Mechanisms of Resistance and Therapeutic Perspectives in Immunotherapy for Advanced Head and Neck Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Immune Resistance to Standard HNSCC Treatment
3. Current Immunotherapy in the Treatment of Recurrent or Metastatic HNSCC
4. Carcinogenesis of HNSCC and Immune Escape Mechanisms
4.1. Intrinsic Mechanisms to Anti-PD-1 Immunotherapy (Table 1, Figure 1)
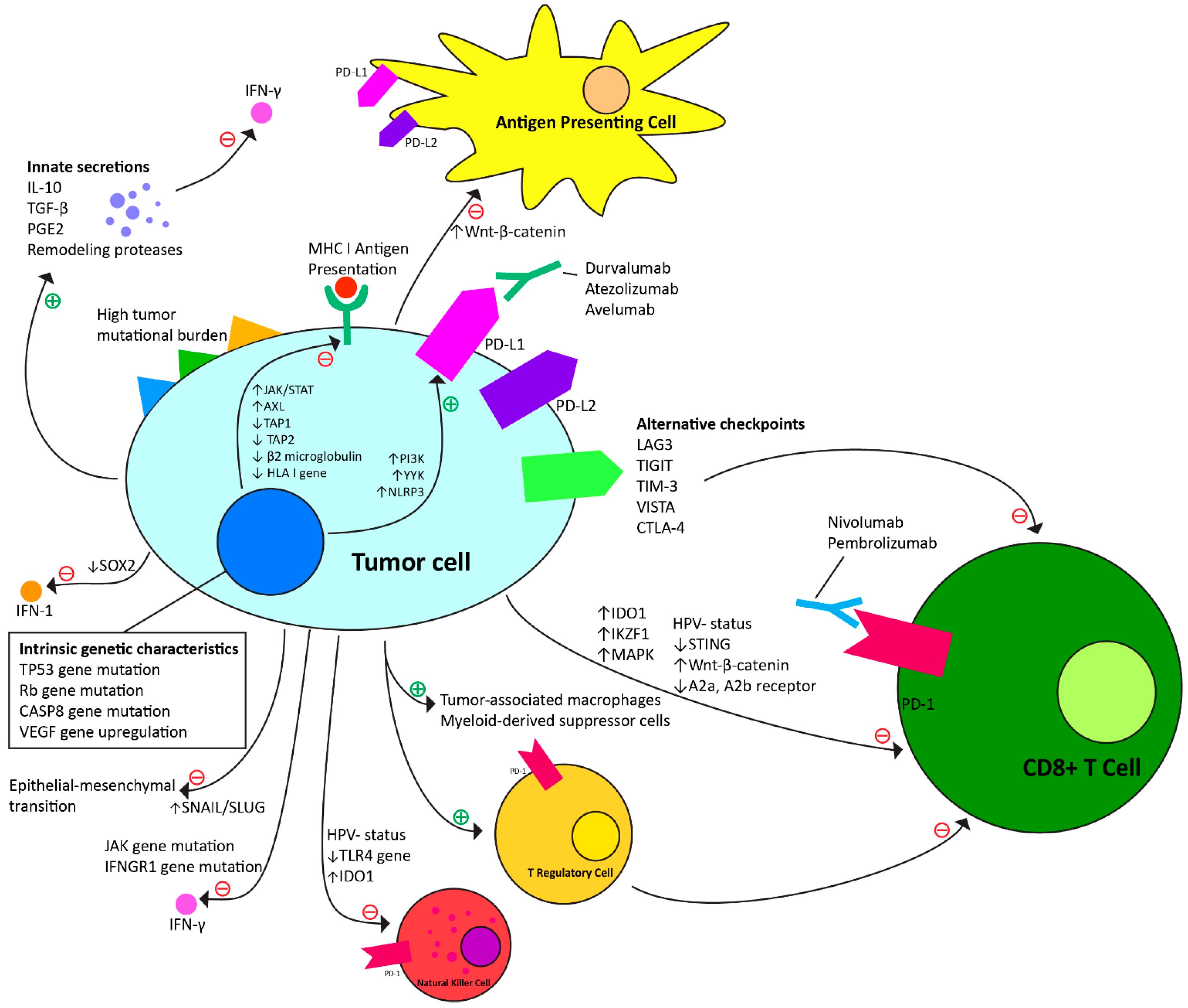
| Characteristic of Head and Neck Cancer | Tumor Immunotherapy Resistance Mechanism |
|---|---|
| Genetic mutations and regulatory changes | |
| HPV-negative status | Lower tumor infiltration by T cells, Tregs, and NK cells [2,44,45]. |
| TP53, Rb gene mutations | G1/S cell cycle dysregulation interfering with normal apoptosis signaling [51]. |
| JAK gene mutation | Decreased sensitivity to pro-inflammatory IFN-γ [46]. |
| IFNGR1 gene mutation | Decreased sensitivity to pro-inflammatory IFN-γ [47]. |
| STING suppression | Reduced CD8+ T cells in tumor microenvironment [48]. |
| CASP8 gene mutation | Procaspase-8 mutation to constitutionally bind to FADD, blocking normal apoptosis signaling [49]. |
| TLR4 gene suppression | Protects from NK cell immune attack via activated NF-κB [50]. |
| VEGF gene upregulation | Contributes to hypoxic environment, driving increased expression of hypoxia-inducible factor 1-alpha, which further drives VEGF expression and glycolysis-based energy production [51]. |
| Presence of epithelial–mesenchymal transition | Increases propensity of metastasis via regulators including transcription factors, hypoxic conditions, acquisition of stem cell properties, growth factors, and cytokines [2]. |
| Tumor neoantigens | |
| High tumor mutational burden | Contributes to both immune stimulation and suppression. Stimulation via increase in the number of antigens the immune system has available for stimulation. Inhibitory due to mutations to the HLA I antigen presentation pathway, leading to reduced CD8+ T cell activation [52,53,54,55]. |
| Soluble tumor secretions | |
| IL-10 | Decreased sensitivity to pro-inflammatory IFN-γ [17]. |
| TGF-β | Decreased sensitivity to pro-inflammatory IFN-γ [17]. |
| PGE2 | Decreased sensitivity to pro-inflammatory IFN-γ [17]. |
| Remodeling proteases | Contribute to hypoxic environment and EMT; can contribute to a physical extracellular matrix that protects the tumor [56,57]. |
| Upregulated cell lines | |
| Tumor-associated macrophages | Secrete IL-10, TGF-β, VEGF, and remodeling proteases [56,57]. |
| Myeloid-derived suppressor cells | Induce CD8+ T cell dysfunction via production of TGF-β and local L-arginine starvation [58]. |
| T regulatory cells | Downregulate T cell immune response via IL-10, TGF-β, and T-lymphocyte antigen-4 release; Induce T cell anergy and apoptosis; release PD-L1 [24]. |
4.2. Adaptive Resistance Mechanisms to Anti-PD-1 Checkpoint Immunotherapy (Table 2, Figure 1)
| Adaptive Modification Pathway | Immunotherapy Resistance Mechanism |
|---|---|
| Oncogenic signaling | |
| Wnt-β-catenin upregulation | Suppresses dendritic cell recruitment and antigen presentation to T cells; decreases T cell gene expression [59,60]. |
| PI3K upregulation | Increases expression of CCL2 and VEGF immunosuppressive cytokines; reduces CD8+ T cell tumor penetration; increased extrinsic PD-L1 expression [61]. |
| YY1 upregulation | Upregulates PI3K; increases extrinsic PD-L1 and variant “decoy” ligand expression [62,63,64]. |
| Alternative checkpoints | |
| LAG3 upregulation | Contributes to impaired T cell proliferation and cytokine production [73]. |
| TIGIT upregulation | Contributes to impaired T cell proliferation and cytokine production [75,76]. |
| TIM-3 upregulation | Contributes to impaired T cell proliferation and cytokine production; contributes to T cell exhaustion [71,75]. |
| VISTA upregulation | Contributes to impaired T cell proliferation and cytokine production [74,77]. |
| CTLA-4 upregulation | Contributes to impaired T cell proliferation, exhaustion, and cytokine production [71,76]. |
| Alternative immunosuppression | |
| NLRP3 upregulation | Upregulates PD-L1 expression via the recruitment of granulocytic myeloid-derived suppressor cells [65]. |
| JAK/STAT upregulation | Contributes to IFN-γ receptor loss on tumor cells and deficits in antigen presentation [66,67,68]. |
| SOX2 downregulation | Inhibits STING gene and contributes to blockade of the IFN-1 inflammatory pathway [69]. |
| TAP1 downregulation | Contributes to suppression of antigen presentation machinery [70]. |
| TAP2 downregulation | Contributes to suppression of antigen presentation machinery [70]. |
| β2-microglobulin downregulation | Contributes to suppression of antigen presentation machinery [70,71]. |
