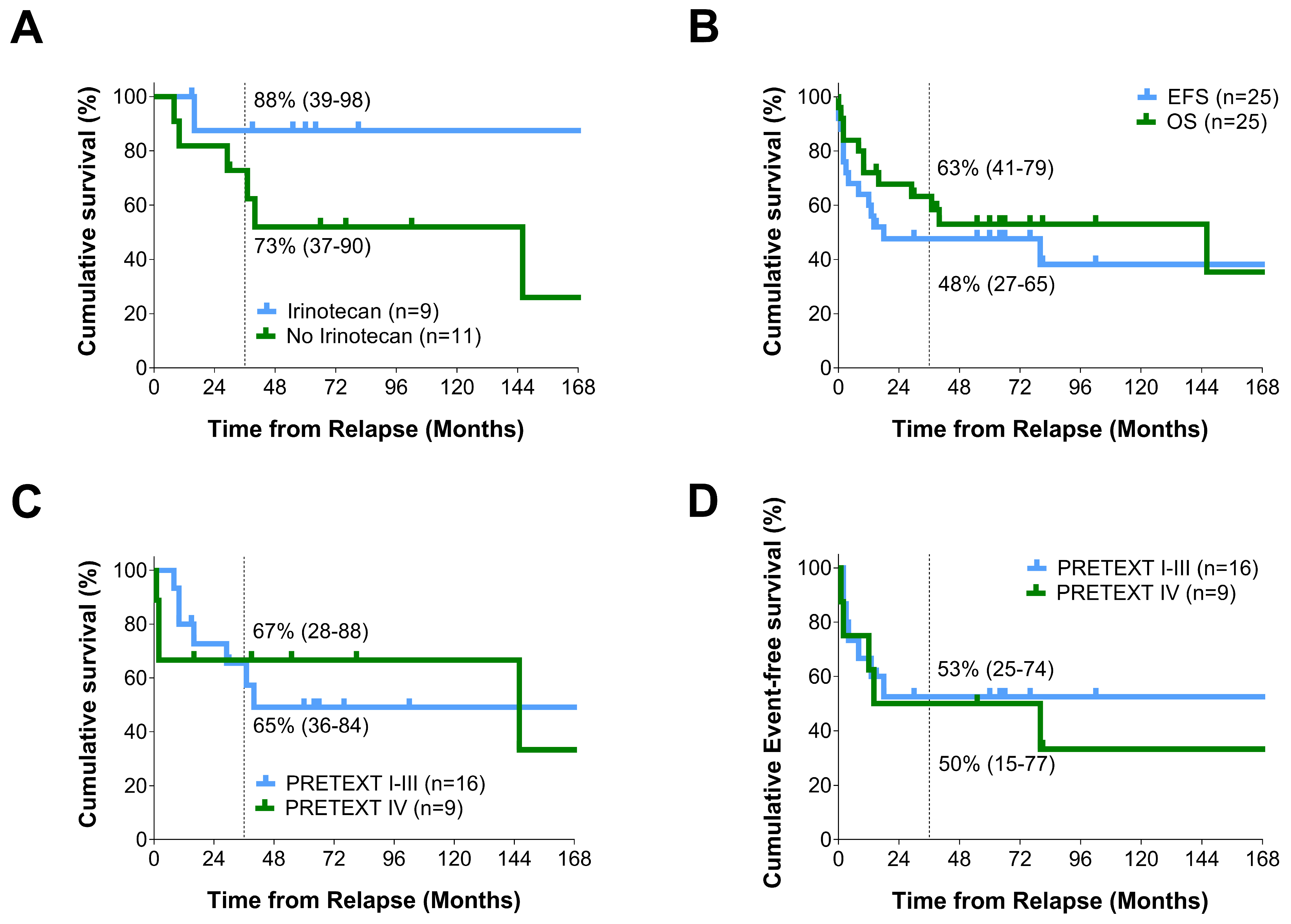
3.3. Outcome after Relapse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hafberg, E.; Borinstein, S.C.; Alexopoulos, S.P. Contemporary management of hepatoblastoma. Curr. Opin. Organ. Transplant. 2019, 24, 113–117. [Google Scholar] [CrossRef]
- Stocker, J.T. Hepatic Tumors in Children. Clin. Liver Dis. 2001, 5, 259–281, viii–ix. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, P.; Lopez-Terrada, D.; Hiyama, E.; Häberle, B.; Malogolowkin, M.H.; Meyers, R.L. Hepatoblastoma state of the art: Pathology, genetics, risk stratification, and chemotherapy. Curr. Opin. Pediatr. 2014, 26, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Kaatsch, P.; Grabow, D.; Spix, C. German Childhood Cancer Registry—Annual Report 2019 (1980–2018); Institute of Medical Biostatistics, Epidemio logy and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz: Mainz, Germany, 2020. [Google Scholar]
- Häberle, B.; Maxwell, R.; von Schweinitz, D.; Schmid, I. High Dose Chemotherapy with Autologous Stem Cell Transplantation in Hepatoblastoma does not Improve Outcome. Results of the GPOH Study HB99. Klin. Padiatr. 2019, 231, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.; Branchereau, S.; Maibach, R.; Zsiros, J.; Casanova, M.; Brock, P.; Domerg, C.; Aronson, D.C.; Zimmermann, A.; Laithier, V.; et al. Relapses in hepatoblastoma patients: Clinical characteristics and outcome—Experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur. J. Cancer 2013, 49, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.Y.; Yeh, T.C.; Huang, T.H.; Sheu, J.C.; Liu, H.C. A retrospective study of clinical features and outcome in patients with refractory or recurrent hepatoblastoma: A single institution experience. Pediatr. Neonatol. 2021, 62, 400–405. [Google Scholar] [CrossRef]
- Trobaugh-Lotrario, A.D.; Meyers, R.L.; Feusner, J.H. Outcomes of Patients with Relapse Hepatoblastoma Enrolled on Children’s Oncology Group (COG) Phase I and II Studies. J. Pediatr. Hematol. Oncol. 2016, 38, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Somers, K.M.; Tabbouche, R.B.; Bondoc, A.; Towbin, A.J.; Ranganathan, S.; Tiao, G.; Geller, J. Retreatment with Cisplatin May Provide a Survival Advantage for Children with Relapsed/Refractory Hepatoblastoma: An Institutional Experience. Cancers 2023, 15, 3921. [Google Scholar] [CrossRef]
