High Engraftment and Metastatic Rates in Orthotopic Xenograft Models of Gastric Cancer via Direct Implantation of Tumor Cell Suspensions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Culture
2.3. Production of Lentiviral Particles and Transduction of Cells
2.4. Orthotopic Implantation of Tumor Fragments
2.5. Orthotopic Implantation of Tumor Cell Suspensions
2.6. In Vivo and Ex Vivo Bioluminescence Imaging
2.7. Tumor Volume Measurement
2.8. H&E and Ki67 Stainings
2.9. Statistical Analysis
3. Results
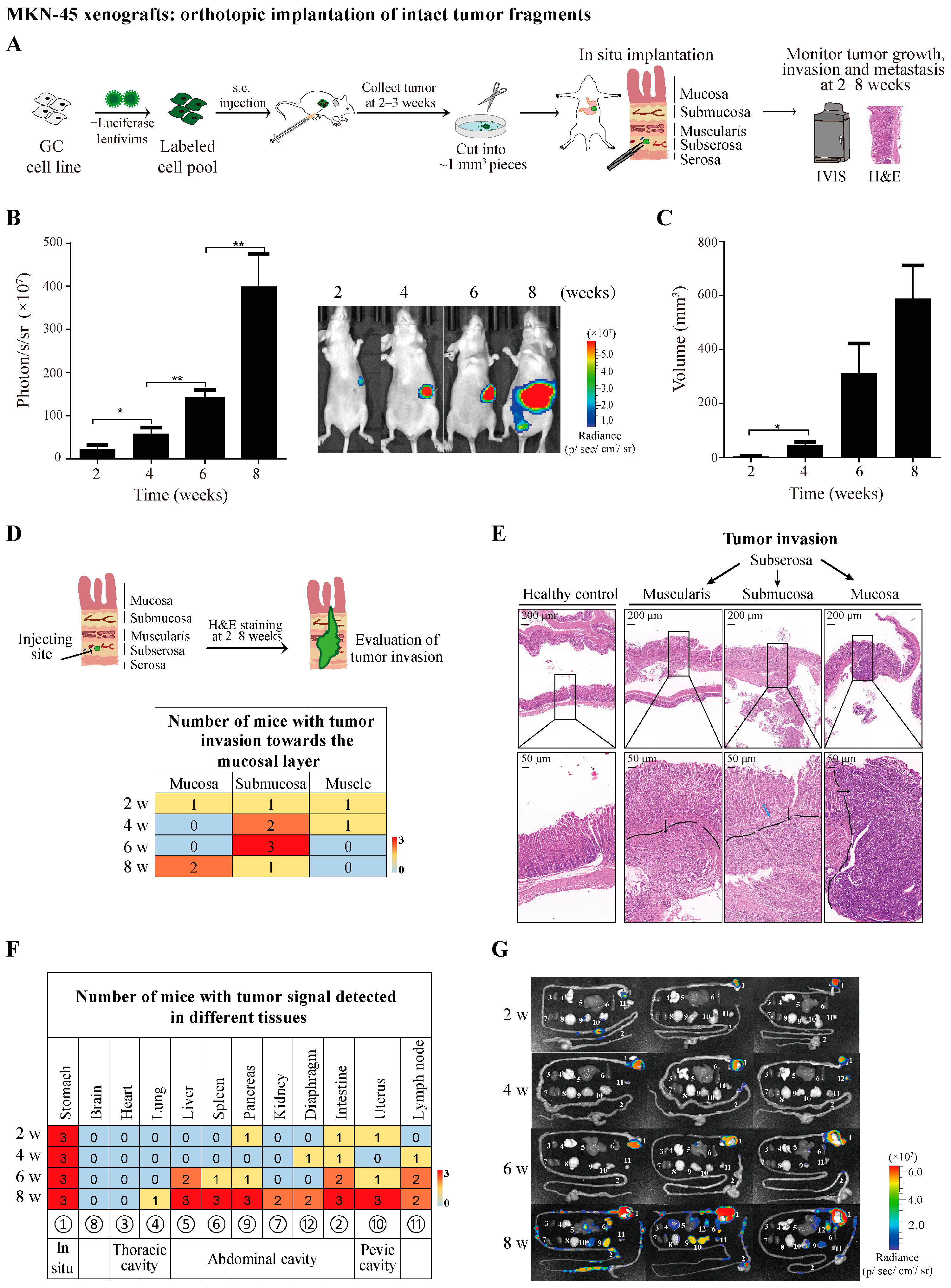
3.1. Establishment of MKN-45 Orthotopic Xenograft Model by Transplanting Subcutaneous Tumor Fragments from Donor Mice
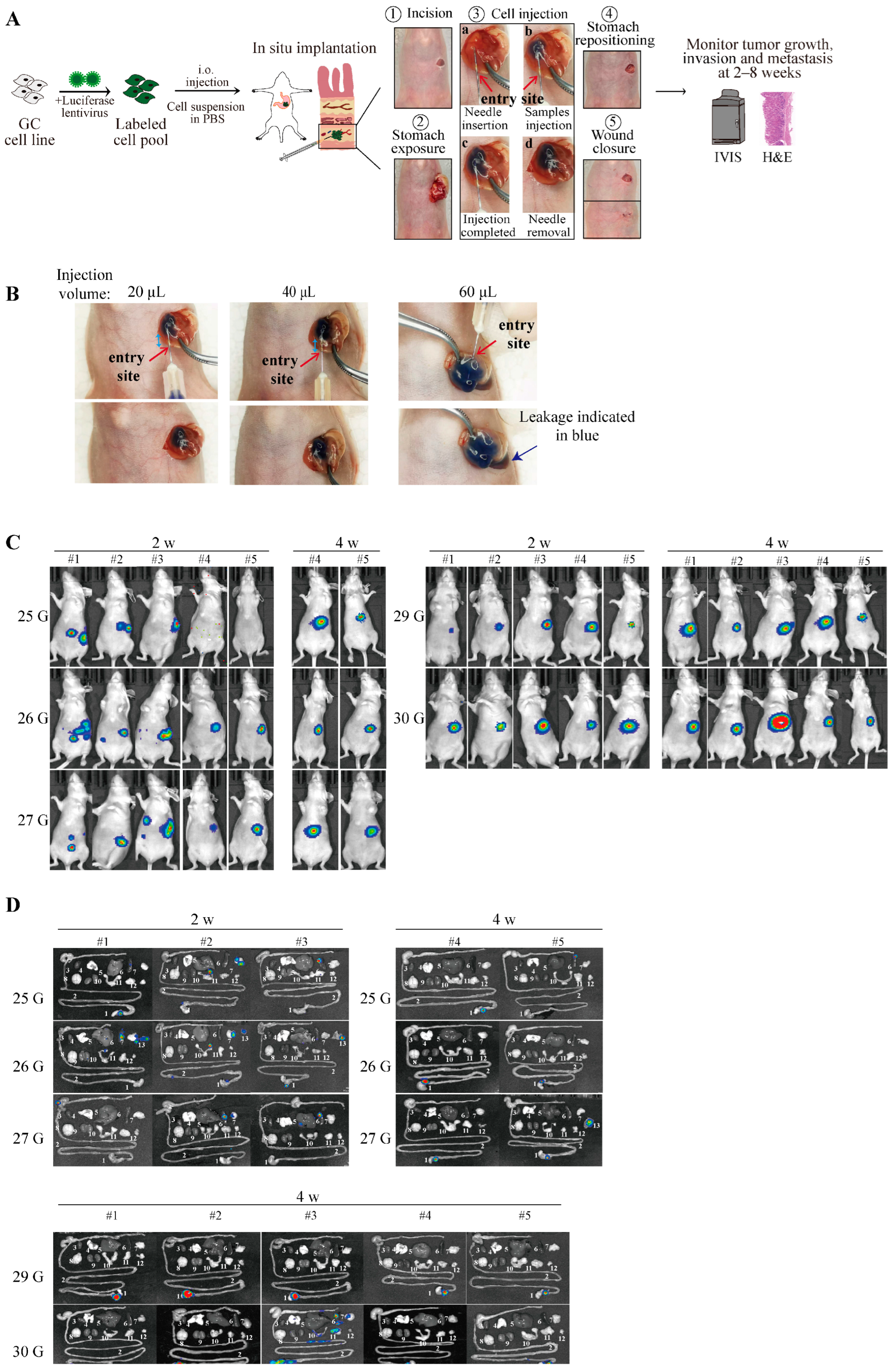
3.2. Minimized Risk of Cell Leakage by Orthotopic Injection of Tumor Cell Suspensions in PBS through Optimized Injection Volume and Appropriate Needle Size
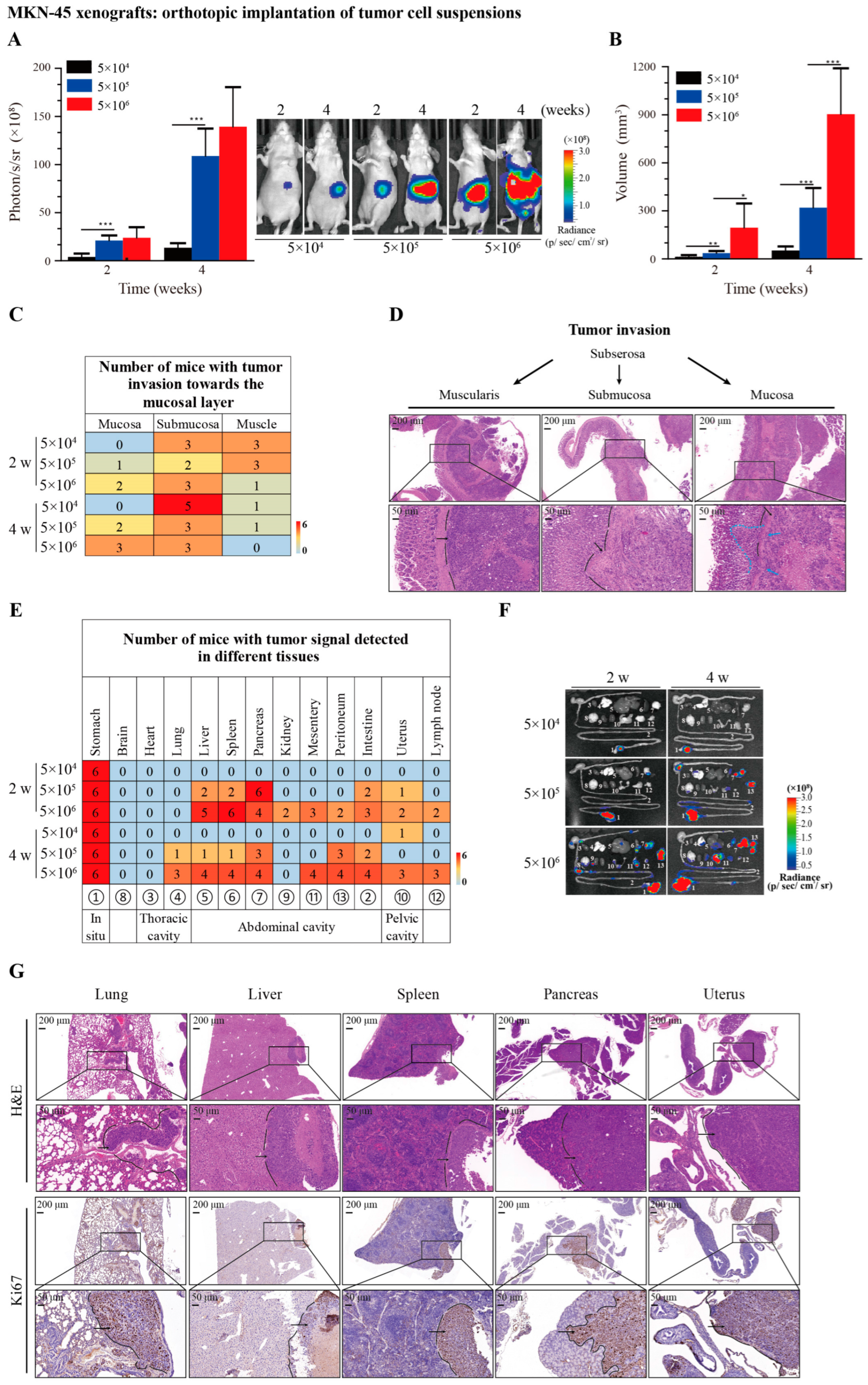
3.3. Recapitulation of Tumor Growth, Invasion, and Metastasis by Orthotopic Implantation of MKN-45 Cells in Suspensions
3.4. High Engraftment Rates in 23132/87 and HGC-27 Orthotopic Xenograft Models via Direct Implantation of Tumor Cell Suspensions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Jiang, L.; Wang, X.Q.; Pan, W.; She, F.F.; Chen, Y.L. Establishment of and comparison between orthotopic xenograft and subcutaneous xenograft models of gallbladder carcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 3747–3752. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, W.; Rachagani, S.; Zhou, Z.; Lele, S.M.; Batra, S.K.; Garrison, J.C. Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy. Sci. Rep. 2019, 9, 11117. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S.; Cornil, I.; Theodorescu, D. Importance of orthotopic transplantation procedures in assessing the effects of transfected genes on human tumor growth and metastasis. Cancer Metastasis Rev. 1991, 10, 201–215. [Google Scholar] [CrossRef]
