RACK1 Promotes Meningioma Progression by Activation of NF-κB Pathway via Preventing CSNK2B from Ubiquitination Degradation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Meningioma Samples
2.2. Cell Lines and Primary Cell Culture
2.3. Tumor Xenograft Experiments
2.4. Cell Cycle Assay
2.5. Colony Formation Assay
2.6. Immunohistochemical Staining
2.7. ELISA Assay
2.8. Luciferase Reporter Assay
2.9. Plasmid Construction and Transfection
2.10. Western Blot
2.11. Quantitative Real-Time PCR
2.12. Statistical Analyses
3. Results
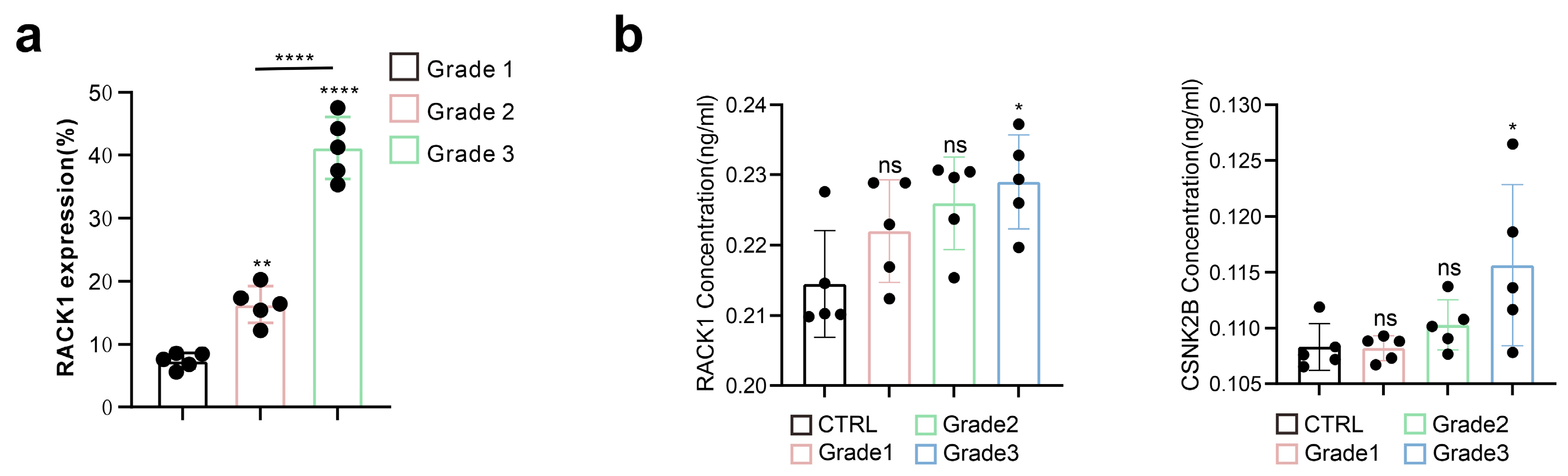
3.1. RACK1 Expression Level Positively Correlates with the Malignancy of Meningiomas
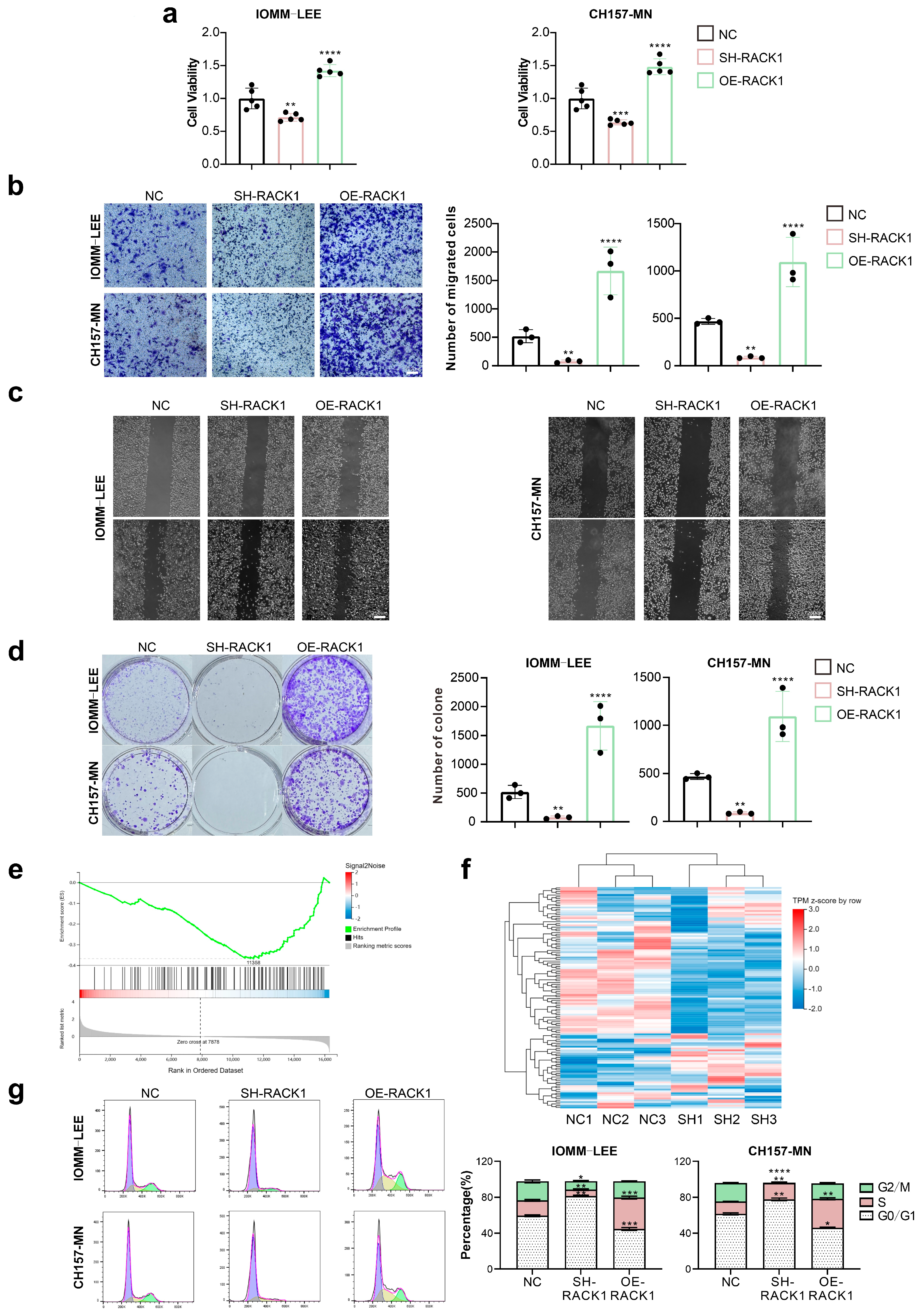
3.2. RACK1 Promotes the Proliferation and Migration Ability of Meningioma Cells and Affects the Cell Cycle
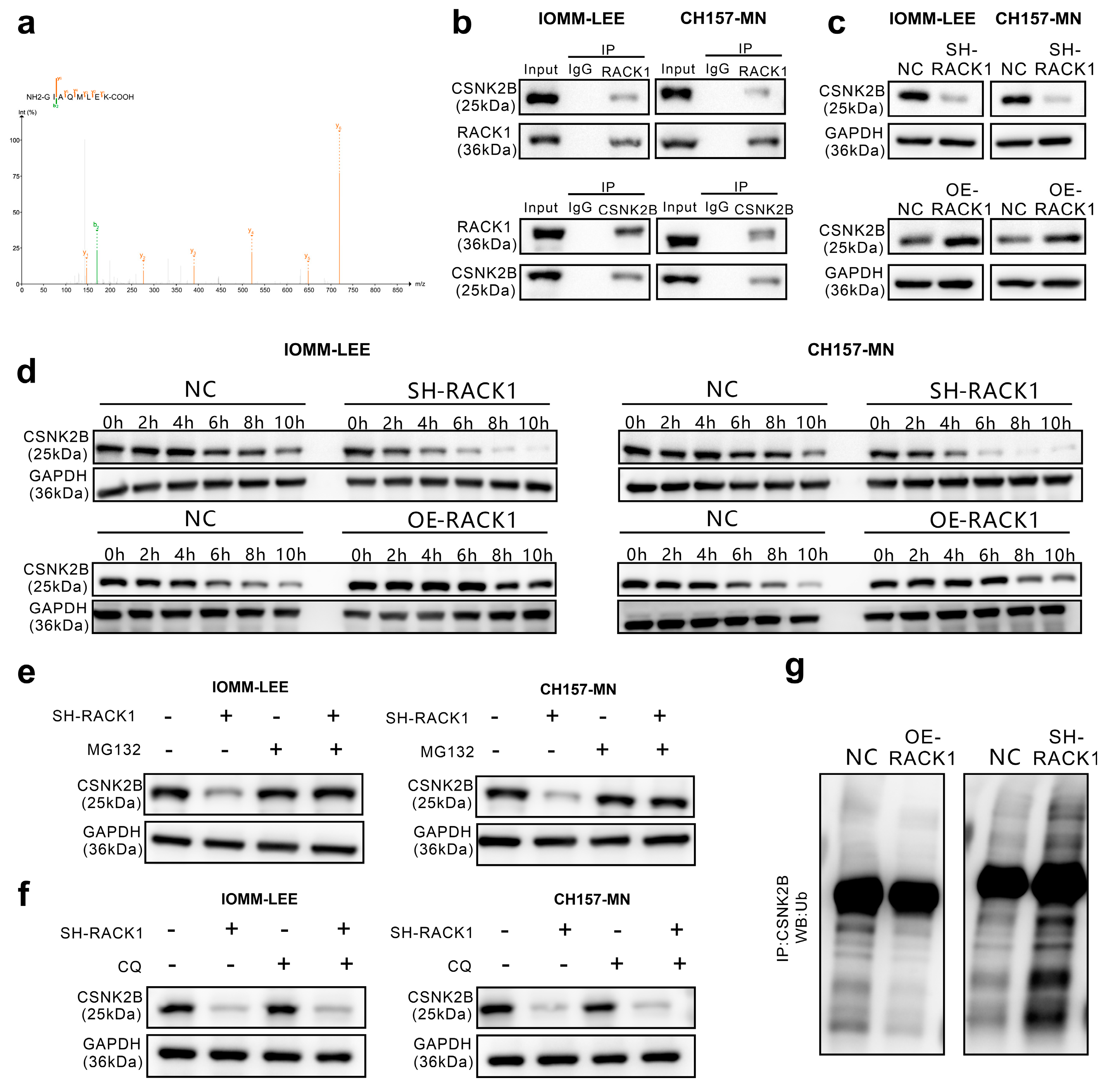
3.3. RACK1 Inhibits its Ubiquitination Degradation by Binding to CSNK2B
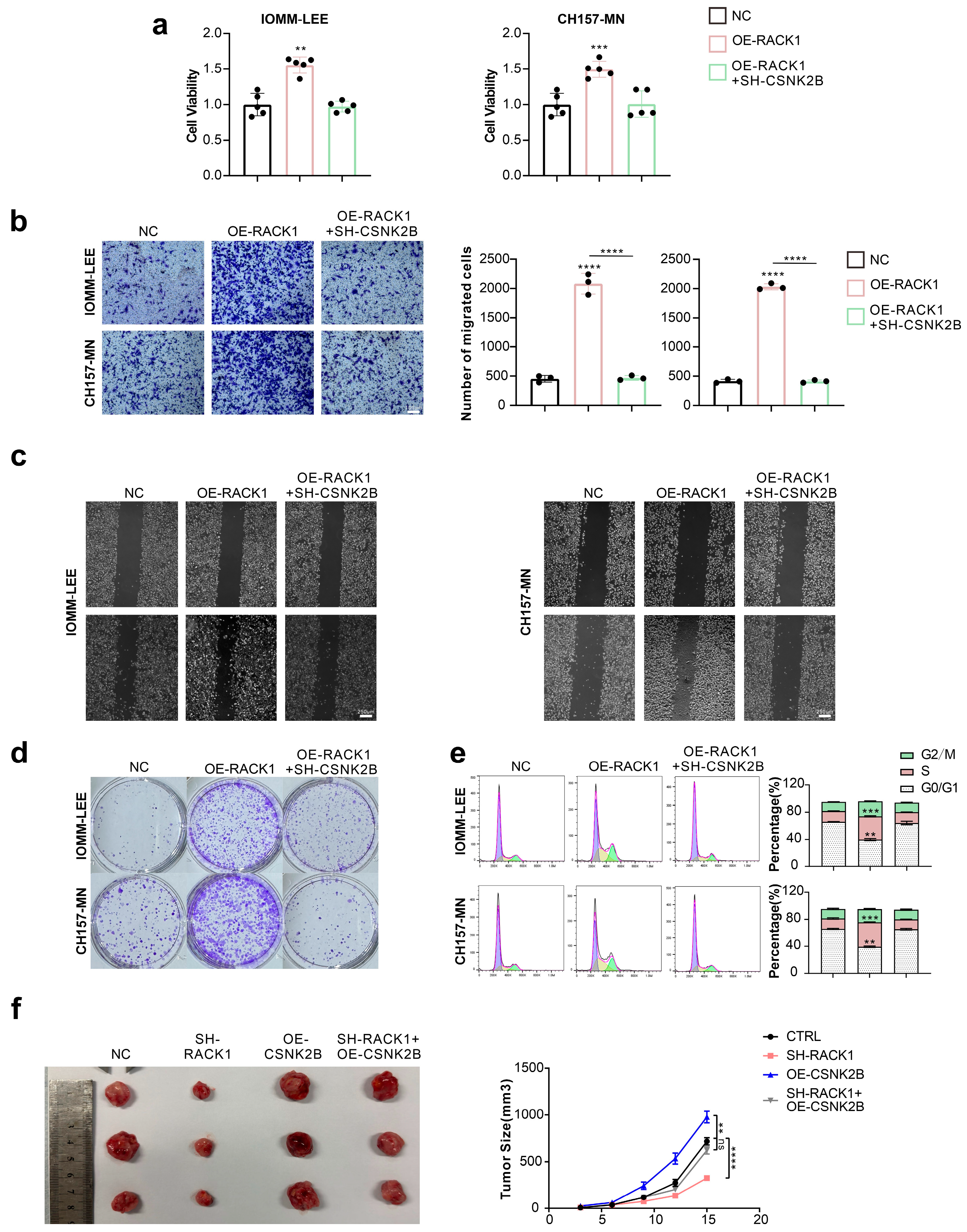
3.4. CSNK2B Promotes the Proliferation and Migration Ability of Meningioma Cells and Affects the Cell Cycle
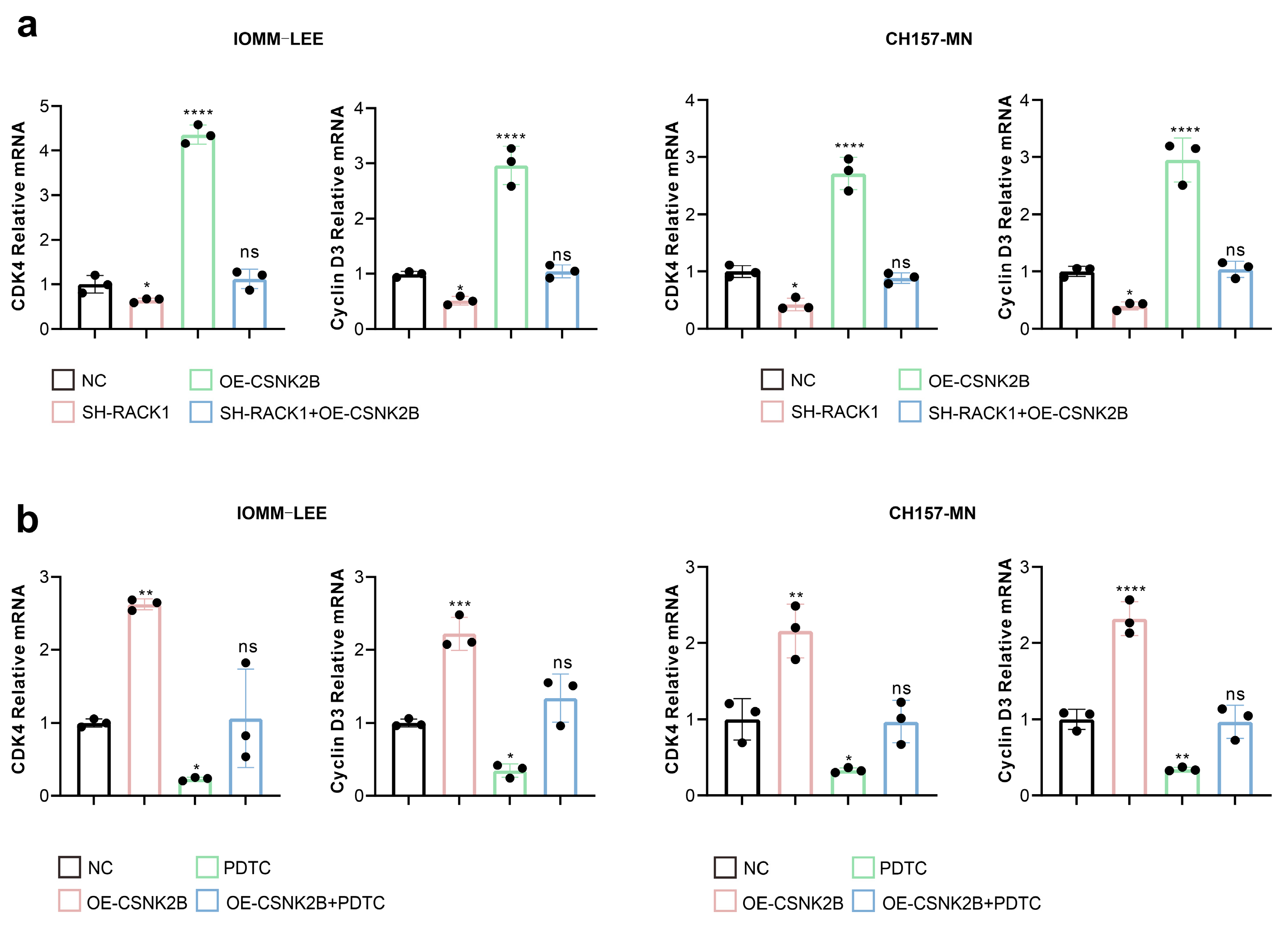
3.5. CSNK2B Promotes the Expression of CDK4 and Cyclin D3 by Activating the NF-κB Pathway
3.6. RACK1 Inhibitor HA Suppresses Value-Added Migration of Meningioma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. 4), iv1–iv62. [Google Scholar] [CrossRef]
