Endoscopic Ultrasound-Guided Fine-Needle Biopsy Versus Aspiration for Tissue Sampling Adequacy for Molecular Testing in Pancreatic Ductal Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Study Endpoints
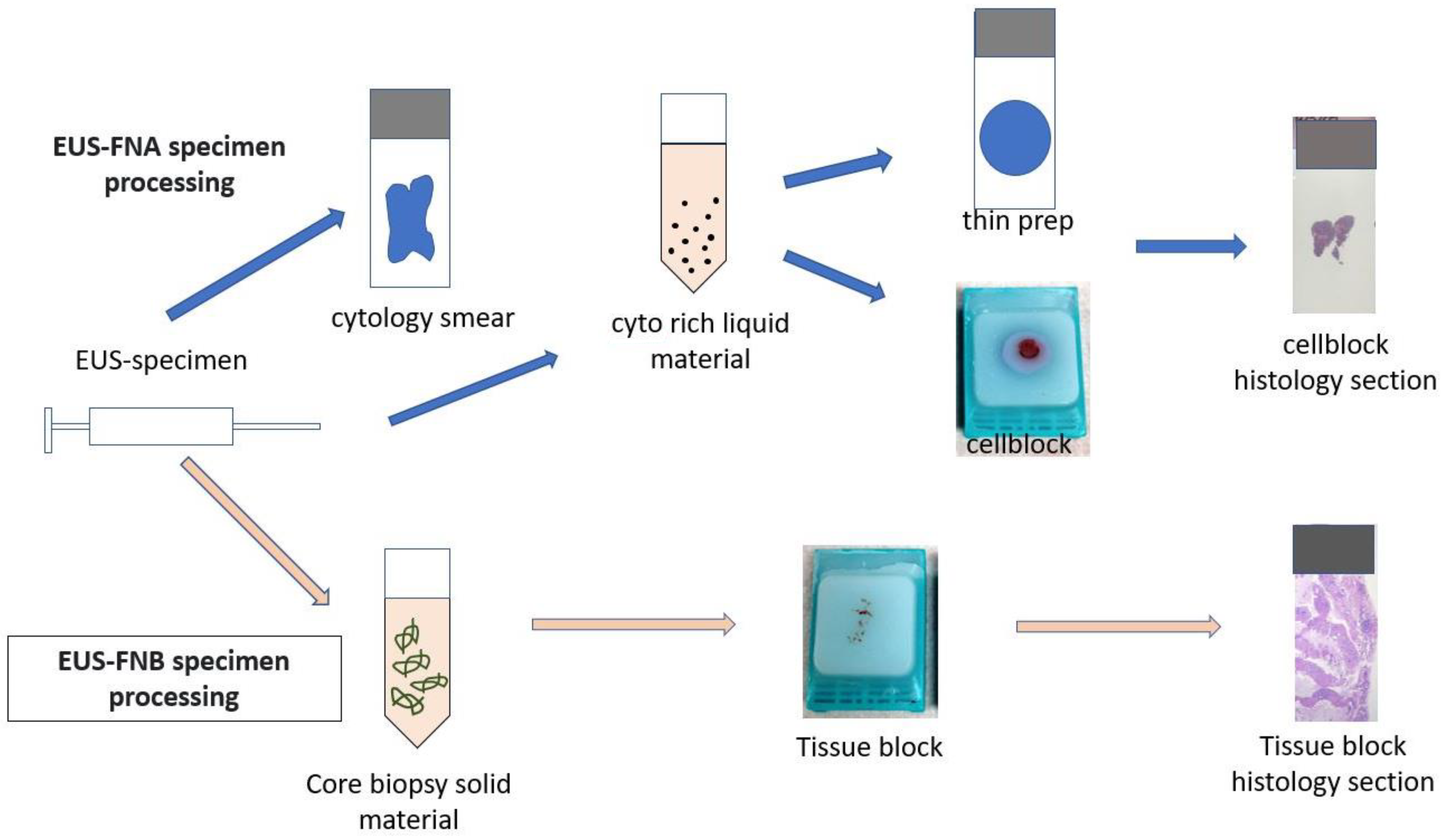
2.3. Procedures Details
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Primary Outcome
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic Ductal Adenocarcinoma |
| CT | Computerized Tomography |
| EUS | Endoscopic Ultrasound |
| MRI | Magnetic Resonance Imaging |
| EUS-TA | Endoscopic Ultrasound Tissue Acquisition |
| FNA | Fine-Needle Aspiration |
| FNB | Fine-Needle Biopsy |
| CNB | Core Needle Biopsy |
| EUS-FNA | Endoscopic Ultrasound Fine-Needle Aspiration |
| EUS-FNB | Endoscopic Ultrasound Fine-Needle Biopsy |
| KC | Kansas City |
| MO | Missouri |
| CA | Cancer Antigen |
| CEA | Carcino-Embryonic Antigen |
| ROSE | Rapid On-site Evaluation |
| RPMI | Roswell Park Memorial Institute |
| Q1, Q3 | Quartile 1, Quartile 3 |
| IgG 4 | Immunoglobin G 4 |
References
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Firpo, M.A.; Adler, D.G.; Mulvihill, S.J. Screening for pancreatic cancer: Why, how, and who? Ann. Surg. 2013, 257, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kim, M.; Hawes, R.; Varadarajulu, S. Changing trends in tissue acquisition in malignant pancreatic neoplasms. J. Gastroenterol. Hepatol. 2016, 31, 501–505. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sadakari, Y.; Shindo, K.; Suenaga, M.; Brant, A.; Almario, J.A.N.; Borges, M.; Barkley, T.; Fesharakizadeh, S.; Ford, M.; et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut 2017, 66, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; Dacic, S.; Ghofrani, M.; Illei, P.B.; Layfield, L.J.; Lee, C.; Michael, C.W.; Miller, R.A.; Mitchell, J.W.; Nikolic, B.; et al. Collection and Handling of Thoracic Small Biopsy and Cytology Specimens for Ancillary Studies: Guideline From the College of American Pathologists in Collaboration With the American College of Chest Physicians, Association for Molecular Pathology, American Society of Cytopathology, American Thoracic Society, Pulmonary Pathology Society, Papanicolaou Society of Cytopathology, Society of Interventional Radiology, and Society of Thoracic Radiology. Arch. Pathol. Lab. Med. 2020, 144, 933–958. [Google Scholar] [CrossRef]
- Sur, Y.K.; Kim, Y.C.; Kim, J.K.; Lee, J.H.; Yoo, B.M.; Kim, Y.B. Comparison of Ultrasound-Guided Core Needle Biopsy and Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Solid Pancreatic Lesions. J. Ultrasound Med. 2015, 34, 2163–2169. [Google Scholar] [CrossRef]
- Yan, L.; Ikemura, K.; Park, J.W. Utility of core biopsy with concurrent ROSE FNA in the diagnosis of pancreatic tumor-does the biopsy add any diagnostic benefit? Diagn. Cytopathol. 2018, 46, 154–159. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Hébert-Magee, S. Is there a role for endoscopic ultrasound-guided fine-needle biopsy in pancreatic cancer? Endoscopy 2015, 47, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Rana, S.S. Endoscopic Ultrasound-Guided Tissue Acquisition: Techniques and Challenges. J. Cytol. 2019, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Elhanafi, S.; Mahmud, N.; Vergara, N.; Kochman, M.L.; Das, K.K.; Ginsberg, G.G.; Rajala, M.; Chandrasekhara, V. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J. Gastroenterol. Hepatol. 2019, 34, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, A.; Beuvon, F.; Grabar, S.; Leblanc, S.; Chaussade, S.; Terris, B.; Barret, M.; Prat, F. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United Eur. Gastroenterol. J. 2015, 3, 343–352. [Google Scholar] [CrossRef] [PubMed]
