ANLN Promotes the Proliferation and Migration of Gallbladder Cancer Cells via STRA6-Mediated Activation of PI3K/AKT Signaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Tissue Samples
2.2. Immunohistochemistry (IHC)
2.3. Cell Culture and Transfection
2.4. Lentivirus Packaging and Infection
2.5. Total RNA Extraction and qRT-PCR
2.6. Western Blot
2.7. CCK8 Assay and Colony Formation Assay
2.8. Transwell Migration Assay and Wound Scratch Assay
2.9. Subcutaneous Xenograft Models
2.10. mRNA Sequencing and Bioinformatic Analysis
2.11. Flow Cytometry: Apoptosis Assay and Cell Cycle Assay
2.12. Statistical Analysis
3. Results
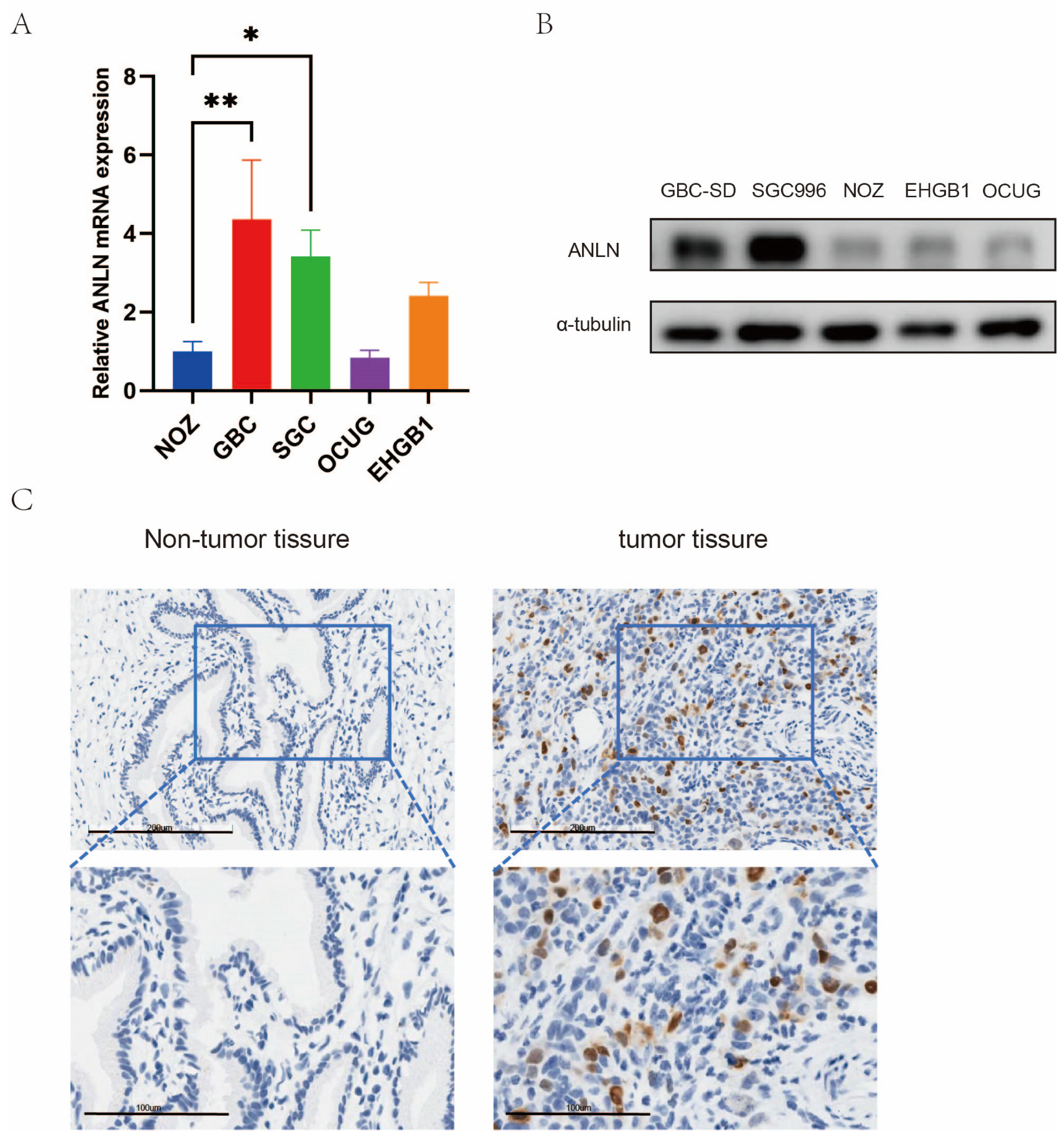
3.1. Expression of ANLN Is Upregulated in Human GBC Tissues and GBC Cell Lines
3.2. Knockdown of ANLN Attenuates GBC Cell Proliferation and Migration
3.3. Knockdown of ANLN Promotes Apoptosis and Cell Cycle Arrest in GBC Cells


3.4. ANLN Facilitates GBC Tumor Growth In Vivo
3.5. Knockdown of ANLN Inhibited the PI3K/AKT Signaling Pathway, Leading to Inhibition of GBC Cell Growth and Migration
3.6. ANLN Activates the PI3K/AKT Signaling Pathway by Regulating STRA6

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, M.A.; Marcano-Bonilla, L.; Roberts, L.R. Gallbladder cancer: Epidemiology and genetic risk associations. Chin. Clin. Oncol. 2019, 8, 31. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, C.; Liu, L.; Hu, Y.; Yang, B.; Qiu, S.; Li, Y.; Cao, D.; Ju, Z.; Ge, J.; et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J. Hepatol. 2021, 75, 1128–1141. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Calomino, N.; Scheiterle, M.L.P.F.; Fusario, D.; La Francesca, N.; Martellucci, I.; Marrelli, D. Porcelain gallbladder and its relationship to cancer. Eur. Surg. 2021, 53, 311–316. [Google Scholar] [CrossRef]
- Rakić, M.; Patrlj, L.; Kopljar, M.; Kliček, R.; Kolovrat, M.; Loncar, B.; Busic, Z. Gallbladder cancer. Hepatobiliary Surg. Nutr. 2014, 3, 221–226. [Google Scholar] [CrossRef][Green Version]
- Baiu, I.; Visser, B. Gallbladder Cancer. JAMA 2018, 320, 1294. [Google Scholar] [CrossRef]
- Gunasekaran, G.; Bekki, Y.; Lourdusamy, V.; Schwartz, M. Surgical Treatments of Hepatobiliary Cancers. Hepatology 2021, 73 (Suppl. S1), 128–136. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, Y.; Li, Y.; Shao, R.; Liu, F.; Liu, Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct. Target. Ther. 2020, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Edeline, J.; McNamara, M.G.; Hubner, R.A.; Nagino, M.; Bridgewater, J.; Primrose, J.; Valle, J.W. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat. Rev. 2020, 84, 101936. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.G.; Field, C.M.; Alberts, B.M. Actin-binding proteins from Drosophila embryos: A complex network of interacting proteins detected by F-actin affinity chromatography. J. Cell Biol. 1989, 109, 2963–2975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Maddox, A.S. Anillin. Curr. Biol. 2010, 20, R135–R136. [Google Scholar] [CrossRef]