- Hoffman, R.M. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: A bridge to the clinic. Investig. New Drugs 1999, 17, 343–359. [Google Scholar] [CrossRef]
- Reddavid, R.; Corso, S.; Moya-Rull, D.; Giordano, S.; Degiuli, M. Patient-Derived Orthotopic Xenograft models in gastric cancer: A systematic review. Updates Surg. 2020, 72, 951–966. [Google Scholar] [CrossRef]
- Kang, W.; Maher, L.; Michaud, M.; Bae, S.W.; Kim, S.; Lee, H.S.; Im, S.A.; Yang, H.K.; Lee, C. Development of a Novel Orthotopic Gastric Cancer Mouse Model. Biol. Proced. Online 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Hotz, B.; Buhr, H.J.; Hotz, H.G. An orthotopic nude mouse model for preclinical research of gastric cardia cancer. Int. J. Color. Dis. 2009, 24, 31–39. [Google Scholar] [CrossRef]
- Song, S.; Xu, Y.; Huo, L.; Zhao, S.; Wang, R.; Li, Y.; Scott, A.W.; Pizzi, M.P.; Wang, Y.; Fan, Y.; et al. Patient-derived cell lines and orthotopic mouse model of peritoneal carcinomatosis recapitulate molecular and phenotypic features of human gastric adenocarcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 207. [Google Scholar] [CrossRef]
- Bettenworth, D.; Mucke, M.M.; Schwegmann, K.; Faust, A.; Poremba, C.; Schafers, M.; Domagk, D.; Lenz, P. Endoscopy-guided orthotopic implantation of colorectal cancer cells results in metastatic colorectal cancer in mice. Clin. Exp. Metastasis 2016, 33, 551–562. [Google Scholar] [CrossRef]
