Lynch Syndrome: From Multidisciplinary Management to Precision Prevention
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Molecular Genetics and Phenotypic Heterogeneity
4. CRC Development in Individuals with LS
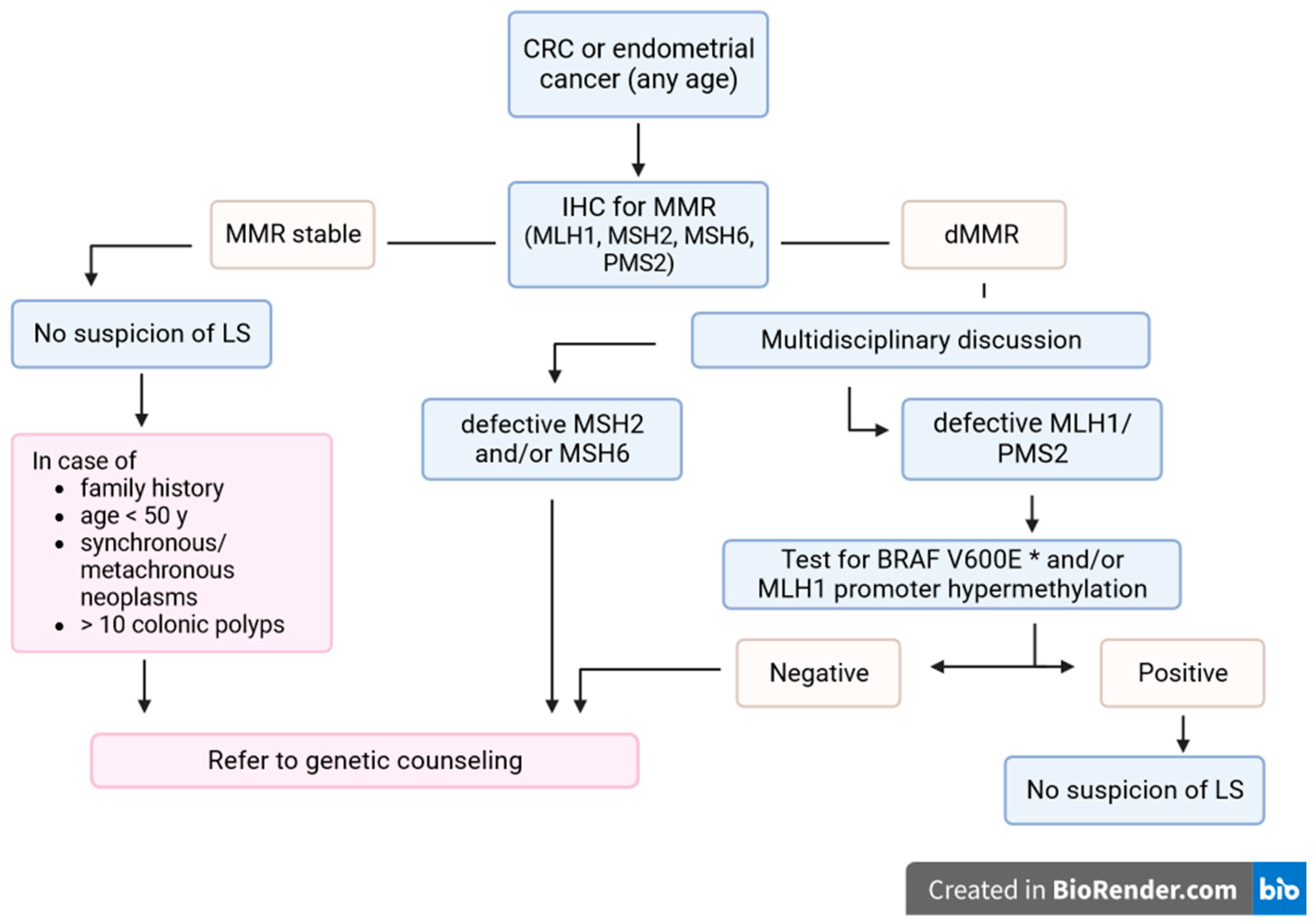
5. Genetic Counselling
6. Surveillance Recommendations
7. Treatment of LS Cancers; Immunotherapy and Immunoprevention
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch Syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef]
- Peltomäki, P.; Nyström, M.; Mecklin, J.P.; Seppälä, T.T. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology 2023, 164, 783–799. [Google Scholar] [CrossRef]
- Win, A.K.; Jenkins, M.A.; Dowty, J.G.; Antoniou, A.C.; Lee, A.; Giles, G.G.; Buchanan, D.D.; Clendenning, M.; Rosty, C.; Ahnen, D.J.; et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 404–412. [Google Scholar] [CrossRef]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer Susceptibility Gene Mutations in Individuals with Colorectal Cancer. J. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef]
- Ring, K.L.; Bruegl, A.S.; Allen, B.A.; Elkin, E.P.; Singh, N.; Hartman, A.R.; Daniels, M.S.; Broaddus, R.R. Germline Multi-Gene Hereditary Cancer Panel Testing in an Unselected Endometrial Cancer Cohort. Mod. Pathol. 2016, 29, 1381–1389. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer Risk and Survival in Path_MMR Carriers by Gene and Gender up to 75 Years of Age: A Report from the Prospective Lynch Syndrome Database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef]
- Sehgal, R.; Sheahan, K.; O’Connell, P.; Hanly, A.; Martin, S.; Winter, D. Lynch Syndrome: An Updated Review. Genes 2014, 5, 497–507. [Google Scholar] [CrossRef]
- Cunningham, L.A.; Gasior, A.; Kalady, M.F. Management of Colorectal Cancer in Hereditary Syndromes. Surg. Oncol. Clin. N. Am. 2022, 31, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Allen, J.I.; Axilbund, J.E.; Boland, R.C.; Burke, C.A.; Burt, R.W.; Church, J.M.; Dominitz, J.A.; Johnson, D.A.; Kaltenbach, T.; et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2014, 109, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Fishel, R.; Lescoe, M.K.; Rao, M.R.S.; Copeland, N.G.; Jenkins, N.A.; Garber, J.; Kane, M.; Kolodner, R. The Human Mutator Gene Homolog MSH2 and Its Association with Hereditary Nonpolyposis Colon Cancer. Cell 1993, 75, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.T.; Ahadova, A.; Crosbie, E.J.; Holinski-Feder, E.; Scott, R.; Haupt, S.; Möslein, G.; Winship, I.; Broeke, S.W.B.T.; et al. Dominantly Inherited Micro-Satellite Instable Cancer—The Four Lynch Syndromes—An EHTG, PLSD Position Statement. Hered. Cancer Clin. Pract. 2023, 21, 19. [Google Scholar] [CrossRef]
- Barnetson, R.A.; Tenesa, A.; Farrington, S.M.; Nicholl, I.D.; Cetnarskyj, R.; Porteous, M.E.; Campbell, H.; Dunlop, M.G. Identification and Survival of Carriers of Mutations in DNA Mismatch-Repair Genes in Colon Cancer. New Engl. J. Med. 2006, 354, 2751–2763. [Google Scholar] [CrossRef]
- Eshleman, J.R.; Markowitz, S.D. Mismatch Repair Defects in Human Carcinogenesis. Hum. Mol. Genet. 1996, 5, 1489–1494. [Google Scholar] [CrossRef]
