Individual Survival Distributions Generated by Multi-Task Logistic Regression Yield a New Perspective on Molecular and Clinical Prognostic Factors in Gastric Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Models and Statistical Analysis
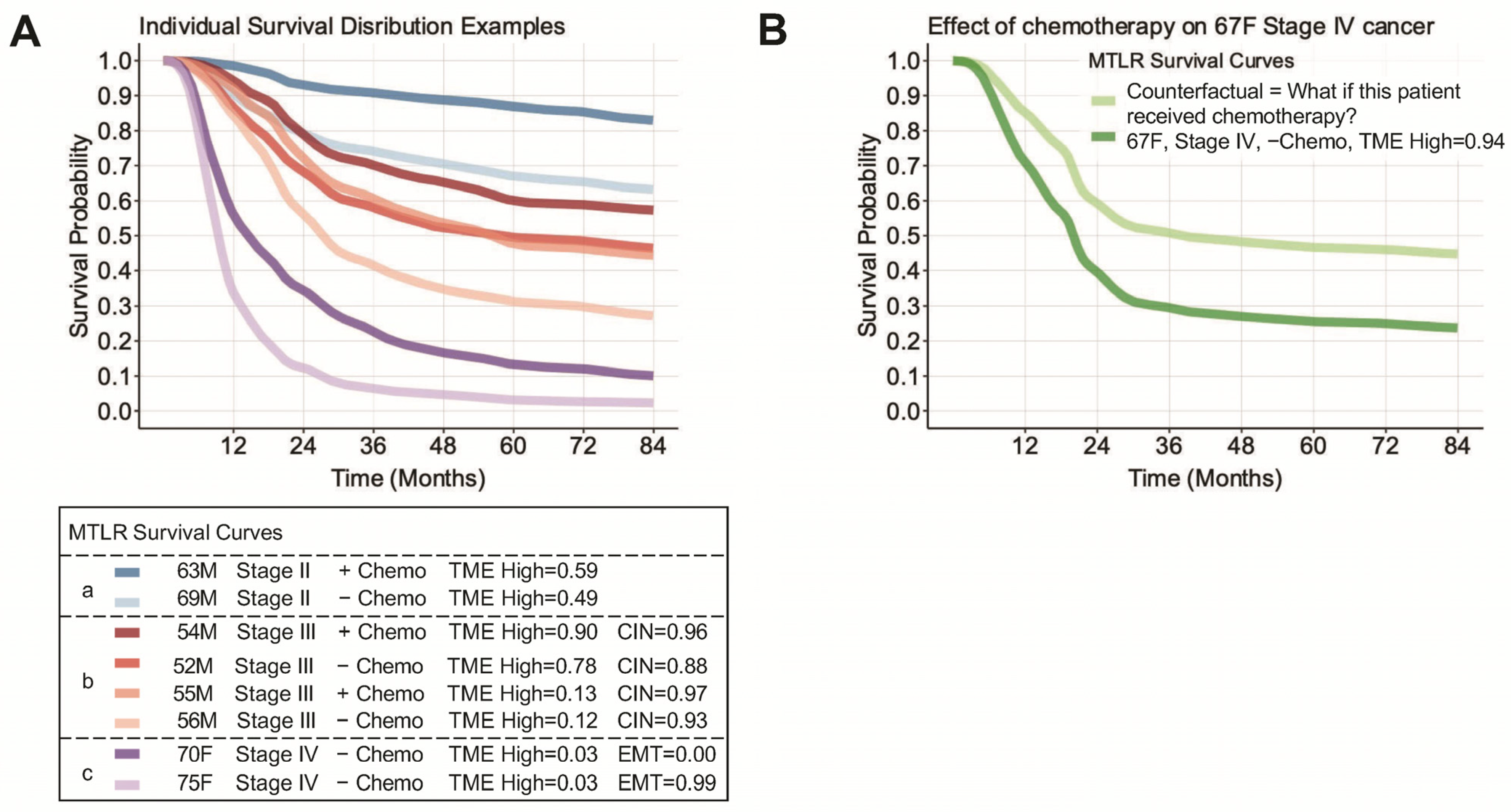
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive Molecular Characterization of Clinical Responses to PD-1 Inhibition in Metastatic Gastric Cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular Analysis of Gastric Cancer Identifies Subtypes Associated with Distinct Clinical Outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Patel, M.A.; Kratz, J.D.; Lubner, S.J.; Loconte, N.K.; Uboha, N.V. Esophagogastric Cancers: Integrating Immunotherapy Therapy into Current Practice. J. Clin. Oncol. 2022, 40, 2751–2762. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- André, T.; André, A.; Tougeron, D.; Piessen, G.; De La Fouchardì, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2022, 41, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, H.; Shin, S.-J.; Kim, H.Y.; Lee, J.; Yang, H.-K.; Kim, W.H.; Kim, Y.-W.; Kook, M.-C.; Park, Y.K.; et al. Microsatellite Instability and Programmed Cell Death-Ligand 1 Expression in Stage II/III Gastric Cancer: Post Hoc Analysis of the CLASSIC Randomized Controlled Study. Ann. Surg. 2019, 270, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability as a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F. Chemotherapy for Resectable Microsatellite Instability-High Gastric Cancer? Lancet Oncol. 2020, 21, 203. [Google Scholar] [CrossRef]