- Christine, M.; Marco, H.; Karl, R.; Michele, R.; Milena, S.; Elena, M.; Charles, V. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Castro, L.N.G. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2021, 128, 47–58. [Google Scholar] [CrossRef]
- Di Nunno, V.; Giannini, C.; Asioli, S.; Conti, A.; Furtner, J.; Balestrini, D.; Tosoni, A. Diagnostic and Therapeutic Strategy in Anaplastic (Malignant) Meningioma, CNS WHO Grade 3. Cancers 2022, 14, 4689. [Google Scholar] [CrossRef]
- Sherman, W.; Raizer, J. Chemotherapy: What is its role in meningioma? Expert Rev. Neurother. 2012, 12, 1189–1196. [Google Scholar] [CrossRef]
- Chamberlain, M.C. The role of chemotherapy and targeted therapy in the treatment of intracranial meningioma. Curr. Opin. Oncol. 2012, 24, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.D.; Meddis, A.; Mirian, C.; Haslund-Vinding, J.; Bartek, J.; Krog, S.M.; Nguyen, T.U.P.; Areškevičiūtė, A.; Melchior, L.C.; Heegaard, S.; et al. Gene expression analysis during progression of malignant meningioma compared to benign meningioma. J. Neurosurg. 2022, 1, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; Shah, M.A. Targeting the Cell Cycle: A New Approach to Cancer Therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2021, 32, 30–44. [Google Scholar] [CrossRef]
- Swanton, C. Cell-cycle targeted therapies. Lancet Oncol. 2004, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, L.; Lv, W.; Dong, C.; Wang, Y.; Zhang, J. Overexpression of cyclin D1 in meningioma is associated with malignancy grade and causes abnormalities in apoptosis, invasion and cell cycle progression. Med Oncol. 2014, 32, 439. [Google Scholar] [CrossRef] [PubMed]