- Chicote, I.; Martinez-Quintanilla, J.; Camara, J.A.; Palmer, H.G. Orthotopic Implantation of Patient-Derived Cancer Cells in Mice Recapitulates Advanced Colorectal Cancer. J. Vis. Exp. 2023, 10, e64629. [Google Scholar] [CrossRef]
- Furukawa, T.; Kubota, T.; Watanabe, M.; Kuo, T.H.; Kitajima, M.; Hoffman, R.M. Differential chemosensitivity of local and metastatic human gastric cancer after orthotopic transplantation of histologically intact tumor tissue in nude mice. Int. J. Cancer 1993, 54, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.A.; Liu, D.S.; Di Costanzo, N.; Schroder, J.; Mitchell, C.; Boussioutas, A. An orthotopic mouse model of gastric cancer invasion and metastasis. Sci. Rep. 2018, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Y.; Zhang, Y.; Liu, H.J.; Dong, X.; Yang, S.C.; Lu, Q.; Meng, F.; Chen, H.Z.; Sun, P.; Fang, C. Characterization of an orthotopic gastric cancer mouse model with lymph node and organ metastases using bioluminescence imaging. Oncol. Lett. 2018, 16, 5179–5185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wei, P.K.; Xu, L.; Su, X.M. Nude mouse model of human gastric carcinoma metastasis constructed by orthotopic transplantation using organism glue paste technique. Ai Zheng 2005, 24, 246–248. [Google Scholar] [PubMed]
- Illert, B.; Otto, C.; Braendlein, S.; Thiede, A.; Timmermann, W. Optimization of a metastasizing human gastric cancer model in nude mice. Microsurgery 2003, 23, 508–512. [Google Scholar] [CrossRef]