- Smyth, E.C. Chemotherapy for Resectable Microsatellite Instability-High Gastric Cancer? Lancet Oncol. 2020, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.; Hoehn, B.; Davis, S.; Greiner, R. Effective Ways to Build and Evaluate Individual Survival Distributions. J. Mach. Learn. Res. 2020, 21, 85. [Google Scholar]
- Haider, H. MTLR: Survival Prediction with Multi-Task Logistic Regression; 2019. Available online: https://github.com/haiderstats/MTLR. (accessed on 16 June 2021).
- Cheung, C.C.; Vittinghoff, E.; Marcus, G.M.; Gerstenfeld, E.P. Beware of the Hazards: Limitations of the Proportional Hazards Assumption. EP Eur. 2021, 23, 2048. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Skubleny, D.; Parkes, M.; Verma, R.; Davis, S.; Kumar, L.; Aissiou, A.; Greiner, R. Learning Individual Survival Models from PanCancer Whole Transcriptome Data. Clin. Cancer Res. 2023, 29, 3924–3936. [Google Scholar] [CrossRef]
- Zeng, D.; Li, M.; Zhou, R.; Zhang, J.; Sun, H.; Shi, M.; Bin, J.; Liao, Y.; Rao, J.; Liao, W. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol. Res. 2019, 7, 737–750. [Google Scholar] [CrossRef]
- Skubleny, D.; Purich, K.; Williams, T.; Wickware, J.; McLean, D.R.; Martins-Filho, S.N.; Buttenschoen, K.; Haase, E.; McCall, M.; Ghosh, S.; et al. The Tumour Immune Microenvironment Drives Survival Outcomes and Therapeutic Response in an Integrated Molecular Analysis of Gastric Adenocarcinoma. Clin. Cancer Res. 2023; submitted. [Google Scholar]
- Qi, S.-A.; Kumar, N.; Farrokh, M.; Sun, W.; Kuan, L.-H.; Ranganath, R.; Henao, R.; Greiner, R. An Effective Meaningful Way to Evaluate Survival Models. Proc. Mach. Learn. Res. 2023, 202, 28244–28276. Available online: https://nyuscholars.nyu.edu/en/publications/an-effective-meaningful-way-to-evaluate-survival-models (accessed on 6 December 2023).