- Hitchins, M.P.; Ward, R.L. Constitutional (Germline) MLH1 Epimutation as an Aetiological Mechanism for Hereditary Non-Polyposis Colorectal Cancer. J. Med. Genet. 2009, 46, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.L.; Yuen, S.T.; Kong, C.K.; Chan, Y.W.; Chan, A.S.Y.; Ng, W.F.; Tsui, W.Y.; Lo, M.W.S.; Tam, W.Y.; Li, V.S.W.; et al. Heritable Germline Epimutation of MSH2 in a Family with Hereditary Nonpolyposis Colorectal Cancer. Nat. Genet. 2006, 38, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, M.J.L.; Kuiper, R.P.; Chan, T.L.; Goossens, M.; Hebeda, K.M.; Voorendt, M.; Lee, T.Y.H.; Bodmer, D.; Hoenselaar, E.; Hendriks-Cornelissen, S.J.B.; et al. Heritable Somatic Methylation and Inactivation of MSH2 in Families with Lynch Syndrome Due to Deletion of the 3′ Exons of TACSTD1. Nat. Genet. 2008, 41, 112–117. [Google Scholar] [CrossRef]
- Kuismanen, S.A.; Holmberg, M.T.; Salovaara, R.; De La Chapelle, A.; Ivi Peltomä, P. Genetic and Epigenetic Modification of MLH1 Accounts for a Major Share of Microsatellite-Unstable Colorectal Cancers. Am. J. Pathol. 2000, 156, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Hitchins, M.P.; Lin, V.A.; Buckle, A.; Cheong, K.; Halani, N.; Ku, S.; Kwok, C.T.; Packham, D.; Suter, C.M.; Meagher, A.; et al. Epigenetic Inactivation of a Cluster of Genes Flanking MLH1 in Microsatellite-Unstable Colorectal Cancer. Cancer Res. 2007, 67, 9107–9116. [Google Scholar] [CrossRef]
- Kwok, C.T.; Vogelaar, I.P.; Van Zelst-Stams, W.A.; Mensenkamp, A.R.; Ligtenberg, M.J.; Rapkins, R.W.; Ward, R.L.; Chun, N.; Ford, J.M.; Ladabaum, U.; et al. The MLH1 c.-27C>A and c.85G>T Variants Are Linked to Dominantly Inherited MLH1 Epimutation and Are Borne on a European Ancestral Haplotype. Eur. J. Hum. Genet. 2014, 22, 617–624. [Google Scholar] [CrossRef]
- Farrell, M.P.; Hughes, D.J.; Berry, I.R.; Gallagher, D.J.; Glogowski, E.A.; Payne, S.J.; Kennedy, M.J.; Clarke, R.M.; White, S.A.; Muldoon, C.B.; et al. Clinical Correlation and Molecular Evaluation Confirm That the MLH1 p.Arg182Gly (c.544A>G) Mutation Is Pathogenic and Causes Lynch Syndrome. Fam. Cancer 2012, 11, 509–518. [Google Scholar] [CrossRef]
- Medina-Arana, V.; Barrios, Y.; Fernández-Peralta, A.; Herrera, M.; Chinea, N.; Lorenzo, N.; Jiménez, A.; Martín-López, J.V.; González-Hermoso, F.; Salido, E.; et al. New Founding Mutation in MSH2 Associated with Hereditary Nonpolyposis Colorectal Cancer Syndrome on the Island of Tenerife. Cancer Lett. 2006, 244, 268–273. [Google Scholar] [CrossRef]
- Thompson, B.A.; Spurdle, A.B.; Plazzer, J.-P.; Greenblatt, M.S.; Akagi, K.; Al-Mulla, F.; Bapat, B.; Bernstein, I.; Capellá, G.; den Dunnen, J.T.; et al. Application of a 5-Tiered Scheme for Standardized Classification of 2,360 Unique Mismatch Repair Gene Variants in the InSiGHT Locus-Specific Database. Nat. Genet. 2014, 46, 107–115. [Google Scholar] [CrossRef]
- Van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Balaguer, F.; Dekker, E.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Ricciardiello, L.; Rupińska, M.; et al. Endoscopic Management of Lynch Syndrome and of Familial Risk of Colorectal Cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Shaw, M.W.; Magnuson, C.W.; Larsen, A.L.; Krush, A.J. Hereditary Factors in Cancer: Study of Two Large Midwestern Kindreds. Arch. Intern. Med. 1966, 117, 206–212. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition: Development of International Criteria for the Determination of Microsatellite Instability in Colorectal Cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Jenkins, M.; Baglietto, L.; Dowty, J.; Vanvliet, C.; Smith, L.; Mead, L.; Macrae, F.; Stjohn, D.; Jass, J.; Giles, G. Cancer Risks for Mismatch Repair Gene Mutation Carriers: A Population-Based Early Onset Case-Family Study. Clin. Gastroenterol. Hepatol. 2006, 4, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, F. Risk of Colorectal and Endometrial Cancer for Carriers of Mutations of the hMLH1 and hMSH2 Gene: Correction for Ascertainment. J. Med. Genet. 2005, 42, 491–496. [Google Scholar] [CrossRef]