- Field, C.M.; Alberts, B.M. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J. Cell Biol. 1995, 131, 165–178. [Google Scholar] [CrossRef]
- Olakowski, M.; Tyszkiewicz, T.; Jarzab, M.; Król, R.; Oczko-Wojciechowska, M.; Kowalska, M.; Kowal, M.; Gala, G.M.; Kajor, M.; Lange, D.; et al. NBL1 and anillin (ANLN) genes over-expression in pancreatic carcinoma. Folia Histochem. Cytobiol. 2009, 47, 249–255. [Google Scholar] [CrossRef]
- Wang, A.; Dai, H.; Gong, Y.; Zhang, C.; Shu, J.; Luo, Y.; Jiang, Y.; Liu, W.; Bie, P. ANLN-induced EZH2 upregulation promotes pancreatic cancer progression by mediating miR-218-5p/LASP1 signaling axis. J. Exp. Clin. Cancer Res. 2019, 38, 347. [Google Scholar] [CrossRef]
- Skrzypski, M.; Jassem, E.; Taron, M.; Sanchez, J.J.; Mendez, P.; Rzyman, W.; Gulida, G.; Raz, D.; Jablons, D.; Provencio, M.; et al. Three-gene expression signature predicts survival in early-stage squamous cell carcinoma of the lung. Clin. Cancer Res. 2008, 14, 4794–4799. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Y.; Long, J.; Guo, K.; Ge, C.; Du, R. microRNA-218 suppresses the proliferation, invasion and promotes apoptosis of pancreatic cancer cells by targeting HMGB1. Chin. J. Cancer Res. 2015, 27, 247–257. [Google Scholar] [CrossRef]
- Li, R.; Yu, S.; Qian, X. Expression of ANLN in intrahepatic cholangiocarcinoma and its effect on cholangiocarcinoma cell proliferation. J. Nanjing Med. Univ. 2019, 39, 26–31. (In Chinese) [Google Scholar]
- Zhou, W.; Wang, Z.; Shen, N.; Pi, W.; Jiang, W.; Huang, J.; Hu, Y.; Li, X.; Sun, L. Knockdown of ANLN by lentivirus inhibits cell growth and migration in human breast cancer. Mol. Cell Biochem. 2015, 398, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Fukushima, P.; DeGraff, W.; Mitchell, J.B.; Stetler Stevenson, M.; Ashkenazi, A.; Steeg, P.S. Radiation and the Apo2L/TRAIL apoptotic pathway preferentially inhibit the colonization of premalignant human breast cells overexpressing cyclin D1. Cancer Res. 2000, 60, 2611–2615. [Google Scholar] [PubMed]