| HLA I gene downregulation | Reduced MHC I antigen-presenting machinery leading to decreased CD8+ T cell activation [52]. |
| AXL upregulation | Contributes to suppression of antigen presentation machinery [72]. |
| A2a and A2b receptor upregulation | Contributes to suppression of CD8+ T cell immune response [79]. |
| SNAIL/SLUG upregulation | Increases expression of TGF-β and matrix metalloproteinases, which are associated with EMT [80,81]. |
| IDO1 upregulation | Reduces proliferation of T and NK cells through expression of arginase-1 by tumor cells (degrades L-arginine needed for cell survival) [82,83]. |
| IKZF1 upregulation | Inhibition of immune infiltrate recruitment [85]. |
| MAPK upregulation | Induces VGEF and IL-8 release inhibiting T cell recruitment; cross-activates JAK/STAT and PI3K [86]. |
| Treg upregulation | Increased recruitment of myeloid-derived suppressor cells, which immunosuppress via the nitric oxide pathway [84]. |
| Antibiotic therapy | Modifies the native microbiome and likely the immune microenvironment as a result [87]. |
4.3. Predictors of Treatment Response
5. Overcoming Resistance to Immunotherapy (Table 3)
5.1. Combination Strategies
5.1.1. Salvage Surgery
5.1.2. Chemotherapy
5.1.3. Radiation Therapy
5.1.4. Combinations with Other Immune Checkpoint Inhibitors
5.1.5. Combinations with Other Immune-Stimulating Molecules
5.1.6. Oncolytic Viral Immunotherapy
5.2. Other Treatments in Development
5.2.1. Cancer Vaccines
5.2.2. Adoptive Cellular Therapy
| Treatment Modality | Mechanism of Action in Overcoming Resistance | Simplified Results of Clinical Trials |
|---|---|---|
| Additional anti-PD-1 antibodies | ||
| Durvalumab | Humanized PD-L1 monoclonal antibody. | Monotherapy is safe but has not shown progression-free survival improvement compared to standard of care [37,38]. |
| Atezolizumab | PD-L1 monoclonal antibody. | Under investigation as adjuvant treatment to surgery. Results pending [39]. |
| Avelumab | Fully human monoclonal anti-PD-1 monoclonal antibody. | No progression-free survival benefit compared to placebo in combination with standard of care [40]. |
| Anti-PD-1 combination with standard of care treatments | ||
| Salvage surgery | Reduction of tumor bulk | Phase II trials have shown safety and improved disease-free survival compared to historical samples [101]. |
| Chemotherapy | Increases TMB; depletes Tregs and MDSCs; normalizes neovasculature; upregulates HLA I; induces cancer cell death; increases sensitivity to IFN-γ. | Phase III trial has shown improvement in overall survival with platinum, 5-FU, and pembrolizumab compared to the EXTREME regimen [34]. |
| Radiotherapy | Reverses T cell exhaustion; propagates oligoclonal T cell expansion; direct anti-tumor activity (in animal models). | Past trials have not shown progression-free survival benefits. Several phase III trials are ongoing [22,104]. |
| Anti-PD-1 combination with non-redundant immune checkpoint inhibitors | ||
| CTLA-4 inhibitors (Ipilimumab) | Blocks binding of CTLA-4 to B7 ligand, which restores antigen presentation via MHC proteins. | Multiple phase III trials have shown no improvement in overall survival when combined with durvalumab or nivolumab [39,107,108]. |
| LAG3 inhibitors (Relalimab) | Blockade restores MHC II function. | Phase I and II trials are underway. Results pending [25,109]. |
| TIGIT inhibitors (Tiragolumab) | Blockade restores T cell immune function against tumor cells. | A phase II trial is underway in combination with atezolizumab compared to placebo. Another phase II trial showed improved ORR compared to atezolizumab alone [110,111]. |
| TIM-3 inhibitors (Cobolimab, MGB453) | Blockade restores production of cytokines and prevents apoptosis of T cells. | Early trials are underway for two different monoclonal antibody blockades of TIM-3. Results pending [102,113]. |
| Anti-PD-1 combinations with other immune stimulating molecules | ||
| EGFR inhibitors (cetuximab) | Inhibition of EGFR promotes antigen presentation an immune response to tumor cells. | Combinations with PD-1 inhibitors have shown improved ORR and overall survival in HPV-related disease [114]. |
| STAT3 inhibitors (AZD9150) | Blockade inhibits immunosuppressive transcription factor. | Phase I studies have shown a tolerable safety profile and suggested anti-tumor activity [115,116]. |
| CXCR2 inhibitors (AZD5069) | Blockade of pro-inflammatory cytokine receptor (IL-8 predominantly). | Tested with durvalumab, the combination did not improve patient ORR and had a high rate of adverse events [117]. |
| IDO1 inhibitors (Epacadostat, Navoximod) | Blockade decreases arginase-1 expression and restores T and NK cell proliferation. | Epacadostat and pembrolizumab have been shown to be safe but did not show positive results in a melanoma phase III trial [119,120]. Navoximod with atezolizumab is in Phase I testing for solid tumors [121]. |