- Aarnio, M.; Sankila, R.; Pukkala, E.; Salovaara, R.; Aaltonen, L.A.; de la Chapelle, A.; Peltomäki, P.; Mecklin, J.P.; Järvinen, H.J. Cancer Risk in Mutation Carriers of DNA-Mismatch-Repair Genes. Int. J. Cancer 1999, 81, 214–218. [Google Scholar] [CrossRef]
- Vasen, H.F.A.; Stormorken, A.; Menko, F.H.; Nagengast, F.M.; Kleibeuker, J.H.; Griffioen, G.; Taal, B.G.; Moller, P.; Wijnen, J.T. MSH2 Mutation Carriers Are at Higher Risk of Cancer Than MLH1 Mutation Carriers: A Study of Hereditary Nonpolyposis Colorectal Cancer Families. J. Clin. Oncol. 2001, 19, 4074–4080. [Google Scholar] [CrossRef]
- Aaltonen, L.A.; Salovaara, R.; Kristo, P.; Canzian, F.; Hemminki, A.; Peltomäki, P.; Chadwick, R.B.; Kääriäinen, H.; Eskelinen, M.; Järvinen, H.; et al. Incidence of Hereditary Nonpolyposis Colorectal Cancer and the Feasibility of Molecular Screening for the Disease. New Engl. J. Med. 1998, 338, 1481–1487. [Google Scholar] [CrossRef]
- Bonadona, V.; Bonaïti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.-J.; Caron, O.; et al. Cancer Risks Associated with Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA 2011, 305, 2304. [Google Scholar] [CrossRef]
- Stoffel, E.; Mukherjee, B.; Raymond, V.M.; Tayob, N.; Kastrinos, F.; Sparr, J.; Wang, F.; Bandipalliam, P.; Syngal, S.; Gruber, S.B. Calculation of Risk of Colorectal and Endometrial Cancer among Patients with Lynch Syndrome. Gastroenterology 2009, 137, 1621–1627. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Cotterchio, M.; McKeown-Eyssen, G.; Neerav, M.; Bapat, B.; Boyd, K.; Gallinger, S.; McLaughlin, J.; Aronson, M.; Briollais, L. Penetrance of Colorectal Cancer among MLH1/MSH2 Carriers Participating in the Colorectal Cancer Familial Registry in Ontario. Hered. Cancer Clin. Pract. 2009, 7, 14. [Google Scholar] [CrossRef]
- Alarcon, F.; Lasset, C.; Carayol, J.; Bonadona, V.; Perdry, H.; Desseigne, F.; Wang, Q.; Bonaïti-Pellié, C. Estimating Cancer Risk in HNPCC by the GRL Method. Eur. J. Hum. Genet. 2007, 15, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M. Cancer Risk Associated with Germline DNA Mismatch Repair Gene Mutations. Hum. Mol. Genet. 1997, 6, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Senter, L.; Clendenning, M.; Sotamaa, K.; Hampel, H.; Green, J.; Potter, J.D.; Lindblom, A.; Lagerstedt, K.; Thibodeau, S.N.; Lindor, N.M.; et al. The Clinical Phenotype of Lynch Syndrome Due to Germ-Line PMS2 Mutations. Gastroenterology 2008, 135, 419–428.e1. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer Incidence and Survival in Lynch Syndrome Patients Receiving Colonoscopic and Gynaecological Surveillance: First Report from the Prospective Lynch Syndrome Database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef]
- Plaschke, J.; Engel, C.; Krüger, S.; Holinski-Feder, E.; Pagenstecher, C.; Mangold, E.; Moeslein, G.; Schulmann, K.; Gebert, J.; von Knebel Doeberitz, M.; et al. Lower Incidence of Colorectal Cancer and Later Age of Disease Onset in 27 Families with Pathogenic MSH6 Germline Mutations Compared with Families with MLH1 or MSH2 Mutations: The German Hereditary Nonpolyposis Colorectal Cancer Consortium. J. Clin. Oncol. 2004, 22, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Lynch Syndrome–Associated Colorectal Cancer. New Engl. J. Med. 2018, 379, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.M.; Shashidharan, M.; Ternent, C.A.; Thorson, A.G.; Blatchford, G.J.; Christensen, M.A.; Lanspa, S.J.; Lemon, S.J.; Watson, P.; Lynch, H.T. Colorectal and Extracolonic Cancer Variations in MLH1/MSH2 Hereditary Nonpolyposis Colorectal Cancer Kindreds and the General Population. Dis. Colon. Rectum 1998, 41, 428–433. [Google Scholar] [CrossRef]