- Young, J.S.; Kidwell, R.L.; Zheng, A.; Haddad, A.F.; Aghi, M.K.; Raleigh, D.R.; Schulte, J.D.; Butowski, N.A. CDK 4/6 inhibitors for the treatment of meningioma. Front. Oncol. 2022, 12, 931371. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Xie, D. RACK1, a versatile hub in cancer. Oncogene 2014, 34, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, H.C.; Kiely, P.A.; O’Connor, R. Effects of RACK1 on cell migration and IGF-I signalling in cardiomyoctes are not dependent on an association with the IGF-IR. Cell. Signal. 2007, 19, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Liu, S.; Liu, J.; Liu, D.; Yin, F.; Wei, Z.; Wang, J.; Zhou, Y.; Jiang, L.; Ji, N.; et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-κB pathway in oral squamous cell carcinoma. Mol. Oncol. 2020, 14, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhao, M.; Liu, J.; Zhang, X.; Pei, Y.; Wang, J.; Yang, X.; Shen, B.; Zhang, J. RACK1 Promotes Self-Renewal and Chemoresistance of Cancer Stem Cells in Human Hepatocellular Carcinoma through Stabilizing Nanog. Theranostics 2019, 9, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Si, W.; Ji, W.; Wang, Z.; Gao, Z.; Tian, R.; Song, W.; Zhang, H.; Niu, R.; Zhang, F. Rack1 mediates tyrosine phosphorylation of Anxa2 by Src and promotes invasion and metastasis in drug-resistant breast cancer cells. Breast Cancer Res. 2019, 21, 66. [Google Scholar] [CrossRef]