| NKG2A inhibitors (Monalizumab) | Blockade restores CD8+ T and NK cell function. | Monalizumab with durvalumab with standard of care treatment has not shown improvements to progression-free survival. Phase II trial results are pending [122]. |
| B7H3 Inhibitors (Enoblituzumab) | Blockade restores CD8+ T cell function. | Combination with retifanlimab (PD-1 inhibitor) has shown an improved ORR. A phase II/III study has so far shown acceptable safety and anti-tumor activity results [124]. |
| ICOS inhibitors (Feladilimab) | Blocks Treg upregulation. | Phase II/III trials have not shown survival benefit [127]. |
| VEGF inhibitors (Lenvatinib) | Inhibition deters hypoxic environment. | A phase III trial combining lenvatinib with pembrolizumab did not show survival benefit [128,129]. |
| Anti-PD-1 combinations with oncolytic virus immunotherapy | ||
| Adenovirus | Stimulate direct oncolysis, systemic anti-tumor immunity, and destruction of tumor vasculature. | Phase 1 studies involving different virus variants and PD-1 inhibitor combinations are underway. Results pending [130,131]. |
| Herpes simplex virus | ||
| Cancer vaccines | ||
| Cell-mediated cytotoxicity vaccines | Induces antigen-specific, cell-medicated cytotoxicity that targets specific tumor antigens. | Over 40 major trials related to therapeutic vaccines are underway for HNSCC, all of which are in various points within Phase I and II phases and many of which are being used in combination with PD-1 checkpoint inhibitors [132]. |
| Direct targeting of immunosuppressive elements | Selectively target and inhibit elements of the immunosuppressive tumor microenvironment. | |
| Adoptive cellular therapy | ||
| CAR-T cell therapy | Genetically re-engineered native T cells target tumor-specific antigens and release pro-inflammatory cytokines. | EGFR targeting with CAR-T causes gastrointestinal, respiratory, and hematological toxicity. New strategies that target local adjacent tumor tissue antigens like FAP, HER3, and NKGD2 are in phase I and II clinical trials to assess safety [138]. |
| Natural killer cell therapy | Genetically re-engineered autologous NK cells target tumor-specific antigens. | In vivo trials have shown efficacy and phase 0, I, and II are underway [140]. |
| Engineered T cell receptor T therapy | T cell receptor isolation and peptide/HLA engineering to recognize intracellular tumor-associated antigens. | No trials yet underway for HNSCC [139]. |
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Veigas, F.; Mahmoud, Y.D.; Merlo, J.; Rinflerch, A.; Rabinovich, G.A.; Girotti, M.R. Immune Checkpoints Pathways in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 1018. [Google Scholar] [CrossRef]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef]
- Fulcher, C.D.; Haigentz, M., Jr.; Ow, T.J. AHNS Series: Do you know your guidelines? Principles of treatment for locally advanced or unresectable head and neck squamous cell carcinoma. Head Neck 2018, 40, 676–686. [Google Scholar] [CrossRef]
- Bhatia, A.; Burtness, B. Treating Head and Neck Cancer in the Age of Immunotherapy: A 2023 Update. Drugs 2023, 83, 217–248. [Google Scholar] [CrossRef]
- Argiris, A.; Harrington, K.J.; Tahara, M.; Schulten, J.; Chomette, P.; Ferreira Castro, A.; Licitra, L. Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2017, 7, 72. [Google Scholar] [CrossRef]
- Botticelli, A.; Cirillo, A.; Strigari, L.; Valentini, F.; Cerbelli, B.; Scagnoli, S.; Cerbelli, E.; Zizzari, I.G.; Rocca, C.D.; D’Amati, G.; et al. Anti-PD-1 and Anti-PD-L1 in Head and Neck Cancer: A Network Meta-Analysis. Front. Immunol. 2021, 12, 705096. [Google Scholar] [CrossRef]
- Kok, V.C. Current Understanding of the Mechanisms Underlying Immune Evasion from PD-1/PD-L1 Immune Checkpoint Blockade in Head and Neck Cancer. Front. Oncol. 2020, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Baste, N.; Neupane, P.C.; Bratland, A.; et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37 (Suppl. S15), 6000. [Google Scholar] [CrossRef]
- Goel, B.; Tiwari, A.K.; Pandey, R.K.; Singh, A.P.; Kumar, S.; Sinha, A.; Jain, S.K.; Khattri, A. Therapeutic approaches for the treatment of head and neck squamous cell carcinoma-An update on clinical trials. Transl. Oncol. 2022, 21, 101426. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, H.; Hamad, A.; Huang, H.; Tsung, A. Surgery-mediated tumor-promoting effects on the immune microenvironment. Semin. Cancer Biol. 2022, 86, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.T.; Judd, N.P.; Bui, J.D.; Uppaluri, R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope 2012, 122, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Gough, M.J.; Crittenden, M.R. The paradox of radiation and T cells in tumors. Neoplasia 2022, 31, 100808. [Google Scholar] [CrossRef] [PubMed]