- Ryan, N.A.; McMahon, R.F.; Ramchander, N.C.; Seif, M.W.; Evans, D.G.; Crosbie, E.J. Lynch Syndrome for the Gynaecologist. Obstet. Gynaecol. 2021, 23, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Jackson, S.A.; Susswein, L.R.; Zeinomar, N.; Ma, X.; Marshall, M.L.; Stettner, A.R.; Milewski, B.; Xu, Z.; Solomon, B.D.; et al. MSH6 and PMS2 Germ-Line Pathogenic Variants Implicated in Lynch Syndrome Are Associated with Breast Cancer. Genet. Med. 2018, 20, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Albanese, L.; Signoriello, G.; Napoli, C.; Molinari, A.M. Pancreatic Cancer with Mutation in BRCA1/2, MLH1, and APC Genes: Phenotype Correlation and Detection of a Novel Germline BRCA2 Mutation. Genes 2022, 13, 321. [Google Scholar] [CrossRef]
- Shrestha, K.S.; Aska, E.M.; Tuominen, M.M.; Kauppi, L. Tissue-Specific Reduction in MLH1 Expression Induces Microsatellite Instability in Intestine of Mlh1+/− Mice. DNA Repair. 2021, 106, 103178. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Mutation and Cancer: Statistical Study of Retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Cejka, P.; Stojic, L.; Mojas, N.; Russell, A.M.; Heinimann, K.; Cannavó, E.; Di Pietro, M.; Marra, G.; Jiricny, J. Methylation-Induced G(2)/M Arrest Requires a Full Complement of the Mismatch Repair Protein hMLH1. EMBO J. 2003, 22, 2245–2254. [Google Scholar] [CrossRef]
- Pussila, M.; Törönen, P.; Einarsdottir, E.; Katayama, S.; Krjutškov, K.; Holm, L.; Kere, J.; Peltomäki, P.J.; Mäkinen, M.; Linden, J.; et al. MLH1 Deficiency in Normal Mouse Colon Mucosa Associates with Chromosomally Unstable Colon Cancer. Carcinogenesis 2018, 39, 788–797. [Google Scholar] [CrossRef]
- Kloor, M.; Huth, C.; Voigt, A.Y.; Benner, A.; Schirmacher, P.; von Knebel Doeberitz, M.; Bläker, H. Prevalence of Mismatch Repair-Deficient Crypt Foci in Lynch Syndrome: A Pathological Study. Lancet Oncol. 2012, 13, 598–606. [Google Scholar] [CrossRef]
- Niskakoski, A.; Pasanen, A.; Lassus, H.; Renkonen-Sinisalo, L.; Kaur, S.; Mecklin, J.P.; Bützow, R.; Peltomäki, P. Molecular Changes Preceding Endometrial and Ovarian Cancer: A Study of Consecutive Endometrial Specimens from Lynch Syndrome Surveillance. Mod. Pathol. 2018, 31, 1291–1301. [Google Scholar] [CrossRef]
- Wong, S.; Hui, P.; Buza, N. Frequent Loss of Mutation-Specific Mismatch Repair Protein Expression in Nonneoplastic Endometrium of Lynch Syndrome Patients. Mod. Pathol. 2020, 33, 1172–1181. [Google Scholar] [CrossRef]
- Lee, B.C.H.; Robinson, P.S.; Coorens, T.H.H.; Yan, H.H.N.; Olafsson, S.; Lee-Six, H.; Sanders, M.A.; Siu, H.C.; Hewinson, J.; Yue, S.S.K.; et al. Mutational Landscape of Normal Epithelial Cells in Lynch Syndrome Patients. Nat. Commun. 2022, 13, 2710. [Google Scholar] [CrossRef]