- Wu, H.; Song, S.; Yan, A.; Guo, X.; Chang, L.; Xu, L.; Hu, L.; Kuang, M.; Liu, B.; He, D.; et al. RACK1 promotes the invasive activities and lymph node metastasis of cervical cancer via galectin-1. Cancer Lett. 2019, 469, 287–300. [Google Scholar] [CrossRef]
- Tian, R.; Tian, J.; Zuo, X.; Ren, S.; Zhang, H.; Liu, H.; Wang, Z.; Cui, Y.; Niu, R.; Zhang, F. RACK1 facilitates breast cancer progression by competitively inhibiting the binding of β-catenin to PSMD2 and enhancing the stability of β-catenin. Cell Death Dis. 2023, 14, 685. [Google Scholar] [CrossRef]
- Pi, Y.; Feng, Q.; Sun, F.; Wang, Z.; Zhao, Y.; Chen, D.; Liu, Y.; Lou, G. Loss of SMURF2 expression enhances RACK1 stability and promotes ovarian cancer progression. Cell Death Differ. 2023, 30, 2382–2392. [Google Scholar] [CrossRef]
- Peng, R.; Jiang, B.; Ma, J.; Ma, Z.; Wan, X.; Liu, H.; Chen, Z.; Cheng, Q.; Chen, R. Forced downregulation of RACK1 inhibits glioma development by suppressing Src/Akt signaling activity. Oncol. Rep. 2013, 30, 2195–2202. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, Y. RACK1 affects glioma cell growth and differentiation through the CNTN2-mediated RTK/Ras/MAPK pathway. Int. J. Mol. Med. 2015, 37, 251–257. [Google Scholar] [CrossRef]