- Roebuck, D.J.; Olsen, O.; Pariente, D. Radiological staging in children with hepatoblastoma. Pediatr. Radiol. 2006, 36, 176–182. [Google Scholar] [CrossRef]
- Perilongo, G.; Maibach, R.; Shafford, E.; Brugieres, L.; Brock, P.; Morland, B.; de Camargo, B.; Zsiros, J.; Roebuck, D.; Zimmermann, A.; et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N. Engl. J. Med. 2009, 361, 1662–1670. [Google Scholar] [CrossRef]
- Zsiros, J.; Maibach, R.; Shafford, E.; Brugieres, L.; Brock, P.; Czauderna, P.; Childs, M.; Zimmermann, A.; Laithier, V.; Otte, J.B.; et al. Successful treatment of childhood high-risk hepatoblastoma with dose-Intensive multiagent chemotherapy and surgery: Final results of the SIOPEL-3HR Study. J. Clin. Oncol. 2010, 28, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Zsiros, J.; Brugieres, L.; Brock, P.; Roebuck, D.; Maibach, R.; Zimmermann, A.; Childs, M.; Pariente, D.; Laithier, V.; Otte, J.B.; et al. International Childhood Liver Tumors Strategy Group (SIOPEL) et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): A prospective, single-arm, feasibility study. Lancet Oncol. 2013, 14, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete oberservations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Fahy, A.S.; Shaikh, F.; Gerstle, J.T. Multifocal hepatoblastoma: What is the risk of recurrent disease in the remnant liver? J. Pediatr. Surg. 2019, 54, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Furch, C.; Schmid, I.; von Schweinitz, D.; Häberle, B. Impact of postoperative complications on overall survival of patients with hepatoblastoma. Pediatr. Blood Cancer 2015, 62, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Von Schweinitz, D.; Faundez, A.; Teichmann, B.; Birnbaum, T.; Koch, A.; Hecker, H.; Glüer, S.; Fuchs, J.; Pietsch, T. Hepatocyte growth-factor-scatter factor can stimulate post-operative tumor- cell proliferation in childhood hepatoblastoma. Int. J. Cancer 2000, 85, 151–159. [Google Scholar] [CrossRef]
- Rojas, Y.; Guillerman, R.P.; Zhang, W.; Vasudevan, S.A.; Nuchtern, J.G.; Thompson, P.A. Relapse surveillance in AFP-positive hepatoblastoma: Re-evaluating the role of imaging. Pediatr. Radiol. 2014, 44, 1275–1280. [Google Scholar] [CrossRef]
- Kawahara, I.; Fukuzawa, H.; Urushihara, N.; Kosaka, Y.; Kuroda, Y.; Fujieda, Y.; Takeuschi, Y.; Uemura, K.; Iwade, T.; Samejima, Y.; et al. AFP-L3 as a Prognostic Predictor of Recurrence in Hepatoblastoma: A Pilot Study. J. Pediatr. Hematol. Oncol. 2021, 43, e76–e79. [Google Scholar] [CrossRef]