- Jennette, K.W.; Lippard, S.J.; Vassiliades, G.A.; Bauer, W.R. Metallointercalation reagents. 2-hydroxyethanethiolato(2,2’,2’-terpyridine)-platinum(II) monocation binds strongly to DNA by intercalation. Proc. Natl. Acad. Sci. USA 1974, 71, 3839–3843. [Google Scholar] [CrossRef]
- Pendleton, K.P.; Grandis, J.R. Cisplatin-Based Chemotherapy Options for Recurrent and/or Metastatic Squamous Cell Cancer of the Head and Neck. Clin. Med. Insights Ther. 2013, 2013, CMT-S10409. [Google Scholar] [CrossRef]
- Wittes, R.E.; Cvitkovic, E.; Shah, J.; Gerold, F.P.; Strong, E.W. CIS-Dichlorodiammineplatinum(II) in the treatment of epidermoid carcinoma of the head and neck. Cancer Treat. Rep. 1977, 61, 359–366. [Google Scholar] [PubMed]
- Dos Santos, L.V.; Abrahão, C.M.; William, W.N., Jr. Overcoming Resistance to Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinomas. Front. Oncol. 2021, 11, 596290. [Google Scholar] [CrossRef] [PubMed]
- Syn, N.L.X.; Roudi, R.; Wang, L.Z.; Wang, L.; Loh, M.; Huang, Y.; Ou, S.H.I.; Soong, R.; Drilon, A.; Wee, I. Immune checkpoint inhibitors plus chemotherapy versus chemotherapy or immunotherapy for first-line treatment of advanced non-small cell lung cancer: A generic protocol. Cochrane Database Syst. Rev. 2018, 2018, CD013009. [Google Scholar] [CrossRef]
- Forster, M.D.; Devlin, M.J. Immune Checkpoint Inhibition in Head and Neck Cancer. Front. Oncol. 2018, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Cristina, V.; Herrera-Gómez, R.G.; Szturz, P.; Espeli, V.; Siano, M. Immunotherapies and Future Combination Strategies for Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 5399. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nihira, N.T.; Bu, X.; Chu, C.; Zhang, J.; Kolodziejczyk, A.; Fan, Y.; Chan, N.T.; Ma, L.; Liu, J.; et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 2020, 22, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Burtness, B.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Brana, I.; Basté, N.; Neupane, P.; et al. Pembrolizumab with or without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J. Clin. Oncol. 2022, 41, 790–802. [Google Scholar] [CrossRef]
- Rischin, D.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Braña, I.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma: Health-related quality-of-life results from KEYNOTE-048. Oral Oncol. 2022, 128, 105815. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.F.; Haddad, R.I.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.-E.; Clement, P.M.; Mesia, R.; Kutukova, S.I.; Zholudeva, L.; et al. EAGLE: A phase 3, randomized, open-label study of durvalumab (D) with or without tremelimumab (T) in patients (pts) with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37 (Suppl. S15), 6012. [Google Scholar] [CrossRef]
- Psyrri, A.; Fayette, J.; Harrington, K.; Gillison, M.; Ahn, M.J.; Takahashi, S.; Weiss, J.; Machiels, J.P.; Baxi, S.; Vasilyev, A.; et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann. Oncol. 2023, 34, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Wong, D.J.; Guo, Y.; Fayette, J.; Cohen, E.E.W.; Kowgier, M.; Sandler, A.; Matheny, C.; Kabbinavar, F.; Raben, D. IMvoke010: Randomized phase III study of atezolizumab (atezo) as adjuvant monotherapy after definitive therapy of squamous cell carcinoma of the head and neck (SCCHN). Ann. Oncol. 2018, 29, viii397. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.-H.; Lin, J.-C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Hunter, A.M.; LaCasse, E.C.; Korneluk, R.G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 2007, 12, 1543–1568. [Google Scholar] [CrossRef]
- Burnet, F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970, 13, 1–27. [Google Scholar] [CrossRef]
- Freiser, M.E.; Serafini, P.; Weed, D.T. The immune system and head and neck squamous cell carcinoma: From carcinogenesis to new therapeutic opportunities. Immunol. Res. 2013, 57, 52–69. [Google Scholar] [CrossRef]
- Partlová, S.; Bouček, J.; Kloudová, K.; Lukešová, E.; Zábrodský, M.; Grega, M.; Fučíková, J.; Truxová, I.; Tachezy, R.; Špíšek, R.; et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015, 4, e965570. [Google Scholar] [CrossRef]
- Solomon, B.; Young, R.J.; Rischin, D. Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin. Cancer Biol. 2018, 52 Pt 2, 228–240. [Google Scholar] [CrossRef]