- Matsumoto, M.; Modliszewski, J.L.; Shinozaki, K.; Maezawa, R.; Perez, V.M.; Ishikawa, Y.; Suzuki, R.; McKnight, K.L.; Masaki, T.; Hirai-Yuki, A.; et al. CSNK2B modulates IRF1 binding to functional DNA elements and promotes basal and agonist-induced antiviral signaling. Nucleic Acids Res. 2023, 51, 4451–4466. [Google Scholar] [CrossRef] [PubMed]
- Azad, P.; Zhou, D.; Tu, H.-C.; Villafuerte, F.C.; Traver, D.; Rana, T.M.; Haddad, G.G. Long noncoding RNA HIKER regulates erythropoiesis in Monge’s disease via CSNK2B. J. Clin. Investig. 2023, 133, e165831. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, S.; Qiu, F.; Ding, X.; Sun, Y.; Wei, C.; Hu, X.; Wei, K.; Long, S.; Xie, L.; et al. Tumor necrosis factor α-induced protein 1 as a novel tumor suppressor through selective downregulation of CSNK2B blocks nuclear factor-κB activation in hepatocellular carcinoma. EBioMedicine 2020, 51, 102603. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.; Albanese, C.; Steer, J.; Fu, M.; Bouzahzah, B.; Pestell, R.G. NF-κB and cell-cycle regulation: The cyclin connection. Cytokine Growth Factor Rev. 2001, 12, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.-Y.; Wu, X.-T.; Chen, C.; Liu, X.-Q.; Zhu, L.; Luo, J.-G.; Kong, L.-Y. Photoaffinity Probe Reveals the Potential Target of Harringtonolide for Cancer Cell Migration Inhibition. ACS Med. Chem. Lett. 2022, 13, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, L.; Chen, C.; Tao, Y.; Zhou, W.; Kong, L.; Luo, J. Semi-Synthesis of Harringtonolide Derivatives and Their Antiproliferative Activity. Molecules 2021, 26, 1380. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Chen, C.; Liu, X.; Wu, X.; Zhu, L.; Luo, J.; Kong, L. Hainanolide inhibits the progression of colon cancer via inducing the cell cycle arrest, cell apoptosis and activation of the MAPK signaling pathway. Toxicol. Appl. Pharmacol. 2022, 454, 116249. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Hawasli, A.H.; Huang, J.; Chicoine, M.R.; Kim, A.H. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg. Focus 2015, 38, E3. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Tang, H.; Yang, K. PER1 suppresses glycolysis and cell proliferation in oral squamous cell carcinoma via the PER1/RACK1/PI3K signaling complex. Cell Death Dis. 2021, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Wang, L.; Xi, Z.; Shen, H.; Jiang, Y.; Zhou, F.; Liu, Y.; Zhou, Y. MYO10 contributes to the malignant phenotypes of colorectal cancer via RACK1 by activating integrin/Src/FAK signaling. Cancer Sci. 2022, 113, 3838–3851. [Google Scholar] [CrossRef]
- Sato, T.; Takahashi, H.; Hatakeyama, S.; Iguchi, A.; Ariga, T. The TRIM-FLMN protein TRIM45 directly interacts with RACK1 and negatively regulates PKC-mediated signaling pathway. Oncogene 2014, 34, 1280–1291. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, X.; Qin, L.; Deng, H.; Wang, J.; Ren, W.; Li, H.; Zhao, L.; Liu, H.; Yan, H.; et al. A novel UBE2T inhibitor suppresses Wnt/β-catenin signaling hyperactivation and gastric cancer progression by blocking RACK1 ubiquitination. Oncogene 2020, 40, 1027–1042. [Google Scholar] [CrossRef]
- Pereira, B.J.A.; Lerario, A.M.; Sola, P.R.; Laurentino, T.d.S.; Mohan, D.R.; de Almeida, A.N.; de Aguiar, P.H.P.; Paiva, W.d.S.; Wakamatsu, A.; Teixeira, M.J.; et al. Impact of a cell cycle and an extracellular matrix remodeling transcriptional signature on tumor progression and correlation with EZH2 expression in meningioma. J. Neurosurg. 2023, 138, 649–662. [Google Scholar] [CrossRef]
- Mondielli, G.; Mougel, G.; Darriet, F.; Roche, C.; Querdray, A.; Lisbonis, C.; Appay, R.; Dufour, H.; Chinot, O.; Graillon, T.; et al. Co-Targeting MAP Kinase and Pi3K-Akt-mTOR Pathways in Meningioma: Preclinical Study of Alpelisib and Trametinib. Cancers 2022, 14, 4448. [Google Scholar] [CrossRef]
- Johnson, M.; O’Connell, M.; Walter, K. STAT3 activation and risk of recurrence in meningiomas. Oncol. Lett. 2017, 13, 2432–2436. [Google Scholar] [CrossRef]
- Bakshi, H.A.; Quinn, G.A.; Nasef, M.M.; Mishra, V.; Aljabali, A.A.A.; El-Tanani, M.; Serrano-Aroca, Á.; Da Silva, M.W.; McCarron, P.A.; Tambuwala, M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells 2022, 11, 1502. [Google Scholar] [CrossRef] [PubMed]