- Venkatramani, R.; Furman, W.L.; Fuchs, J.; Warmann, S.W.; Malogolowkin, M.H. Current and future mangement strategies for relapsed or progressive hepatoblastoma. Pediatr. Drugs 2012, 14, 221–232. [Google Scholar] [CrossRef]
- Koh, K.N.; Park, M.; Kim, B.E.; Bae, K.W.; Kim, K.M.; Im, H.J.; Seo, J.J. Prognostic implications of serum alpha-fetoprotein response during treatment of hepatoblastoma. Pediatr. Blood Cancer 2011, 57, 554–560. [Google Scholar] [CrossRef]
- Nguyen, R.; McCarville, M.B.; Sykes, A.; Mao, S.; Wu, J.; Langham, M.R., Jr.; Furman, W.L. Rapid decrease of serum alpha-fetoprotein and tumor volume predicts outcome in children with hepatoblastoma treated with neoadjuvant chemotherapy. Int. J. Clin. Oncol. 2018, 23, 900–907. [Google Scholar] [CrossRef]
- Zsiros, J.; Brugieres, L.; Brock, P.; Roebuck, D.; Maibach, R.; Child, M.; Morland, B.; Casanova, M.; Pariente, D.; Paris, C.; et al. Efficacy of irinotecan single drug treatment in children with refractory or recurrent hepatoblastoma—A phase II trial of the childhood liver tumour strategy group (SIOPEL). Eur. J. Cancer 2012, 48, 3456–3464. [Google Scholar] [CrossRef]
- Trobaugh-Lotrario, A.; Feusner, J.H. Relapsed hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 813–817. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, L.; Zhong, X.; Wang, L.; Chang, J. Vincristine and irinotecan in children with relapsed hepatoblastoma: A single-Institution experience. Paediatr. Hematol. Oncol. 2015, 32, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.B.; Pritchard, J.; Aronson, D.C.; Brown, J.; Czauderna, P.; Maibach, R.; Perilongo, G.; Shafford, E.; Plaschkes, J. Liver Transplantation for Hepatoblastoma: Results from the International Society of Paediatric Oncology (SIOP) Study SIOPEL-1 and Review of World Experience. Pediatr. Blood Cancer 2004, 42, 74–83. [Google Scholar] [CrossRef] [PubMed]
- De Ville de Goyet, J.; Baumann, U.; Karam, V.; Adam, R.; Nadalin, S.; Heaton, N.; Reding, R.; Branchereau, S.; Mirza, D.; Klempnauer, J.L.; et al. European Liver Transplant Registry: Donor and transplant surgery aspects of 16,641 liver transplantations in children. Hepatology 2022, 75, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Vokuhl, C.; Oyen, F.; Häberle, B.; von Schweinitz, D.; Schneppenheim, R.; Leuschner, I. Small cell undifferentiated (SCUD) hepatoblastomas: All malignant rhabdoid