- Albacker, L.A.; Wu, J.; Smith, P.; Warmuth, M.; Stephens, P.J.; Zhu, P.; Yu, L.; Chmielecki, J. Loss of function JAK1 mutations occur at high frequency in cancers with microsatellite instability and are suggestive of immune evasion. PLoS ONE 2017, 12, e0176181. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Escalante, N.K.; Jude, K.M.; Sotolongo Bellon, J.; Su, L.; Horton, T.M.; Tsutsumi, N.; Berardinelli, S.J.; Haltiwanger, R.S.; Piehler, J.; et al. Structure of the IFNγ receptor complex guides design of biased agonists. Nature 2019, 567, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cao, J.; Cai, W.L.; Lang, S.M.; Horton, J.R.; Jansen, D.J.; Liu, Z.Z.; Chen, J.F.; Zhang, M.; Mott, B.T.; et al. KDM5 histone demethylases repress immune response via suppression of STING. PLoS Biol. 2018, 16, e2006134. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Allen, C.T. Mechanisms of resistance to T cell-based immunotherapy in head and neck cancer. Head Neck 2020, 42, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, M.J.; Czystowska, M.; Szajnik, M.; Harasymczuk, M.; Boyiadzis, M.; Kruk-Zagajewska, A.; Szyfter, W.; Zeromski, J.; Whiteside, T.L. Triggering of Toll-like Receptor 4 Expressed on Human Head and Neck Squamous Cell Carcinoma Promotes Tumor Development and Protects the Tumor from Immune Attack. Cancer Res. 2009, 69, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef]
- Concha-Benavente, F.; Srivastava, R.; Ferrone, S.; Ferris, R.L. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016, 58, 52–58. [Google Scholar] [CrossRef]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef]
- Ogino, T.; Shigyo, H.; Ishii, H.; Katayama, A.; Miyokawa, N.; Harabuchi, Y.; Ferrone, S. HLA Class I Antigen Down-regulation in Primary Laryngeal Squamous Cell Carcinoma Lesions as a Poor Prognostic Marker. Cancer Res. 2006, 66, 9281–9289. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Voillet, V.; McAfee, M.S.; Hunter, D.S.; Wagener, F.D.; Perdicchio, M.; Valente, W.J.; Koelle, S.J.; Church, C.D.; Vandeven, N.; et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat. Commun. 2018, 9, 3868. [Google Scholar] [CrossRef] [PubMed]
- Pahler, J.C.; Tazzyman, S.; Erez, N.; Chen, Y.Y.; Murdoch, C.; Nozawa, H.; Lewis, C.E.; Hanahan, D. Plasticity in tumor-promoting inflammation: Impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia 2008, 10, 329–340. [Google Scholar] [CrossRef]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Pak, A.S.; Wright, M.A.; Matthews, J.P.; Collins, S.L.; Petruzzelli, G.J.; Young, M.R. Mechanisms of immune suppression in patients with head and neck cancer: Presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res. 1995, 1, 95–103. [Google Scholar] [PubMed]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell–Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Hays, E.; Bonavida, B. YY1 regulates cancer cell immune resistance by modulating PD-L1 expression. Drug Resist. Updates 2019, 43, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Bui, I.; Bonavida, B. Role of YY1 in the Regulation of Anti-Apoptotic Gene Products in Drug-Resistant Cancer Cells. Cancers 2023, 15, 4267. [Google Scholar] [CrossRef]
- Gong, B.; Kiyotani, K.; Sakata, S.; Nagano, S.; Kumehara, S.; Baba, S.; Besse, B.; Yanagitani, N.; Friboulet, L.; Nishio, M.; et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non–small cell lung cancer. J. Exp. Med. 2019, 216, 982–1000. [Google Scholar] [CrossRef] [PubMed]
- Theivanthiran, B.; Evans, K.S.; DeVito, N.C.; Plebanek, M.; Sturdivant, M.; Wachsmuth, L.P.; Salama, A.K.; Kang, Y.; Hsu, D.; Balko, J.M.; et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J. Clin. Investig. 2020, 130, 2570–2586. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, M.S.; Andrade Filho, P.A.; Ferrone, S.; Ferris, R.L. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol. Immunother. 2011, 60, 525–535. [Google Scholar] [CrossRef]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Sucker, A.; Zhao, F.; Pieper, N.; Heeke, C.; Maltaner, R.; Stadtler, N.; Real, B.; Bielefeld, N.; Howe, S.; Weide, B.; et al. Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 2017, 8, 15440. [Google Scholar] [CrossRef]
- Tan, Y.S.; Sansanaphongpricha, K.; Xie, Y.; Donnelly, C.R.; Luo, X.; Heath, B.R.; Zhao, X.; Bellile, E.; Hu, H.; Chen, H.; et al. Mitigating SOX2-potentiated Immune Escape of Head and Neck Squamous Cell Carcinoma with a STING-inducing Nanosatellite Vaccine. Clin. Cancer Res. 2018, 24, 4242–4255. [Google Scholar] [CrossRef]
- Jagadeeshan, S.; Prasad, M.; Ortiz-Cuaran, S.; Gregoire, V.; Saintigny, P.; Elkabets, M. Adaptive Responses to Monotherapy in Head and Neck Cancer: Interventions for Rationale-Based Therapeutic Combinations. Trends Cancer 2019, 5, 365–390. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, T.A.; Rafat, M.; Castellini, L.; Shehade, H.; Kariolis, M.S.; Hui, A.B.; Stehr, H.; von Eyben, R.; Jiang, D.; Ellies, L.G.; et al. Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat. Commun. 2016, 7, 13898. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ohno, T.; Nishii, N.; Harada, K.; Yagita, H.; Azuma, M. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol. 2016, 57, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A.; Jin, J.Y.; Lemaire, V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann. Oncol. 2018, 29, 71–83. [Google Scholar] [CrossRef]