- Jones-Bolin, S.; Ruggeri, B. Orthotopic models of human gastric carcinoma in nude mice: Applications for study of tumor growth and progression. Curr. Protoc. Pharmacol. 2007, 37, 14. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Lee, C.L.; Wang, Q.; Zhou, Z.W.; Yang, F.; Jin, C.; Fu, D.L. Establishment of an orthotopic pancreatic cancer mouse model: Cells suspended and injected in Matrigel. World J. Gastroenterol. 2014, 20, 9476–9485. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Kimoto, T. Human cell line (HGC-27) derived from the metastatic lymph node of gastric cancer. Acta Med. Okayama 1976, 30, 215–219. [Google Scholar] [PubMed]
- Scarpa, E.S.; Tasini, F.; Crinelli, R.; Ceccarini, C.; Magnani, M.; Bianchi, M. The Ubiquitin Gene Expression Pattern and Sensitivity to UBB and UBC Knockdown Differentiate Primary 23132/87 and Metastatic MKN45 Gastric Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Yu, W.X.; Zheng, M.; Liao, X.H.; Wang, J.C.; Yang, D.Y.; Lu, W.X.; Wang, L.; Zhang, S.; Liu, H.K.; et al. PIN1 Inhibition Sensitizes Chemotherapy in Gastric Cancer Cells by Targeting Stem Cell-like Traits and Multiple Biomarkers. Mol. Cancer Ther. 2020, 19, 906–919. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Karsch-Bluman, A.; Feiglin, A.; Arbib, E.; Stern, T.; Shoval, H.; Schwob, O.; Berger, M.; Benny, O. Tissue necrosis and its role in cancer progression. Oncogene 2019, 38, 1920–1935. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.T.; Shin, S.J.; Cheong, J.H.; Choi, Y.Y. Genomic and evolutionary characteristics of metastatic gastric cancer by routes. Br. J. Cancer 2023, 129, 672–682. [Google Scholar] [CrossRef]
- Giraud, J.; Bouriez, D.; Seeneevassen, L.; Rousseau, B.; Sifre, E.; Giese, A.; Megraud, F.; Lehours, P.; Dubus, P.; Gronnier, C.; et al. Orthotopic Patient-Derived Xenografts of Gastric Cancer to Decipher Drugs Effects on Cancer Stem Cells and Metastatic Dissemination. Cancers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Lai, D.W.; Lan, K.H.; Shen, C.C.; Wu, S.M.; Chiu, C.S.; Wang, K.B.; Sheu, M.L. Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model. Carcinogenesis 2013, 34, 2568–2579. [Google Scholar] [CrossRef] [PubMed]