- De Simone, V.; Franzè, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2014, 34, 3493–3503. [Google Scholar] [CrossRef]
- Dolezal, E.; Infantino, S.; Drepper, F.; Börsig, T.; Singh, A.; Wossning, T.; Fiala, G.J.; Minguet, S.; Warscheid, B.; Tarlinton, D.M.; et al. The BTG2-PRMT1 module limits pre-B cell expansion by regulating the CDK4-Cyclin-D3 complex. Nat. Immunol. 2017, 18, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, S.I.; Pelletier, L.A.; Espada, A.; Gutiérrez, J.; Sanfeliciano, S.M.G.; Rauch, C.T.; Ganado, M.P.; Baquero, C.; Zapatero, E.; Zhang, A.; et al. Crystal structure of active CDK4-cyclin D and mechanistic basis for abemaciclib efficacy. NPJ Breast Cancer 2022, 8, 126. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maalim, A.A.; Wang, Z.; Huang, Y.; Lei, T. RACK1 Promotes Meningioma Progression by Activation of NF-κB Pathway via Preventing CSNK2B from Ubiquitination Degradation. Cancers 2024, 16, 767. https://doi.org/10.3390/cancers16040767
Maalim AA, Wang Z, Huang Y, Lei T. RACK1 Promotes Meningioma Progression by Activation of NF-κB Pathway via Preventing CSNK2B from Ubiquitination Degradation. Cancers. 2024; 16(4):767. https://doi.org/10.3390/cancers16040767
Chicago/Turabian StyleMaalim, Ali Abdi, Zihan Wang, Yimin Huang, and Ting Lei. 2024. "RACK1 Promotes Meningioma Progression by Activation of NF-κB Pathway via Preventing CSNK2B from Ubiquitination Degradation" Cancers 16, no. 4: 767. https://doi.org/10.3390/cancers16040767