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemesbow, S. Goodness of Fit Tests for the Multiple Logistic Regression Model. Commun. Stat. Theory Methods 1980, 9, 1043–1069. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. Marginal Likelihoods Based on Cox’s Regression and Life Model. Biometrika 1973, 60, 267–278. [Google Scholar] [CrossRef]
- Simon, N.; Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J. Stat. Softw. 2011, 39, 1–13. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection via the Elastic Net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random Survival Forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Stute, W. Consistent Estimation Under Random Censorship When Covariables Are Present. J. Multivar. Anal. 1993, 45, 89–103. [Google Scholar] [CrossRef]
- Yu, C.-N.; Greiner, R.; Lin, H.-C.; Baracos, V. Learning Patient-Specific Cancer Survival Distributions as a Sequence of Dependent Regressors. In Advances in Neural Information Processing Systems, Proceedings of the 24th International Conference on Neural Information Processing Systems, Granada, Spain, 12–15 December 2011; Shawe-Taylor, J., Zemel, R., Bartlett, P., Pereira, F., Weinberger, K.Q., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2011; Volume 24. [Google Scholar]
- Hyman, J.M. Accurate Monotonicity Preserving Cubic Interpolation. SIAM J. Sci. Stat. Comput. 1983, 4, 645–654. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Kim, G.H.; Bang, S.J.; Ende, A.R.; Hwang, J.H. Is Screening and Surveillance for Early Detection of Gastric Cancer Needed in Korean Americans? Korean J. Intern. Med. 2015, 30, 747–758. [Google Scholar] [CrossRef]
- Shah, S.C.; Canakis, A.; Peek, R.M.; Saumoy, M. Endoscopy for Gastric Cancer Screening Is Cost Effective for Asian Americans in the United States. Clin. Gastroenterol. Hepatol. 2020, 18, 3026–3039. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Liang, P.S.; Bang, S.J.; Hwang, J.H. Screening and Surveillance for Gastric Cancer in the United States: Is It Needed? Gastrointest. Endosc. 2016, 84, 18–28. [Google Scholar] [CrossRef]
- Park, Y.H.; Lal, S.; Lee, J.E.; Choi, Y.L.; Wen, J.; Ram, S.; Ding, Y.; Lee, S.H.; Powell, E.; Lee, S.K.; et al. Chemotherapy Induces Dynamic Immune Responses in Breast Cancers That Impact Treatment Outcome. Nat. Commun. 2020, 11, 6175. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T.; et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Lagorce Pagès, C.; Marliot, F.; Custers, P.A.; Dizdarevic, E.; Sroussi, M.; Castillo-Martin, M.; Haicheur, N.; Dermani, M.; et al. International Validation of the Immunoscore Biopsy in Patients with Rectal Cancer Managed by a Watch-and-Wait Strategy. J. Clin. Oncol. 2023, 42, 70–80. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Saw, P.E.; Song, E. Turning Cold Tumors Hot: From Molecular Mechanisms to Clinical Applications. Trends Immunol. 2022, 43, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA Neoantigen Vaccines Stimulate T Cells in Pancreatic Cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Algazi, A.P.; Twitty, C.G.; Tsai, K.K.; Le, M.; Pierce, R.; Browning, E.; Hermiz, R.; Canton, D.A.; Bannavong, D.; Oglesby, A.; et al. Phase II Trial of IL-12 Plasmid Transfection and PD-1 Blockade in Immunologically Quiescent Melanoma. Clin. Cancer Res. 2020, 26, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 Antagonist, in Combination with Pembrolizumab and Chemotherapy for Pancreatic Cancer: The COMBAT Trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in Oncology: More than Meets the Eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Qi, S.-A.; Sun, W.; Greiner, R. SurvivalEVAL: A Comprehensive Open-Source Python Package for Evaluating Individual Survival Distributions. In Proceedings of the 2023 AAAI Fall Symposia, Arlington, VA, USA, 25–27 October 2023. [Google Scholar] [CrossRef]