- de Biase, D.; Visani, M.; Acquaviva, G.; Fornelli, A.; Masetti, M.; Fabbri, C.; Pession, A.; Tallini, G. The Role of Next-Generation Sequencing in the Cytologic Diagnosis of Pancreatic Lesions. Arch. Pathol. Lab. Med. 2018, 142, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Todaro, P.; Crino, S.F.; Barresi, V.; Tuccari, G. Endoscopic ultrasound-guided fine-needle aspiration cytology in pancreaticobiliary carcinomas: Diagnostic efficacy of cell-block immunocytochemistry. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Wang, L.; Arcila, M.E.; Balasubramanian, S.; Greenbowe, J.R.; Ross, J.S.; Stephens, P.; Lipson, D.; Miller, V.A.; Kris, M.G.; et al. Broad, Hybrid Capture-Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clin. Cancer Res. 2015, 21, 3631–3639. [Google Scholar] [CrossRef]
- Klapman, J.B.; Logrono, R.; Dye, C.E.; Waxman, I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am. J. Gastroenterol. 2003, 98, 1289–1294. [Google Scholar] [CrossRef]
- Hewitt, S.M.; Lewis, F.A.; Cao, Y.; Conrad, R.C.; Cronin, M.; Danenberg, K.D.; Goralski, T.J.; Langmore, J.P.; Raja, R.G.; Williams, P.M.; et al. Tissue handling and specimen preparation in surgical pathology: Issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch. Pathol. Lab. Med. 2008, 132, 1929–1935. [Google Scholar] [CrossRef]
- Nakhleh, R.E.; Nowak, J.A. Mining formalin-fixed, paraffin-embedded tissues: A wealth of knowledge or fool’s gold? Arch. Pathol. Lab. Med. 2014, 138, 1426–1427. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M.; Moisini, I.; Hicks, D.G. Molecular Pathology and Pre-Analytic Variables: Impact on Clinical Practice From a Breast Pathology Perspective. Curr. Pathobiol. Rep. 2018, 6, 125–134. [Google Scholar] [CrossRef]
- Compton, C.C.; Robb, J.A.; Anderson, M.W.; Berry, A.B.; Birdsong, G.G.; Bloom, K.J.; Branton, P.A.; Crothers, J.W.; Cushman-Vokoun, A.M.; Hicks, D.G.; et al. Preanalytics and Precision Pathology: Pathology Practices to Ensure Molecular Integrity of Cancer Patient Biospecimens for Precision Medicine. Arch. Pathol. Lab. Med. 2019, 143, 1346–1363. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Longo, D.L. Precision medicine--personalized, problematic, and promising. N. Engl. J. Med. 2015, 372, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Guidi, G.C.; Mattiuzzi, C.; Plebani, M. Preanalytical variability: The dark side of the moon in laboratory testing. Clin. Chem. Lab. Med. 2006, 44, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Chance, J.J.; Church, S.; Dazzi, P.; Fontana, R.; Giavarina, D.; Grankvist, K.; Huisman, W.; Kouri, T.; Palicka, V.; et al. Preanalytical quality improvement: From dream to reality. Clin. Chem. Lab. Med. 2011, 49, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Carraro, P.; Zago, T.; Plebani, M. Exploring the initial steps of the testing process: Frequency and nature of pre-preanalytic errors. Clin. Chem. 2012, 58, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Navina, S.; McGrath, K.; Chennat, J.; Singh, V.; Pal, T.; Zeh, H.; Krasinskas, A.M. Adequacy assessment of endoscopic ultrasound-guided, fine-needle aspirations of pancreatic masses for theranostic studies: Optimization of current practices is warranted. Arch. Pathol. Lab. Med. 2014, 138, 923–928. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Fockens, P.; Hawes, R.H. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin. Gastroenterol. Hepatol. 2012, 10, 697–703. [Google Scholar] [CrossRef]
- Clarke, D.L.; Clarke, B.A.; Thomson, S.R.; Garden, O.J.; Lazarus, N.G. The role of preoperative biopsy in pancreatic cancer. HPB 2004, 6, 144–153. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wu, X.; Yin, P.; Guo, Q.; Hou, W.; Li, Y.; Wang, Y.; Cheng, B. Comparing endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) versus fine needle biopsy (FNB) in the diagnosis of solid lesions: Study protocol for a randomized controlled trial. Trials 2016, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tang, A.L.; Zhang, L.; Liu, X.W.; Li, J.B.; Wang, F.; Shen, S.R.; Wang, X.Y. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: A prospective comparison study. Surg. Endosc. 2018, 32, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Torisu, Y.; Chiba, M.; Kinoshita, Y.; Akasu, T.; Shimamoto, N.; Abe, T.; Kanazawa, K.; Takakura, K.; Tsukinaga, S.; et al. Endoscopic ultrasound-guided fine-needle biopsy histology with a 22-gauge Franseen needle and fine-needle aspiration liquid-based cytology with a conventional 25-gauge needle provide comparable diagnostic accuracy in solid pancreatic lesions. JGH Open 2021, 5, 1092–1096. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Thorburn, D.; Toumpanakis, C.; Frazzoni, L.; Johnson, G.; Vessal, S.; Luong, T.V.; Caplin, M.; Pereira, S.P. Endoscopic ultrasound-guided fine-needle aspiration vs fine-needle biopsy for the diagnosis of pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, e1393–e1399. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.S.; Jeffus, S.K.; Sauer, B.G.; Wang, A.Y.; Stelow, E.B.; Shami, V.M. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn. Cytopathol. 2014, 42, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Renelus, B.D.; Jamorabo, D.S.; Boston, I.; Briggs, W.M.; Poneros, J.M. Endoscopic Ultrasound-Guided Fine Needle Biopsy Needles Provide Higher Diagnostic Yield Compared to Endoscopic Ultrasound-Guided Fine Needle Aspiration Needles When Sampling Solid Pancreatic Lesions: A Meta-Analysis. Clin. Endosc. 2021, 54, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Bajwa, H.S.; Menon, K.; Buccino, V.R.; Muscatiello, N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc. Ultrasound 2020, 9, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Muthusamy, V.R.; McGrath, C.M.; Sepulveda, A.R.; Das, A.; Messersmith, W.; Kochman, M.L.; Shah, J. AGA White Paper: Optimizing Endoscopic Ultrasound-Guided Tissue Acquisition and Future Directions. Clin. Gastroenterol. Hepatol. 2018, 16, 318–327. [Google Scholar] [CrossRef]