- Wu, L.; Deng, W.-W.; Huang, C.-F.; Bu, L.-L.; Yu, G.-T.; Mao, L.; Zhang, W.-F.; Liu, B.; Sun, Z.-J. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol. Immunother. 2017, 66, 627–636. [Google Scholar] [CrossRef]
- Yuan, Y.; Adam, A.; Zhao, C.; Chen, H. Recent Advancements in the Mechanisms Underlying Resistance to PD-1/PD-L1 Blockade Immunotherapy. Cancers 2021, 13, 663. [Google Scholar] [CrossRef]
- Chen, L.; Diao, L.; Yang, Y.; Yi, X.; Rodriguez, B.L.; Li, Y.; Villalobos, P.A.; Cascone, T.; Liu, X.; Tan, L.; et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov. 2018, 8, 1156–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Evans, K.; Xiao, C.; DeVito, N.; Theivanthiran, B.; Holtzhausen, A.; Siska, P.J.; Blobe, G.C.; Hanks, B.A. Stromal Fibroblasts Mediate Anti–PD-1 Resistance via MMP-9 and Dictate TGFβ Inhibitor Sequencing in Melanoma. Cancer Immunol. Res. 2018, 6, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Johnson, N.W.; Gao, J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. Int. J. Oncol. 2010, 37, 663–668. [Google Scholar] [CrossRef]
- Meliante, P.G.; Barbato, C.; Zoccali, F.; Ralli, M.; Greco, A.; de Vincentiis, M.; Colizza, A.; Petrella, C.; Ferraguti, G.; Minni, A.; et al. Programmed Cell Death-Ligand 1 in Head and Neck Squamous Cell Carcinoma: Molecular Insights, Preclinical and Clinical Data, and Therapies. Int. J. Mol. Sci. 2022, 23, 15384. [Google Scholar] [CrossRef]
- Laimer, K.; Troester, B.; Kloss, F.; Schafer, G.; Obrist, P.; Perathoner, A.; Laimer, J.; Brandacher, G.; Rasse, M.; Margreiter, R.; et al. Expression and prognostic impact of indoleamine 2,3-dioxygenase in oral squamous cell carcinomas. Oral Oncol. 2011, 47, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; Moore, E.C.; Clavijo, P.E.; Friedman, J.; Cash, H.; Chen, Z.; Silvin, C.; Van Waes, C.; Allen, C. Anti-PD-L1 Efficacy Can Be Enhanced by Inhibition of Myeloid-Derived Suppressor Cells with a Selective Inhibitor of PI3Kδ/γ. Cancer Res. 2017, 77, 2607–2619. [Google Scholar] [CrossRef]
- Chen, J.C.; Perez-Lorenzo, R.; Saenger, Y.M.; Drake, C.G.; Christiano, A.M. IKZF1 Enhances Immune Infiltrate Recruitment in Solid Tumors and Susceptibility to Immunotherapy. Cell Syst. 2018, 7, 92–103.e4. [Google Scholar] [CrossRef] [PubMed]
- Ngan, H.-L.; Law, C.-H.; Choi, Y.C.Y.; Chan, J.Y.-S.; Lui, V.W.Y. Precision drugging of the MAPK pathway in head and neck cancer. npj Genom. Med. 2022, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Dorobisz, K.; Dorobisz, T.; Zatoński, T. The Microbiome’s Influence on Head and Neck Cancers. Curr. Oncol. Rep. 2023, 25, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Ismaila, N.; Bauman, J.E.; Dabney, R.; Gan, G.; Jordan, R.; Kaufman, M.; Kirtane, K.; McBride, S.M.; Old, M.O.; et al. Immunotherapy and Biomarker Testing in Recurrent and Metastatic Head and Neck Cancers: ASCO Guideline. J. Clin. Oncol. 2023, 41, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Meliante, P.G.; Zoccali, F.; de Vincentiis, M.; Ralli, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Diagnostic Predictors of Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Diagnostics 2023, 13, 862. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Youngnak, P.; Kozono, Y.; Kozono, H.; Iwai, H.; Otsuki, N.; Jin, H.; Omura, K.; Yagita, H.; Pardoll, D.M.; Chen, L.; et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem. Biophys. Res. Commun. 2003, 307, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, J.; Gao, Z.; Sun, H.; Mei, M.; Wang, Y.; Ren, Y.; Zhou, X. Evolving landscape of PD-L2: Bring new light to checkpoint immunotherapy. Br. J. Cancer 2023, 128, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Piccioni, D.; Kato, S.; Boichard, A.; Wang, H.Y.; Frampton, G.; Lippman, S.M.; Connelly, C.; Fabrizio, D.; Miller, V.; et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol. 2018, 4, 1237–1244. [Google Scholar] [CrossRef]
- Hanna, G.J.; Lizotte, P.; Cavanaugh, M.; Kuo, F.C.; Shivdasani, P.; Frieden, A.; Chau, N.G.; Schoenfeld, J.D.; Lorch, J.H.; Uppaluri, R.; et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight 2018, 3, e98811. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593. [Google Scholar] [CrossRef]
- Jamieson, N.B.; Maker, A.V. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther. 2017, 24, 134–140. [Google Scholar] [CrossRef]
- Hsieh, R.W.; Borson, S.; Tsagianni, A.; Zandberg, D.P. Immunotherapy in Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2021, 11, 705614. [Google Scholar] [CrossRef] [PubMed]