| Characteristic | n/N (Missing %) | N = 1043 1 |
|---|---|---|
| Age | 1043/1043 (0%) | 59 (49, 67) |
| Stage | 1043/1043 (0%) | |
| I | 170 (16%) | |
| II | 330 (32%) | |
| III | 339 (33%) | |
| IV | 204 (20%) | |
| Sex | 1043/1043 (0%) | |
| Female | 359 (34%) | |
| Male | 684 (66%) | |
| TCGA Subtype | 1043/1043 (0%) | |
| Chromosomal Instability | 824 (79%) | |
| Epstein–Barr Virus Type | 43 (4.1%) | |
| Genomically Stable | 66 (6.3%) | |
| Microsatellite Instability | 110 (11%) | |
| ACRG Subtype | 1043/1043 (0%) | |
| Epithelial-to-Mesenchymal Transition | 118 (11%) | |
| Microsatellite Instability | 162 (16%) | |
| Microsatellite Stable TP53 Negative | 412 (40%) | |
| Microsatellite Stable TP53 Positive | 351 (34%) | |
| TME Subtype | 1043/1043 (0%) | |
| High | 478 (46%) | |
| Low | 565 (54%) | |
| Lauren Classification | 1043/1043 (0%) | |
| Diffuse | 495 (47%) | |
| Intestinal | 504 (48%) | |
| Mixed | 44 (4.2%) | |
| Tumour Location | 1043/1043 (0%) | |
| Distal | 537 (51%) | |
| Proximal | 482 (46%) | |
| Whole | 24 (2.3%) | |
| Treatment | 1043/1043 (0%) | |
| No | 299 (29%) | |
| Yes | 744 (71%) | |
| Study | 1043/1043 (0%) | |
| ACRG | 219 (21%) | |
| Kosin | 98 (9.4%) | |
| KUGH | 82 (7.9%) | |
| Samsung | 432 (41%) | |
| TCGA | 151 (14%) | |
| Yonsei MDACC | 61 (5.8%) |
| Model | |||||
|---|---|---|---|---|---|
| Metric | AFT | CoxKP | CoxKPEN | MTLR | RSF |
| Concordance 1 | 0.720 ± 0.028 | 0.721 ± 0.028 | 0.722 ± 0.029 | 0.721 ± 0.033 | 0.699 ± 0.048 |
| D-Calibration 2 | 0.425 | 0.993 | 0.994 | 0.980 | 0.866 |
| 1-Calibration 10th 2 | 0.147 | 0.447 | 0.898 | 0.086 | 0.354 |
| 1-Calibration 25th 2 | 0.024 | 0.470 | 0.477 | 0.506 | 0.026 |
| 1-Calibration 50th 2 | 0.000 | 0.011 | 0.027 | 0.042 | 0.447 |
| 1-Calibration 75th 2 | 0.000 | 0.002 | 0.006 | 0.243 | 0.655 |
| 1-Calibration 90th 2 | 0.000 | 0.009 | 0.027 | 0.132 | 0.050 |
| Integrated Brier 1 | 0.176 ± 0.015 | 0.169 ± 0.011 | 0.169 ± 0.011 | 0.178 ± 0.015 | 0.178 ± 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skubleny, D.; Spratlin, J.; Ghosh, S.; Greiner, R.; Schiller, D.E.; Rayat, G.R. Individual Survival Distributions Generated by Multi-Task Logistic Regression Yield a New Perspective on Molecular and Clinical Prognostic Factors in Gastric Adenocarcinoma. Cancers 2024, 16, 786. https://doi.org/10.3390/cancers16040786
Skubleny D, Spratlin J, Ghosh S, Greiner R, Schiller DE, Rayat GR. Individual Survival Distributions Generated by Multi-Task Logistic Regression Yield a New Perspective on Molecular and Clinical Prognostic Factors in Gastric Adenocarcinoma. Cancers. 2024; 16(4):786. https://doi.org/10.3390/cancers16040786
Chicago/Turabian StyleSkubleny, Daniel, Jennifer Spratlin, Sunita Ghosh, Russell Greiner, Daniel E. Schiller, and Gina R. Rayat. 2024. "Individual Survival Distributions Generated by Multi-Task Logistic Regression Yield a New Perspective on Molecular and Clinical Prognostic Factors in Gastric Adenocarcinoma" Cancers 16, no. 4: 786. https://doi.org/10.3390/cancers16040786
APA StyleSkubleny, D., Spratlin, J., Ghosh, S., Greiner, R., Schiller, D. E., & Rayat, G. R. (2024). Individual Survival Distributions Generated by Multi-Task Logistic Regression Yield a New Perspective on Molecular and Clinical Prognostic Factors in Gastric Adenocarcinoma. Cancers, 16(4), 786. https://doi.org/10.3390/cancers16040786