- Lee, L.S.; Andersen, D.K.; Ashida, R.; Brugge, W.R.; Canto, M.I.; Chang, K.J.; Chari, S.T.; DeWitt, J.; Hwang, J.H.; Khashab, M.A.; et al. EUS and related technologies for the diagnosis and treatment of pancreatic disease: Research gaps and opportunities-Summary of a National Institute of Diabetes and Digestive and Kidney Diseases workshop. Gastrointest. Endosc. 2017, 86, 768–778. [Google Scholar] [CrossRef]
- Asokkumar, R.; Yung Ka, C.; Loh, T.; Kah Ling, L.; Gek San, T.; Ying, H.; Tan, D.; Khor, C.; Lim, T.; Soetikno, R. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): A randomized study. Endosc. Int. Open 2019, 7, e955–e963. [Google Scholar] [CrossRef]
- Dwyer, J.; Pantanowitz, L.; Ohori, N.P.; Pai, R.K.; Vrbin, C.; Brand, R.E.; Monaco, S.E. Endoscopic ultrasound-guided FNA and ProCore biopsy in sampling pancreatic and intra-abdominal masses. Cancer Cytopathol. 2016, 124, 110–121. [Google Scholar] [CrossRef]
- Kandel, P.; Nassar, A.; Gomez, V.; Raimondo, M.; Woodward, T.A.; Crook, J.E.; Fares, N.S.; Wallace, M.B. Comparison of endoscopic ultrasound-guided fine-needle biopsy versus fine-needle aspiration for genomic profiling and DNA yield in pancreatic cancer: A randomized crossover trial. Endoscopy 2021, 53, 376–382. [Google Scholar] [CrossRef]
- Lin, M.; Hair, C.D.; Green, L.K.; Vela, S.A.; Patel, K.K.; Qureshi, W.A.; Shaib, Y.H. Endoscopic ultrasound-guided fine-needle aspiration with on-site cytopathology versus core biopsy: A comparison of both techniques performed at the same endoscopic session. Endosc. Int. Open 2014, 2, e220–e223. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Grimm, I.S.; Ali, B.; Nollan, R.; Tombazzi, C.; Ismail, M.K.; Baron, T.H. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc. Int. Open 2017, 5, e363–e375. [Google Scholar] [CrossRef]
- Adler, D.G.; Jacobson, B.C.; Davila, R.E.; Hirota, W.K.; Leighton, J.A.; Qureshi, W.A.; Rajan, E.; Zuckerman, M.J.; Fanelli, R.D.; Baron, T.H.; et al. ASGE guideline: Complications of EUS. Gastrointest. Endosc. 2005, 61, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Taylor, C.R. Practicing pathology in the era of big data and personalized medicine. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Walk, E.E. The role of pathologists in the era of personalized medicine. Arch. Pathol. Lab. Med. 2009, 133, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Tonellato, P.J.; Crawford, J.M.; Boguski, M.S.; Saffitz, J.E. A national agenda for the future of pathology in personalized medicine: Report of the proceedings of a meeting at the Banbury Conference Center on genome-era pathology, precision diagnostics, and preemptive care: A stakeholder summit. Am. J. Clin. Pathol. 2011, 135, 668–672. [Google Scholar] [CrossRef]
- Matloff, E.; Caplan, A. Direct to confusion: Lessons learned from marketing BRCA testing. Am. J. Bioeth. 2008, 8, 5–8. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848.e5. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | FNB (n = 48) | FNA (n = 76) | FNA and FNB (n = 8) |
|---|---|---|---|
| Mean age, years (±SD) | 69.88 ± 10.38 | 72.11 ± 10.86 | 67.25 ± 9.44 |