- Leddon, J.L.; Gulati, S.; Haque, S.; Allen, C.; Palackdharry, S.; Mathews, M.; Kurtzweil, N.; Riaz, M.K.; Takiar, V.; Nagasaka, M.; et al. Phase II Trial of Adjuvant Nivolumab Following Salvage Resection in Patients with Recurrent Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2022, 28, 3464–3472. [Google Scholar] [CrossRef]
- Julian, R.; Savani, M.; Bauman, J.E. Immunotherapy Approaches in HPV-Associated Head and Neck Cancer. Cancers 2021, 13, 5889. [Google Scholar] [CrossRef]
- Burtness, B.; Rischin, D.; Greil, R.; Soulieres, D.; Tahara, M.; Castro, G.d.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A. Efficacy of first-line (1L) pembrolizumab by PD-L1 combined positive score< 1, 1-19, and>= 20 in recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): KEYNOTE-048 subgroup analysis. In Cancer Research; American Association for Cancer Research: Philadelphia, PA, USA, 2020. [Google Scholar]
- Hui, C.; Chau, B.; Gan, G.; Stokes, W.; Karam, S.D.; Amini, A. Overcoming Resistance to Immunotherapy in Head and Neck Cancer Using Radiation: A Review. Front. Oncol. 2021, 11, 592319. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef]
- Ferris, R.L.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.E.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Siu, L.L.; Even, C.; Mesía, R.; Remenar, E.; Daste, A.; Delord, J.P.; Krauss, J.; Saba, N.F.; Nabell, L.; Ready, N.E.; et al. Safety and Efficacy of Durvalumab with or without Tremelimumab in Patients with PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2019, 5, 195–203. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Z. LAG3-PD-1 Combo Overcome the Disadvantage of Drug Resistance. Front. Oncol. 2022, 12, 831407. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer 2023, 22, 93. [Google Scholar] [CrossRef]
- Cohen, E.; Fayette, J.; Harrington, K.J.; Johnson, M.L.; Kao, H.F.; Lee, S.H.; Licitra, L.F.; Ngamphaiboon, N.; Psyrri, A.; Wong, D.J.; et al. 927TiP SKYSCRAPER-09: A phase II, randomised, double-blinded study of atezolizumab (Atezo) + tiragolumab (Tira) and atezo + placebo as first-line (1L) therapy for recurrent/metastatic (R/M) PD-L1+ squamous cell carcinoma of the head and neck (SCCHN). Ann. Oncol. 2021, 32, S814–S815. [Google Scholar] [CrossRef]
- Yang, F.; Zeng, Z.; Li, J.; Ren, X.; Wei, F. TIM-3 and CEACAM1 are Prognostic Factors in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2021, 8, 619765. [Google Scholar] [CrossRef]
- Falchook, G.S.; Ribas, A.; Davar, D.; Eroglu, Z.; Wang, J.S.; Luke, J.J.; Hamilton, E.P.; Di Pace, B.; Wang, T.; Ghosh, S.; et al. Phase 1 trial of TIM-3 inhibitor cobolimab monotherapy and in combination with PD-1 inhibitors nivolumab or dostarlimab (AMBER). J. Clin. Oncol. 2022, 40 (Suppl. S16), 2504. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, M.; Nie, D.; Xu, L.; Tian, H.; Wang, M.; Liu, W.; Feng, Z.; Han, F. Efficacy of cetuximab plus PD-1 inhibitor differs by HPV status in head and neck squamous cell carcinoma: A systematic review and meta-analysis. J. Immunother. Cancer 2022, 10, e005158. [Google Scholar] [CrossRef]
- Nishina, T.; Fujita, T.; Yoshizuka, N.; Sugibayashi, K.; Murayama, K.; Kuboki, Y. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: A phase 1 study. BMJ Open 2022, 12, e055718. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Harrington, K.J.; Hong, D.S.; Mesia, R.; Brana, I.; Perez Segura, P.; Wise-Draper, T.; Scott, M.L.; Mitchell, P.D.; Mugundu, G.M.; et al. A phase Ib/II study (SCORES) of durvalumab (D) plus danvatirsen (DAN; AZD9150) or AZD5069 (CX2i) in advanced solid malignancies and recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC): Updated results. Ann. Oncol. 2018, 29, viii372. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Rischin, D.; Pfister, D.G.; Vermorken, J.B.; Zhao, Y.; Gowda, H.; Ge, J.Y.; Jin, F.; Harrington, K.J. A phase 3, randomized, open-label study of epacadostat plus pembrolizumab, pembrolizumab monotherapy, and the EXTREME regimen as first-line treatment for recurrent/metastatic head and neck squamous cell carcinoma (R/M SCCHN): ECHO-304/KEYNOTE-669. J. Clin. Oncol. 2018, 36 (Suppl. S15), TPS6090. [Google Scholar] [CrossRef]
- Jung, K.H.; LoRusso, P.; Burris, H.; Gordon, M.; Bang, Y.J.; Hellmann, M.D.; Cervantes, A.; Ochoa de Olza, M.; Marabelle, A.; Hodi, F.S.; et al. Phase I Study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 3220–3228. [Google Scholar] [CrossRef]
- Galot, R.; Le Tourneau, C.; Licitra, L.F.L.; Even, C.; Daste, A.; Henry, S.; Borel, C.; Abdeddaim, C.; Seront, E.; Prevost, J.B.; et al. 935P A phase II study of monalizumab and durvalumab in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN): Results of the I2 cohort of the EORTC-HNCG-1559 trial (UPSTREAM). Ann. Oncol. 2023, 34, S588–S589. [Google Scholar] [CrossRef]