tumors? Genes Chromosomes Cancer 2016, 55, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Terrada, D.; Alaggio, R.; de Davila, M.T.; Czauderna, P.; Hiyama, E.; Katzenstein, H.; Leuschner, I.; Malogolowkin, M.; Meyers, R.; Ranganathan, S.; et al. Towards an international pediatric liver tumor concensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Mod. Pathol. 2014, 27, 472–491. [Google Scholar] [CrossRef]
| All Relapses (25) (6L, 17M, 2C) | SIOPEL 1–3 Relapses [6] (59) (21L, 32M, 5C, 1MD) | All HB99 Patients (142) | All LTR Patients (Till 30 August 2019) (220) | All SIOPEL 1–3 Patients [6] (695) | |
|---|---|---|---|---|---|
| Median Age and Range (months) | 24 (3–158) | MD 84L, 21.5M, 9C | 16 | 18 (0–145) | 17.2 (0–185) |
| Gender (Male/Female) | 19/6 (3.2/1) | 42/17 (2.5/1) | 98/44 (2.2/1) | 127/93 (1.4/1) | 423/272 (1.6/1) |
| PRETEXT | MD 2 | MD 7 | |||
| I | 1 (4%) | 2 (3%) | 4 (3%) | 20 (9%) | 34 (5%) |
| II | 5 (20%) | 15 (26%) | 48 (34%) | 73 (33%) | 256 (37%) |
| III | 10 (40%) | 24 (41%) | 70 (49%) | 86 (39%) | 266 (38%) |
| IV | 9 (36%) | 17 (29%) | 20 (14%) | 39 (18%) | 132 (20%) |
| Risk Group | MD 2 | MD 12 | |||
| SR | 5 (20%) | 26 (45%) | 85 (60%) | 100 (45%) | 430 (62%) |
| HR | 20 (80%) | 32 (55%) | 57 (40%) | 118 (54%) | 253 (36%) |
| Annotations Factors | |||||
| M+ | 10 (40%) | 17 (29%) | 29 (21%) (MD 1) | 40 (18%) (MD 11) | 125 (18%) |
| V+ | 4 (16%) | 4 (7%) | 9 (6%) | 23 (10%) (MD 21) | NA |
| P+ | 6 (24%) | 7 (12%) | 12 (9%) | 28 (13%) (MD 13) | NA |
| E+ | 2 (8%) | NA | 5 (4%) (MD 7) | NA | NA |
| R+ | 3 (12%) | NA | 8 (7%) (MD 20) | NA | NA |
| F+ | 8 (32%) | 21 (36%) | 35 (25%) (MD 1) | 71 (32%) (MD 2) | 114 (16%) (MD 37) |
| AFP at Diagnosis (ng/mL) | |||||
| ≤100 | 2 (8%) | NA | 10 (7%) | NA | NA |
| 101 > 1 Mill | 23 (92%) | NA | 132 (93%) | NA | NA |
| Histology | NA | NA | |||
| Epithelial | 9 (36%) | 28 (47%) | 71 (50%) | ||
| Mixed | 7 (28%) | 12 (20%) | 48 (34%) | ||
| Macrotrabecular | 1 (4%) | 3 (5%) | - | ||
| HB (DD HCC) | 4 (16%) | - | - | ||
| SCUD | 2 a (8%) | 10 (17%) | 4 (3%) | ||
| MD | 2 (8%) | - | - |
| Pat. No. | Age (m) | PRETEXT | Histology | Chemo | Surgery | Time Dx to Relapse (m) |
|---|---|---|---|---|---|---|
| 1 (D) | 104 | III N+ | HB (DD HCC) | Carbo, Etop, Cis, Dox, Ifo | (1) Liver (R1) | 19 |
| 2 (D) | 36 | III M+ | HB | Carbo, Etop, Melp | (1) Liver (R0), (2) Lung (MD), (3) Lung (R0) | 33 |