- Topi, S.; Santacroce, L.; Bottalico, L.; Ballini, A.; Inchingolo, A.D.; Dipalma, G.; Charitos, I.A.; Inchingolo, F. Gastric Cancer in History: A Perspective Interdisciplinary Study. Cancers 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Xie, G.-M.; Zhang, L.-P.; Yan, S.; Xu, J.-L.; Han, Y.-L.; Luo, M.-J.; Gong, J.-N. High Engraftment and Metastatic Rates in Orthotopic Xenograft Models of Gastric Cancer via Direct Implantation of Tumor Cell Suspensions. Cancers 2024, 16, 759. https://doi.org/10.3390/cancers16040759
Wang C, Xie G-M, Zhang L-P, Yan S, Xu J-L, Han Y-L, Luo M-J, Gong J-N. High Engraftment and Metastatic Rates in Orthotopic Xenograft Models of Gastric Cancer via Direct Implantation of Tumor Cell Suspensions. Cancers. 2024; 16(4):759. https://doi.org/10.3390/cancers16040759
Chicago/Turabian StyleWang, Chao, Guo-Min Xie, Li-Ping Zhang, Shuo Yan, Jia-Li Xu, Yun-Lin Han, Ming-Jie Luo, and Jia-Nan Gong. 2024. "High Engraftment and Metastatic Rates in Orthotopic Xenograft Models of Gastric Cancer via Direct Implantation of Tumor Cell Suspensions" Cancers 16, no. 4: 759. https://doi.org/10.3390/cancers16040759
APA StyleWang, C., Xie, G.-M., Zhang, L.-P., Yan, S., Xu, J.-L., Han, Y.-L., Luo, M.-J., & Gong, J.-N. (2024). High Engraftment and Metastatic Rates in Orthotopic Xenograft Models of Gastric Cancer via Direct Implantation of Tumor Cell Suspensions. Cancers, 16(4), 759. https://doi.org/10.3390/cancers16040759