- Suh, W.K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.; et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; Zandberg, D.P.; et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: Interim results from a multicenter phase I/II trial. J. Immunother. Cancer. 2022, 10, e004424. [Google Scholar] [CrossRef]
- Amatore, F.; Gorvel, L.; Olive, D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin. Ther. Targets 2018, 22, 343–351. [Google Scholar] [CrossRef]
- Marinelli, O.; Nabissi, M.; Morelli, M.B.; Torquati, L.; Amantini, C.; Santoni, G. ICOS-L as a Potential Therapeutic Target for Cancer Immunotherapy. Curr. Protein Pept. Sci. 2018, 19, 1107–1113. [Google Scholar] [CrossRef]
- Hansen, A.R.; Stanton, T.S.; Hong, M.H.; Cohen, E.E.W.; Mehanna, H.M.; Chisamore, M.J.; Turner, D.; Yadavilli, S.; Bell, K.; Baccan, C.; et al. INDUCE-3: A randomized, double-blind study of GSK3359609 (GSK609), an inducible T-cell co-stimulatory (ICOS) agonist antibody, plus pembrolizumab (PE) versus placebo (PL) plus PE for first-line treatment of PD-L1-positive recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2020, 38 (Suppl. S15), TPS6591. [Google Scholar] [CrossRef]
- Chen, T.H.; Chang, P.M.; Yang, M.H. Combination of pembrolizumab and lenvatinib is a potential treatment option for heavily pretreated recurrent and metastatic head and neck cancer. J. Chin. Med. Assoc. 2021, 84, 361–367. [Google Scholar] [CrossRef]
- Siu, L.L.; Burtness, B.; Cohen, E.E.W.; Harrington, K.J.; Licitra, L.F.; Rischin, D.; Zhu, Y.; Lee, C.P.; Pinheiro, C.; Swaby, R.F.; et al. Phase III LEAP-010 study: First-line pembrolizumab with or without lenvatinib in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2020, 38 (Suppl. S15), TPS6589. [Google Scholar] [CrossRef]
- Dong, H.; Li, M.; Yang, C.; Wei, W.; He, X.; Cheng, G.; Wang, S. Combination therapy with oncolytic viruses and immune checkpoint inhibitors in head and neck squamous cell carcinomas: An approach of complementary advantages. Cancer Cell Int. 2023, 23, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, P.; Li, Z.; Xiao, S. Clinical Advances and Future Directions of Oncolytic Virotherapy for Head and Neck Cancer. Cancers 2023, 15, 5291. [Google Scholar] [CrossRef]
- Devaraja, K.; Aggarwal, S.; Singh, M. Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma—A Review. Vaccines 2023, 11, 634. [Google Scholar] [CrossRef]
- Meliante, P.G.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Head and Neck Squamous Cell Carcinoma Vaccine: Current Landscape and Perspectives. Curr. Issues Mol. Biol. 2023, 45, 9215–9233. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Butterfield, L.H. Lessons learned from cancer vaccine trials and target antigen choice. Cancer Immunol. Immunother. 2016, 65, 805–812. [Google Scholar] [CrossRef]
- Harrington, K.J.; Kong, A.; Mach, N.; Chesney, J.A.; Fernandez, B.C.; Rischin, D.; Cohen, E.E.W.; Radcliffe, H.S.; Gumuscu, B.; Cheng, J.; et al. Talimogene Laherparepvec and Pembrolizumab in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (MASTERKEY-232): A Multicenter, Phase 1b Study. Clin. Cancer Res. 2020, 26, 5153–5161. [Google Scholar] [CrossRef]
- Wood, L.; Chintakuntlawar, A.V.; Price, K.; Kaczmar, J.; Conn, G.; Bedu-Addo, F.K.; Weiss, J. Preliminary Safety of PDS0101 (Versamune +HPVmix) and Pembrolizumab Combination Therapy in Subjects with Recurrent/Metastatic Human Papillomavirus-16 Positive Oropharyngeal Squamous Cell Carcinoma (OPSCC). Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, e37–e38. [Google Scholar] [CrossRef]
- Wang, H.Q.; Fu, R.; Man, Q.W.; Yang, G.; Liu, B.; Bu, L.L. Advances in CAR-T Cell Therapy in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 2173. [Google Scholar] [CrossRef]
- Ecsedi, M.; McAfee, M.S.; Chapuis, A.G. The Anticancer Potential of T Cell Receptor-Engineered T Cells. Trends Cancer 2021, 7, 48–56. [Google Scholar] [CrossRef]
- Gong, Y.; Klein Wolterink, R.G.J.; Wang, J.; Bos, G.M.J.; Germeraad, W.T.V. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meci, A.; Goyal, N.; Slonimsky, G. Mechanisms of Resistance and Therapeutic Perspectives in Immunotherapy for Advanced Head and Neck Cancers. Cancers 2024, 16, 703. https://doi.org/10.3390/cancers16040703
Meci A, Goyal N, Slonimsky G. Mechanisms of Resistance and Therapeutic Perspectives in Immunotherapy for Advanced Head and Neck Cancers. Cancers. 2024; 16(4):703. https://doi.org/10.3390/cancers16040703
Chicago/Turabian StyleMeci, Andrew, Neerav Goyal, and Guy Slonimsky. 2024. "Mechanisms of Resistance and Therapeutic Perspectives in Immunotherapy for Advanced Head and Neck Cancers" Cancers 16, no. 4: 703. https://doi.org/10.3390/cancers16040703
APA StyleMeci, A., Goyal, N., & Slonimsky, G. (2024). Mechanisms of Resistance and Therapeutic Perspectives in Immunotherapy for Advanced Head and Neck Cancers. Cancers, 16(4), 703. https://doi.org/10.3390/cancers16040703