| 3 (A) | 16 | III M+F+ | HB | Carbo, Etop | (1) Liver (R0) (+Lung—NAD) | 22 |
| 4 (D) | 4 | IV M+E+ | HB | Cis, Dox, Ifo, Carbo, Etop, Ir | (1) Liver (R0), (2) Lung (MD) | 7 |
| 5 (A) | 26 | III M+ | HB | Cis, Do, Ifo, Carbo, Etop | (1) Liver (R0), (2) Lung R + L (R0) | 19 |
| 6 (D) | 13 | IV M+ | HB | Cis, Dox, Ifo, Carbo, Etop | (1) Liver (R0) | 8 |
| 7 (D) | 3 | III R+ | SCUD (INI1 MD) | Cis, Dox, Ifo | (1) Liver (R1) | 9 |
| 8 (A) | 10 | IV R+F+ | HB | Carbo, Etop | (1) Liver (R0) | 7 |
| 9 (A) | 12 | II | HB | Cis, Dox, Ifo | (1) Liver (R0) | 21 |
| 10 (D) | 117 | II R+ | HB (DD HCC) | Cis, Dox, Ifo | (1) Liver (R0) | 45 |
| 11 (D) | 53 | IV M+P+ | HB (DD HCC) | Carbo, Etop | (1) Liver (R1), (2) Lung (R0), (3) Lung (R0) | 12 |
| 12 (A) | 158 | II P+ | HB (Macrotrab) | Cis, Dox, Ifo | (1) Liver (R0) | 21 |
| 13 (D) | 40 | III | HB | Cis | (1) Liver (R0) | 5 |
| 14 (A) | 18 | I M+E+ | HB | Cis, Carbo, Dox, Etop, Ifo, Vin, Ir | (1) Liver (R0), (2) Lung (R1), (3) Lung (R0) | 66 |
| 15 (A) | 23 | III M+P+ | HB | Cis, Carbo, Dox | (1) Liver (R1) | 13 |
| 16 (D) | 24 | II | HB | Cis, 5-FU, Vin | (1) Liver (R0) | 10 |
| 17 (A) | 94 | IV M+F+ | HB | Cis, Carbo, Dox | (1) Liver (R0) | 21 |
| 18 (D) | 35 | IV P+F+ | HB | Cis, Carbo, Dox | (1) LTx (R1) | 10 |
| 19 (D) | 21 | III V+P+F+ | HB | Cis, Carbo, Dox | (1) Liver (R0), (2) Lung (R0) | 8 |
| 20 (A) | 8 | II | HB | Cis | (1) Liver (R0) | 10 |
| 21 (A) | 15 | III | HB | Cis, Carbo, Dox, Vin, Ir | (1) Liver (R0), (2) Lung (R0), (3) Lung (R0) | 10 |
| 22 (A) | 29 | IV M+V+P+F+ | HB | Cis, Carbo, Dox, Vin, Ir, Ifo, Etop | (1) Lung R (MD) and L (R0), (2) LTx (R2) | 35 |
| 23 (A) | 75 | IV V+F+ | HB (DD HCC) | Cis, Carbo, Dox, Sor, Vin, Ir | (1) Liver (R1), (2) Lung (R1) | 12 |
| 24 (A) | 51 | IV F+ | HB/SCUD (INI1+) | Cis, Carbo, Dox | (1) Liver (R0) | 26 |
| 25 (D) | 4 | III V+ | HB | Cis, Carbo, Dox, Vin, Ir, Etop | (1) Liver (R2), (2) LTx (R0) | 13 |
| Pat. No. | Relapse Location | Relapse Chemo | Surgery for Relapse | EFS/OS from Relapse (m) | Status |
|---|---|---|---|---|---|
| 1 | Local | Dox, To | Liver (R0) | 13/40 | D—FR |
| 2 | Met-P | Tro, Id (Pall) | Peritoneum (R2) | 3/10 | D-Prog |
| 3 | Met-L | Cis, Dox, Ifo | Lung (R0) | 102/102 | 2nd CR |
| 4 | Met-P | No Chemo | Peritoneum (R0) | 0/2 | D-Prog |
| 5 | Met-L | Cis, Melp, Ir, Id, Bu | Lung (R0) | 171/171 | 2nd CR |
| 6 | Met-P | Cis, Dox, Ifo (Pall) | Peritoneum (R2) | 2/2 | D-Prog |