| Male, n (%) | 22 (45.8%) | 36 (47.4%) | 6 (75.0%) |
| CEA level, n Median (Q1, Q3) | 15 5.40 (2.70, 47.90) | 27 5.10 (1.70, 16.90) | 2 8.70 (6.60, 10.80) |
| CA 19-9 level, n Median (Q1, Q3) | 35 952.00 (129.00, 4483.00) | 53 312.00 (43.00, 1661.00) | 5 195.00 (174.00, 223.00) |
| Complications, n (%) | 0 | 0 | 0 |
| FNB (n = 56) | FNA (n = 84) | p-value | |
| Pancreatic lesion size in cm2 | 0.713 | ||
| < 2 cm2 | 4 (7.1%) | 4 (4.8%) | |
| ≥ 2 cm2 | 52 (92.9%) | 80 (95.2%) | |
| Pancreatic lesion location, n (%) | 0.719 | ||
| Body | 7 (12.5%) | 11 (13.1%) | |
| Body and tail | 7 (12.5%) | 5 (6.0%) | |
| Head/uncinate process | 28 (50.0%) | 50 (59.5%) | |
| Head and neck | 5 (8.9%) | 4 (4.8%) | |
| Neck and body | 1 (1.8%) | 3 (3.6%) | |
| Neck/genu | 2 (3.6%) | 2 (2.4%) | |
| Tail | 6 (10.7%) | 9 (10.7%) | |
| Needle gauge, n (%) * | < 0.001 | ||
| 19 G | 22 (40.0%) | 10 (11.9%) | |
| 22 G | 32 (58.2%) | 69 (82.1%) | |
| 25 G | 1 (1.8%) | 5 (6.0%) |
| Outcome Measure | FNB (n = 56) | FNA (n = 84) | p-Value * |
|---|---|---|---|
| Mean pass counts (±SD) ** | 2.58 ± 1.06 | 2.49 ± 1.07 | 0.5096 |
| Sample adequacy for molecular testing, n (%) | 35 (71.4%) | 26 (32.1%) | < 0.001 |
| Genomic testing performed, n (%) | 26 (46.4%) | 20 (23.8%) | 0.005 |
| Characteristic | FNB (n = 56) | FNA (n = 84) | p-Value |
|---|---|---|---|
| Tumor Surface Area in mm2, n Median (Q1, Q3) | 49 25.00 (4.00, 100.00) | 81 4.00 (1.00, 25.00) | <0.001 |
| Tumor cellularity * <20% 20–49% >49% | 9 (18.4%) 25 (51.0%) 15 (30.6%) | 30 (37.0%) 32 (39.5%) 19 (23.5%) | 0.079 |
| Mean number of smear slides (±SD) | 4.30 ± 2.82 | 4.49 ± 2.80 | 0.704 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, W.T.; Jahagirdar, V.; Jaber, F.; Ahmed, M.K.; Fatima, I.; Bierman, T.; Fu, Z.; Jones, P.G.; Hassan, A.F.; Faber, E.; et al. Endoscopic Ultrasound-Guided Fine-Needle Biopsy Versus Aspiration for Tissue Sampling Adequacy for Molecular Testing in Pancreatic Ductal Adenocarcinoma. Cancers 2024, 16, 761. https://doi.org/10.3390/cancers16040761
Mohamed WT, Jahagirdar V, Jaber F, Ahmed MK, Fatima I, Bierman T, Fu Z, Jones PG, Hassan AF, Faber E, et al. Endoscopic Ultrasound-Guided Fine-Needle Biopsy Versus Aspiration for Tissue Sampling Adequacy for Molecular Testing in Pancreatic Ductal Adenocarcinoma. Cancers. 2024; 16(4):761. https://doi.org/10.3390/cancers16040761
Chicago/Turabian StyleMohamed, Wael T., Vinay Jahagirdar, Fouad Jaber, Mohamed K. Ahmed, Ifrah Fatima, Thomas Bierman, Zhuxuan Fu, Philip G. Jones, Amira F. Hassan, Erin Faber, and et al. 2024. "Endoscopic Ultrasound-Guided Fine-Needle Biopsy Versus Aspiration for Tissue Sampling Adequacy for Molecular Testing in Pancreatic Ductal Adenocarcinoma" Cancers 16, no. 4: 761. https://doi.org/10.3390/cancers16040761
APA StyleMohamed, W. T., Jahagirdar, V., Jaber, F., Ahmed, M. K., Fatima, I., Bierman, T., Fu, Z., Jones, P. G., Hassan, A. F., Faber, E., Clarkston, W. K., Ghoz, H., Tawfik, O. W., & Jonnalagadda, S. (2024). Endoscopic Ultrasound-Guided Fine-Needle Biopsy Versus Aspiration for Tissue Sampling Adequacy for Molecular Testing in Pancreatic Ductal Adenocarcinoma. Cancers, 16(4), 761. https://doi.org/10.3390/cancers16040761