- Ahadova, A.; Seppälä, T.T.; Engel, C.; Gallon, R.; Burn, J.; Holinski-Feder, E.; Steinke-Lange, V.; Möslein, G.; Nielsen, M.; ten Broeke, S.W.; et al. The “Unnatural” History of Colorectal Cancer in Lynch Syndrome: Lessons from Colonoscopy Surveillance. Int. J. Cancer 2021, 148, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Yurgelun, M.B.; Goel, A.; Hornick, J.L.; Sen, A.; Turgeon, D.K.; Ruffin IV, M.T.; Marcon, N.E.; Baron, J.A.; Bresalier, R.S.; Syngal, S.; et al. Microsatellite Instability and DNA Mismatch Repair Protein Deficiency in Lynch Syndrome Colorectal Polyps. Cancer Prev. Res. 2012, 5, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Ahadova, A.; Seppälä, T.T.; Aretz, S.; Bigirwamungu-Bargeman, M.; Bläker, H.; Bucksch, K.; Büttner, R.; de Vos tot Nederveen Cappel, W.T.; Endris, V.; et al. Associations of Pathogenic Variants in MLH1, MSH2, and MSH6 With Risk of Colorectal Adenomas and Tumors and With Somatic Mutations in Patients with Lynch Syndrome. Gastroenterology 2020, 158, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Ahadova, A.; Pfuderer, P.L.; Ahtiainen, M.; Ballhausen, A.; Bohaumilitzky, L.; Kösegi, S.; Müller, N.; Tang, Y.L.; Kosmalla, K.; Witt, J.; et al. Distinct Mutational Profile of Lynch Syndrome Colorectal Cancers Diagnosed under Regular Colonoscopy Surveillance. J. Clin. Med. 2021, 10, 2458. [Google Scholar] [CrossRef]
- Ahadova, A.; von Knebel Doeberitz, M.; Bläker, H.; Kloor, M. CTNNB1-Mutant Colorectal Carcinomas with Immediate Invasive Growth: A Model of Interval Cancers in Lynch Syndrome. Fam. Cancer 2016, 15, 579–586. [Google Scholar] [CrossRef]
- Ten Broeke, S.W.; van Bavel, T.C.; Jansen, A.M.L.; Gómez-García, E.; Hes, F.J.; van Hest, L.P.; Letteboer, T.G.W.; Olderode-Berends, M.J.W.; Ruano, D.; Spruijt, L.; et al. Molecular Background of Colorectal Tumors from Patients with Lynch Syndrome Associated with Germline Variants in PMS2. Gastroenterology 2018, 155, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Helderman, N.C.; Bajwa-ten Broeke, S.W.; Morreau, H.; Suerink, M.; Terlouw, D.; van der Werf-’ t Lam, A.S.; van Wezel, T.; Nielsen, M. The Diverse Molecular Profiles of Lynch Syndrome-Associated Colorectal Cancers Are (Highly) Dependent on Underlying Germline Mismatch Repair Mutations. Crit. Rev. Oncol. Hematol. 2021, 163, 103338. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. New Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.-P.; Möslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; et al. Cancer Prevention with Aspirin in Hereditary Colorectal Cancer (Lynch Syndrome), 10-Year Follow-up and Registry-Based 20-Year Data in the CAPP2 Study: A Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Reyes-Uribe, L.; Wu, W.; Gelincik, O.; Bommi, P.V.; Francisco-Cruz, A.; Solis, L.M.; Lynch, P.M.; Lim, R.; Stoffel, E.M.; Kanth, P.; et al. Naproxen Chemoprevention Promotes Immune Activation in Lynch Syndrome Colorectal Mucosa. Gut 2021, 70, 555–566. [Google Scholar] [CrossRef]
- Bohaumilitzky, L.; Kluck, K.; Hüneburg, R.; Gallon, R.; Nattermann, J.; Kirchner, M.; Kristiansen, G.; Hommerding, O.; Pfuderer, P.L.; Wagner, L.; et al. The Different Immune Profiles of Normal Colonic Mucosa in Cancer-Free Lynch Syndrome Carriers and Lynch Syndrome Colorectal Cancer Patients. Gastroenterology 2022, 162, 907–919.e10. [Google Scholar] [CrossRef]
- Ballhausen, A.; Przybilla, M.J.; Jendrusch, M.; Haupt, S.; Pfaffendorf, E.; Seidler, F.; Witt, J.; Hernandez Sanchez, A.; Urban, K.; Draxlbauer, M.; et al. The Shared Frameshift Mutation Landscape of Microsatellite-Unstable Cancers Suggests Immunoediting during Tumor Evolution. Nat. Commun. 2020, 11, 4740. [Google Scholar] [CrossRef]
- Seth, S.; Ager, A.; Arends, M.J.; Frayling, I.M. Lynch Syndrome—Cancer Pathways, Heterogeneity and Immune Escape. J. Pathol. 2018, 246, 129–133. [Google Scholar] [CrossRef]
- Cerretelli, G.; Ager, A.; Arends, M.J.; Frayling, I.M. Molecular Pathology of Lynch Syndrome. J. Pathol. 2020, 250, 518–531. [Google Scholar] [CrossRef]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the Management of Hereditary Colorectal Cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef]