- Lin, L.; Xiao, J.; Shi, L.; Chen, W.; Ge, Y.; Jiang, M.; Li, Z.; Fan, H.; Yang, L.; Xu, Z. STRA6 exerts oncogenic role in gastric tumorigenesis by acting as a crucial target of miR-873. J. Exp. Clin. Cancer Res. 2019, 38, 452. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.; Jia, X.; Song, W.; Wu, H.; Zhu, H.; Xuan, Z.; Du, Y.; Zhu, X.; Song, G.; et al. Targeting anillin inhibits tumorigenesis and tumor growth in hepatocellular carcinoma via impairing cytokinesis fidelity. Oncogene 2022, 41, 3118–3130. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, K.; Yu, S.J.; Jang, E.S.; Yu, J.; Cho, G.; Yoon, J.H.; Kim, Y. Development of biomarkers for screening hepatocellular carcinoma using global data mining and multiple reaction monitoring. PLoS ONE 2013, 8, e63468. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.F.; Huang, Y.L.; Wang, J.L.; Deng, M.H.; Xia, T.L.; Zeng, M.S.; Chen, M.S.; Wang, H.B.; Huang, Y.H. Anillin is required for tumor growth and regulated by miR-15a/miR-16-1 in HBV-related hepatocellular carcinoma. Aging 2018, 10, 1884–1901. [Google Scholar] [CrossRef]
- Khan, S.A.; Thomas, H.C.; Davidson, B.R.; Taylor-Robinson, S.D. Cholangiocarcinoma. Lancet 2005, 366, 1303–1314. [Google Scholar] [CrossRef]
- Hall, P.A.; Todd, C.B.; Hyland, P.L.; McDade, S.S.; Grabsch, H.; Dattani, M.; Hillan, K.J.; Russell, S.E. The septin-binding protein anillin is overexpressed in diverse human tumors. Clin. Cancer Res. 2005, 11, 6780–6786. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Z.; Xiong, Y.; Liu, S.; Cai, C.; Shao, Z.; Zhu, Y.; Song, X.; Shen, W.; Wang, X.; et al. Successful conversion surgery for locally advanced gallbladder cancer after gemcitabine and nab-paclitaxel chemotherapy. Front. Oncol. 2022, 12, 977963. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C.; Zuo, B.; Gong, W.; Zhang, Y.; Yang, Y.; Zhou, D.; Weng, M.; Qin, Y.; Jiang, A.; et al. Trends of gallbladder cancer incidence, mortality, and diagnostic approach in urban Shanghai between 1973 and 2009. Tumori 2020, 106, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Gani, F.; Buettner, S.; Amini, N.; Sasaki, K.; Andreatos, N.; Ethun, C.G.; Poultsides, G.; Tran, T.; Idrees, K.; et al. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: A multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB 2016, 18, 872–878. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Topatana, W.; Lv, X.; Cao, J.; Hu, J.; Lin, J.; Juengpanich, S.; Shen, J.; Cai, X. Development and Validation of a Nomogram for Predicting Survival in Gallbladder Cancer Patients With Recurrence After Surgery. Front. Oncol. 2020, 10, 537789. [Google Scholar] [CrossRef]
- Zhao, W.M.; Fang, G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J. Biol. Chem. 2005, 280, 33516–33524. [Google Scholar] [CrossRef]
- Kiyomitsu, T.; Cheeseman, I.M. Cortical dynein and asymmetric membrane elongation coordinately position the spindle in anaphase. Cell 2013, 154, 391–402. [Google Scholar] [CrossRef]