| 7 | Met-P | Carbo, Etop | Peritoneum (R1) | 18/29 | D—FR |
| 8 | Local | Cis, Carbo, Dox, Ifo | Liver (R0) | 186/186 | 2nd CR |
| 9 | Met-L | Cis, Carbo, Dox, Ifo, Etop | Lung (R0) | 76/76 | 2nd CR |
| 10 | Local + P | Carbo, Ifo, Etop, Beva, Sor, Sun | Liver + Peritoneum (MD), Liver (MD) | 8/37 | D—Prog |
| 11 | Met-L | Carbo, Dox, Ifo | No Op | 80/146 | D—FR |
| 12 | Local | Carbo, Etop, Ir, Beva | Liver (R0), LTx (R0) | 64/64 | 2nd CR |
| 13 | Local + P + L | Carbo, Dox, Ifo, Etop, 5FU, Vin, Im | No Op (R3) | 2/8 | D—Prog |
| 14 | Met-L | Carbo, Ifo, Etop, Ir, Vin | Lung (R0) | 15/15 | 2nd CR |
| 15 | Met-L | Ir, Vin | Lung (R0) | 60/60 | 2nd CR |
| 16 | Met-CNS | No Chemo | No Op | 0/0 | D |
| 17 | Local | Ir, Vin | LTx (R0) | 55/55 | 2nd CR |
| 18 | Local | No Chemo | LTx (R1) | 1/1 | D |
| 19 | Met-L | Cis, Carbo, Dox, Vin, Ir | Lung (R1), Lung (NAD), Liver (R0) | 4/16 | D—Prog |
| 20 | Met-L | Carbo, Dox | Lung (R0) | 66/66 | 2nd CR |
| 21 | Met-L | Cis, Dox, 5FU, Vin | Lung (R0) | 30/30 | 2nd CR |
| 22 | Met-L | Vin (oral), Ir | Lung (R0), Lung (MD) | 14/39 | 5th CR |
| 23 | Met-CNS | Carbo, Etop, Melp, Ir, Vin | CNS (R1) | 12/16 | 2nd Rel |
| 24 | Local | Carbo, Etop, Vin, Ir | Liver (R0) | 81/81 | 2nd CR |
| 25 | Met-L | Carbo, Etop | Lung (R0) | 2/10 | D—Prog |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxwell, R.; Häberle, B.; Kappler, R.; von Schweinitz, D.; Rassner, M.; von Frowein, J.; Schmid, I. Hepatoblastoma Relapse—Findings from the German HB99 Trial and the German Liver Tumor Registry. Cancers 2024, 16, 696. https://doi.org/10.3390/cancers16040696
Maxwell R, Häberle B, Kappler R, von Schweinitz D, Rassner M, von Frowein J, Schmid I. Hepatoblastoma Relapse—Findings from the German HB99 Trial and the German Liver Tumor Registry. Cancers. 2024; 16(4):696. https://doi.org/10.3390/cancers16040696
Chicago/Turabian StyleMaxwell, Rebecca, Beate Häberle, Roland Kappler, Dietrich von Schweinitz, Mark Rassner, Julia von Frowein, and Irene Schmid. 2024. "Hepatoblastoma Relapse—Findings from the German HB99 Trial and the German Liver Tumor Registry" Cancers 16, no. 4: 696. https://doi.org/10.3390/cancers16040696
APA StyleMaxwell, R., Häberle, B., Kappler, R., von Schweinitz, D., Rassner, M., von Frowein, J., & Schmid, I. (2024). Hepatoblastoma Relapse—Findings from the German HB99 Trial and the German Liver Tumor Registry. Cancers, 16(4), 696. https://doi.org/10.3390/cancers16040696