- Dove-Edwin, I.; Sasieni, P.; Adams, J.; Thomas, H.J.W. Prevention of Colorectal Cancer by Colonoscopic Surveillance in Individuals with a Family History of Colorectal Cancer: 16 Year, Prospective, Follow-up Study. BMJ 2005, 331, 1047. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.F.A.; Abdirahman, M.; Brohet, R.; Langers, A.M.J.; Kleibeuker, J.H.; van Kouwen, M.; Koornstra, J.J.; Boot, H.; Cats, A.; Dekker, E.; et al. One to 2-Year Surveillance Intervals Reduce Risk of Colorectal Cancer in Families With Lynch Syndrome. Gastroenterology 2010, 138, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; de la Chapelle, A. The Search for Unaffected Individuals with Lynch Syndrome: Do the Ends Justify the Means? Cancer Prev. Res. 2011, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.A.; Liu, M.; Etzel, C.J.; Bannon, S.A.; Mork, M.E.; Ready, K.; Saraiya, D.S.; Grubbs, E.G.; Perrier, N.D.; Lu, K.H.; et al. Comparison of Attitudes Regarding Preimplantation Genetic Diagnosis among Patients with Hereditary Cancer Syndromes. Fam. Cancer 2014, 13, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bigas, M.A.; Boland, C.R.; Hamilton, S.R.; Henson, D.E.; Srivastava, S.; Jass, J.R.; Khan, P.M.; Lynch, H.; Smyrk, T.; Perucho, M.; et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: Meeting Highlights and Bethesda Guidelines. J. Natl. Cancer Inst. 1997, 89, 1758–1762. [Google Scholar] [CrossRef]
- Sjursen, W.; Haukanes, B.I.; Grindedal, E.M.; Aarset, H.; Stormorken, A.; Engebretsen, L.F.; Jonsrud, C.; Bjornevoll, I.; Andresen, P.A.; Ariansen, S.; et al. Current Clinical Criteria for Lynch Syndrome Are Not Sensitive Enough to Identify MSH6 Mutation Carriers. J. Med. Genet. 2010, 47, 579–585. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Lee, S.; Nafa, K.; Lee, J.; Romans, K.; Watson, P.; Gruber, S.B.; Euhus, D.; Kinzler, K.W.; et al. Prediction of Germline Mutations and Cancer Risk in the Lynch Syndrome. JAMA 2006, 296, 1479. [Google Scholar] [CrossRef]
- Green, R.C.; Parfrey, P.S.; Woods, M.O.; Younghusband, H.B. Prediction of Lynch Syndrome in Consecutive Patients with Colorectal Cancer. J. Natl. Cancer Inst. 2009, 101, 331–340. [Google Scholar] [CrossRef]
- Kastrinos, F.; Steyerberg, E.W.; Mercado, R.; Balmaña, J.; Holter, S.; Gallinger, S.; Siegmund, K.D.; Church, J.M.; Jenkins, M.A.; Lindor, N.M.; et al. The PREMM1,2,6 Model Predicts Risk of MLH1, MSH2, and MSH6 Germline Mutations Based on Cancer History. Gastroenterology 2011, 140, 73–81.e5. [Google Scholar] [CrossRef]
- Kastrinos, F.; Ojha, R.P.; Leenen, C.; Alvero, C.; Mercado, R.C.; Balmaña, J.; Valenzuela, I.; Balaguer, F.; Green, R.; Lindor, N.M.; et al. Comparison of Prediction Models for Lynch Syndrome Among Individuals with Colorectal Cancer. J. Natl. Cancer Inst. 2016, 108, djv308. [Google Scholar] [CrossRef]
- Dinh, T.A.; Rosner, B.I.; Atwood, J.C.; Boland, C.R.; Syngal, S.; Vasen, H.F.A.; Gruber, S.B.; Burt, R.W. Health Benefits and Cost-Effectiveness of Primary Genetic Screening for Lynch Syndrome in the General Population. Cancer Prev. Res. 2011, 4, 9–22. [Google Scholar] [CrossRef]
- NICE. Molecular Testing Strategies for Lynch Syndrome in People with Colorectal Cancer Diagnostics Guidance; The National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; Chapelle, A.D.L.; Ruschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Vasen, H.; Watson, P.; Mecklin, J.; Lynch, H. New Clinical Criteria for Hereditary Nonpolyposis Colorectal Cancer (HNPCC, Lynch Syndrome) Proposed by the International Collaborative Group on HNPCC. Gastroenterology 1999, 116, 1453–1456. [Google Scholar] [CrossRef]