- Sheng, L.; Kang, Y.; Chen, D.; Shi, L. Knockdown of ANLN inhibits the progression of lung adenocarcinoma via pyroptosis activation. Mol. Med. Rep. 2023, 28, 13064. [Google Scholar] [CrossRef]
- Wang, G.; Shen, W.; Cui, L.; Chen, W.; Hu, X.; Fu, J. Overexpression of Anillin (ANLN) is correlated with colorectal cancer progression and poor prognosis. Cancer Biomark. 2016, 16, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.X.; Xu, W.; Fei, X.; Hao, F.; Wang, N.; Chen, Y.; Wang, J. Anillin facilitates cell proliferation and induces tumor growth of hepatocellular carcinoma via miR-138/SOX4 axis regulation. Transl. Oncol. 2020, 13, 100815. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, Z.J.; Liang, B.; Wang, Z.G.; Tao, Y.P.; Huang, S.Y.; Ni, J.S.; Li, H.F.; Yang, L.; Yuan, S.X.; et al. N(6)-Methyladenosine Modification of ANLN Enhances Hepatocellular Carcinoma Bone Metastasis. Int. J. Biol. Sci. 2023, 19, 1009–1023. [Google Scholar] [CrossRef]
- Cao, Y.F.; Xie, L.; Tong, B.B.; Chu, M.Y.; Shi, W.Q.; Li, X.; He, J.Z.; Wang, S.H.; Wu, Z.Y.; Deng, D.X.; et al. Targeting USP10 induces degradation of oncogenic ANLN in esophageal squamous cell carcinoma. Cell Death Differ. 2023, 30, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Zhang, L.; Wen, X.; Wang, X.; Cheng, X.; Du, H.; Hu, Y.; Li, L.; Dong, B.; Li, Z.; et al. PP242 suppresses cell proliferation, metastasis, and angiogenesis of gastric cancer through inhibition of the PI3K/AKT/mTOR pathway. Anticancer Drugs 2014, 25, 1129–1140. [Google Scholar] [CrossRef]
- Narayanankutty, A. PI3K/ Akt/ mTOR Pathway as a Therapeutic Target for Colorectal Cancer: A Review of Preclinical and Clinical Evidence. Curr. Drug Targets 2019, 20, 1217–1226. [Google Scholar] [CrossRef]
- Cheng, H.W.; Chen, Y.F.; Wong, J.M.; Weng, C.W.; Chen, H.Y.; Yu, S.L.; Chen, H.W.; Yuan, A.; Chen, J.J. Cancer cells increase endothelial cell tube formation and survival by activating the PI3K/Akt signalling pathway. J. Exp. Clin. Cancer Res. 2017, 36, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Li, X.; Ye, J.; Wu, X.; Tan, Z.; Liu, C.; Shen, B.; Wang, X.A.; Wu, W.; et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat. Genet. 2014, 46, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, A.; Kumari, N.; Krishnani, N.; Rastogi, N. Mutational frequency of KRAS, NRAS, IDH2, PIK3CA, and EGFR in North Indian gallbladder cancer patients. Ecancermedicalscience 2017, 11, 757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, T.Y.; Feng, X.F.; Fang, Z.; Cui, X.W.; Lin, Y.K.; Pan, Y.F.; Yang, C.; Ding, Z.W.; Zhang, Y.J.; Tan, Y.X.; et al. PTEN deficiency facilitates the therapeutic vulnerability to proteasome inhibitor bortezomib in gallbladder cancer. Cancer Lett. 2021, 501, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Su, H.; Wang, G.; Li, B.; Shen, G.; Gao, Q. Anti-tumor Activity of Bufalin by Inhibiting c-MET Mediated MEK/ERK and PI3K/AKT Signaling Pathways in Gallbladder Cancer. J. Cancer 2020, 11, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Z.; Liang, H.; Zhang, W.; Ye, Y.; Li, H.; Hu, Y.; Zhang, Y.; Weng, H.; Lu, J.; et al. Dioscin Induces Gallbladder Cancer Apoptosis by Inhibiting ROS-Mediated PI3K/AKT Signalling. Int. J. Biol. Sci. 2017, 13, 782–793. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Nacerddine, K.; Beaudry, J.B.; Ginjala, V.; Westerman, B.; Mattiroli, F.; Song, J.Y.; van der Poel, H.; Ponz, O.B.; Pritchard, C.; Cornelissen-Steijger, P.; et al. Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. J. Clin. Investig. 2012, 122, 1920–1932. [Google Scholar] [CrossRef]
- Gan, W.; Liu, P.; Wei, W. Akt promotes tumorigenesis in part through modulating genomic instability via phosphorylating XLF. Nucleus 2015, 6, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.M.; Leal, J.F.; Seger, R.; Taya, Y.; Oren, M. Cross-talk between Akt, p53 and Mdm2: Possible implications for the regulation of apoptosis. Oncogene 2002, 21, 1299–1303. [Google Scholar] [CrossRef]
- Strozyk, E.; Kulms, D. The role of AKT/mTOR pathway in stress response to UV-irradiation: Implication in skin carcinogenesis by regulation of apoptosis, autophagy and senescence. Int. J. Mol. Sci. 2013, 14, 15260–15285. [Google Scholar] [CrossRef]
- Berry, D.C.; O’Byrne, S.M.; Vreeland, A.C.; Blaner, W.S.; Noy, N. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol. Cell Biol. 2012, 32, 3164–3175. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Ge, J.; Wang, X.; Lin, B.; Yu, S.; Li, Y.; Hong, S.; Xiao, H. STRA6 regulates tumor immune microenvironment and is a prognostic marker in BRAF-mutant papillary thyroid carcinoma. Front. Endocrinol. 2023, 14, 1076640. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cheng, Z.; Huo, Z.; Lin, B.; Wang, X.; Sun, Y.; Yu, S.; Cao, S.; Xue, J.; Liu, R.; et al. STRA6 Promotes Thyroid Carcinoma Progression via Activation of the ILK/AKT/mTOR Axis in Cells and Female Nude Mice. Endocrinology 2023, 164, bqac215. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, P.; Yao, S.; Li, M.; Bian, Z.; Huang, Z. Up-regulation of STRA6 predicts poor prognosis and contributes to oxaliplatin resistance in colorectal cancer. Pathol. Res. Pract. 2023, 243, 154352. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Hernández, S.; Velázquez-Fernández, J.B.; Díaz-Chávez, J.; Mondragón-Fonseca, O.; Mayén-Lobo, Y.; Ortega, A.; López-López, M.; Arrieta, O. STRA6 Polymorphisms Are Associated With EGFR Mutations in Locally-Advanced and Metastatic Non-Small Cell Lung Cancer Patients. Front. Oncol. 