- Moreira, L.; Balaguer, F.; Lindor, N.; de la Chapelle, A.; Hampel, H.; Aaltonen, L.A.; Hopper, J.L.; Le Marchand, L.; Gallinger, S.; Newcomb, P.A.; et al. Identification of Lynch Syndrome Among Patients with Colorectal Cancer. JAMA 2012, 308, 1555. [Google Scholar] [CrossRef]
- Stewart, A. Genetic Testing Strategies in Newly Diagnosed Endometrial Cancer Patients Aimed at Reducing Morbidity or Mortality from Lynch Syndrome in the Index Case or Her Relatives. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Hampel, H.; Frankel, W.; Panescu, J.; Lockman, J.; Sotamaa, K.; Fix, D.; Comeras, I.; La Jeunesse, J.; Nakagawa, H.; Westman, J.A.; et al. Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2006, 66, 7810–7817. [Google Scholar] [CrossRef]
- Seppälä, T.T.; Latchford, A.; Negoi, I.; Sampaio Soares, A.; Jimenez-Rodriguez, R.; Sánchez-Guillén, L.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; et al. European Guidelines from the EHTG and ESCP for Lynch Syndrome: An Updated Third Edition of the Mallorca Guidelines Based on Gene and Gender. Br. J. Surg. 2021, 108, 484–498. [Google Scholar] [CrossRef]
- Holtzman, N.A. Promoting Safe and Effective Genetic Tests in the United States: Work of the Task Force on Genetic Testing. Clin. Chem. 1999, 45, 732–738. [Google Scholar] [CrossRef]
- Statement of the American Society of Clinical Oncology: Genetic Testing for Cancer Susceptibility, Adopted on February 20, 1996. J. Clin. Oncol. 1996, 14, 1730–1736. [CrossRef]
- Tutika, R.K.; Bennett, J.A.; Abraham, J.; Snape, K.; Tatton-Brown, K.; Kemp, Z.; Copson, E.; Openshaw, M.R. Mainstreaming of Genomics in Oncology: A Nationwide Survey of the Genomics Training Needs of UK Oncologists. Clin. Med. 2023, 23, 9–15. [Google Scholar] [CrossRef]
- Ishida, H.; Yamaguchi, T.; Tanakaya, K.; Akagi, K.; Inoue, Y.; Kumamoto, K.; Shimodaira, H.; Sekine, S.; Tanaka, T.; Chino, A.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2016 for the Clinical Practice of Hereditary Colorectal Cancer (Translated Version). J. Anus Rectum Colon 2018, 2, S1–S51. [Google Scholar] [CrossRef]
- Bisschops, R.; Tejpar, S.; Willekens, H.; De Hertogh, G.; Van Cutsem, E. Virtual Chromoendoscopy (I-SCAN) Detects More Polyps in Patients with Lynch Syndrome: A Randomized Controlled Crossover Trial. Endoscopy 2017, 49, 342–350. [Google Scholar] [CrossRef]
- Engel, C.; Vasen, H.F.; Seppälä, T.; Aretz, S.; Bigirwamungu-Bargeman, M.; de Boer, S.Y.; Bucksch, K.; Büttner, R.; Holinski-Feder, E.; Holzapfel, S.; et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy Among 3 Countries With Different Lynch Syndrome Surveillance Policies. Gastroenterology 2018, 155, 1400–1409.e2. [Google Scholar] [CrossRef]
- Sánchez, A.; Roos, V.H.; Navarro, M.; Pineda, M.; Caballol, B.; Moreno, L.; Carballal, S.; Rodríguez-Alonso, L.; Ramon y Cajal, T.; Llort, G.; et al. Quality of Colonoscopy Is Associated with Adenoma Detection and Postcolonoscopy Colorectal Cancer Prevention in Lynch Syndrome. Clin. Gastroenterol. Hepatol. 2022, 20, 611–621.e9. [Google Scholar] [CrossRef]
- Dinjens, W.N.M.; Dubbink, H.J.; Wagner, A. Guidelines on Genetic Evaluation and Management of Lynch Syndrome. Gastrointest. Endosc. 2015, 81, 243–244. [Google Scholar] [CrossRef]