2020, 10, 579561. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kanda, M.; Shimizu, D.; Sawaki, K.; Tanaka, C.; Hattori, N.; Hayashi, M.; Yamada, S.; Nakayama, G.; Omae, K.; et al. STRA6 Expression Serves as a Prognostic Biomarker of Gastric Cancer. Cancer Genom. Proteom. 2020, 17, 509–516. [Google Scholar] [CrossRef]


| Sense 5′-3′ | Antisense 5′-3′ | |
|---|---|---|
| ANLN-si1 | GCAUCUGCUAGGAUCAAUA | UAUUGAUCCUAGCAGAUGC |
| ANLN-si2 | CAGUGAUGUCCUAGAGGAA | UUCCUCUAGGACAUCACUG |
| STRA6-si1 | GCUCUGGAAGUGUGCUACA | UGUAGCACACUUCCAGAGC |
| STRA6-si2 | CCAAGAUCUACAAGUACUA | UAGUACUUGUAGAUCUUGG |
| Forward | Reverse | |
|---|---|---|
| ANLN | TGCACCATTGGCACAAACAG | CCAGATTCAGCTCGAGGGAC |
| STRA6 | AGACCAGGTCCCACACTGA | TTCATAATAGCCAAAGGCATAAAAA |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| Antibody | Application | Dilution | Company |
|---|---|---|---|
| ANLN | IHC | 1:100 | Abcam (Cambridge, UK) |
| Ki67 | IHC | 1:100 | Cell Signaling Technology (Danvers, MA, USA) |
| ANLN | WB | 1:1000 | Abcam (Cambridge, UK) |
| ANLN | WB | 1:1000 | Proteintech (Wuhan, China) |
| STRA6 | WB | 1:1000 | proteintech (Wuhan, China) |
| PI3K | WB | 1:10,000 | ABclonal (Wuhan, China) |
| P-PI3K | WB | 1:1000 | ABclonal (Wuhan, China) |
| Pan-AKT | WB | 1:2000 | ABclonal (Wuhan, China) |
| P-AKT | WB | 1:2000 | ABclonal (Wuhan, China) |
| GAPDH | WB | 1:25,000 | proteintech (Wuhan, China) |
| α-tubulin | WB | 1:20,000 | proteintech (Wuhan, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Zhang, Y.; Bian, R.; Zhu, J.; Shi, W.; Ye, Y. ANLN Promotes the Proliferation and Migration of Gallbladder Cancer Cells via STRA6-Mediated Activation of PI3K/AKT Signaling. Cancers 2024, 16, 752. https://doi.org/10.3390/cancers16040752
Zhu X, Zhang Y, Bian R, Zhu J, Shi W, Ye Y. ANLN Promotes the Proliferation and Migration of Gallbladder Cancer Cells via STRA6-Mediated Activation of PI3K/AKT Signaling. Cancers. 2024; 16(4):752. https://doi.org/10.3390/cancers16040752
Chicago/Turabian StyleZhu, Xiang, Yong Zhang, Rui Bian, Jiyue Zhu, Weibin Shi, and Yuanyuan Ye. 2024. "ANLN Promotes the Proliferation and Migration of Gallbladder Cancer Cells via STRA6-Mediated Activation of PI3K/AKT Signaling" Cancers 16, no. 4: 752. https://doi.org/10.3390/cancers16040752
APA StyleZhu, X., Zhang, Y., Bian, R., Zhu, J., Shi, W., & Ye, Y. (2024). ANLN Promotes the Proliferation and Migration of Gallbladder Cancer Cells via STRA6-Mediated Activation of PI3K/AKT Signaling. Cancers, 16(4), 752. https://doi.org/10.3390/cancers16040752