- Hüneburg, R.; Lammert, F.; Rabe, C.; Rahner, N.; Kahl, P.; Büttner, R.; Propping, P.; Sauerbruch, T.; Lamberti, C. Chromocolonoscopy Detects More Adenomas than White Light Colonoscopy or Narrow Band Imaging Colonoscopy in Hereditary Nonpolyposis Colorectal Cancer Screening. Endoscopy 2009, 41, 316–322. [Google Scholar] [CrossRef]
- Rahmi, G.; Lecomte, T.; Malka, D.; Maniere, T.; Le Rhun, M.; Guimbaud, R.; Lapalus, M.G.; Le Sidaner, A.; Moussata, D.; Caron, O.; et al. Impact of Chromoscopy on Adenoma Detection in Patients with Lynch Syndrome: A Prospective, Multicenter, Blinded, Tandem Colonoscopy Study. Am. J. Gastroenterol. 2015, 110, 288–298. [Google Scholar] [CrossRef]
- Lecomte, T.; Cellier, C.; Meatchi, T.; Barbier, J.P.; Cugnenc, P.H.; Jian, R.; Laurent-Puig, P.; Landi, B. Chromoendoscopic Colonoscopy for Detecting Preneoplastic Lesions in Hereditary Nonpolyposis Colorectal Cancer Syndrome. Clin. Gastroenterol. Hepatol. 2005, 3, 897–902. [Google Scholar] [CrossRef]
- Hurlstone, D.P.; Karajeh, M.; Cross, S.S.; McAlindon, M.E.; Brown, S.; Hunter, M.D.; Sanders, D.S. The Role of High-Magnification-Chromoscopic Colonoscopy in Hereditary Nonpolyposis Colorectal Cancer Screening: A Prospective “Back-to-Back” Endoscopic Study. Am. J. Gastroenterol. 2005, 100, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Houwen, B.B.S.L.; Hazewinkel, Y.; Pellisé, M.; Rivero-Sánchez, L.; Balaguer, F.; Bisschops, R.; Tejpar, S.; Repici, A.; Ramsoekh, D.; Jacobs, M.A.J.M.; et al. Linked Colour Imaging for the Detection of Polyps in Patients with Lynch Syndrome: A Multicentre, Parallel Randomised Controlled Trial. Gut 2022, 71, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Haanstra, J.F.; Dekker, E.; Cats, A.; Nagengast, F.M.; Hardwick, J.C.; Vanhoutvin, S.A.; de Vos tot Nederveen Cappel, W.H.; Vasen, H.F.; Kleibeuker, J.H.; Koornstra, J.J. Effect of Chromoendoscopy in the Proximal Colon on Colorectal Neoplasia Detection in Lynch Syndrome: A Multicenter Randomized Controlled Trial. Gastrointest. Endosc. 2019, 90, 624–632. [Google Scholar] [CrossRef]
- Rivero-Sánchez, L.; Arnau-Collell, C.; Herrero, J.; Remedios, D.; Cubiella, J.; García-Cougil, M.; Alvarez, V.; Albéniz, E.; Calvo, P.; Gordillo, J.; et al. White-Light Endoscopy Is Adequate for Lynch Syndrome Surveillance in a Randomized and Noninferiority Study. Gastroenterology 2020, 158, 895–904.e1. [Google Scholar] [CrossRef]
- Hassan, C.; Spadaccini, M.; Iannone, A.; Maselli, R.; Jovani, M.; Chandrasekar, V.T.; Antonelli, G.; Yu, H.; Areia, M.; Dinis-Ribeiro, M.; et al. Performance of Artificial Intelligence in Colonoscopy for Adenoma and Polyp Detection: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2021, 93, 77–85.e6. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Berzin, T.M.; Glissen Brown, J.R.; Liu, P.; Zhou, C.; Lei, L.; Li, L.; Guo, Z.; Lei, S.; et al. Effect of a Deep-Learning Computer-Aided Detection System on Adenoma Detection during Colonoscopy (CADe-DB Trial): A Double-Blind Randomised Study. Lancet Gastroenterol. Hepatol. 2020, 5, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520.e7. [Google Scholar] [CrossRef]
- Repici, A.; Spadaccini, M.; Antonelli, G.; Correale, L.; Maselli, R.; Galtieri, P.A.; Pellegatta, G.; Capogreco, A.; Milluzzo, S.M.; Lollo, G.; et al. Artificial Intelligence and Colonoscopy Experience: Lessons from Two Randomised Trials. Gut 2022, 71, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Hüneburg, R.; Bucksch, K.; Schmeißer, F.; Heling, D.; Marwitz, T.; Aretz, S.; Kaczmarek, D.J.; Kristiansen, G.; Hommerding, O.; Strassburg, C.P.; et al. Real-time Use of Artificial Intelligence (CADEYE) in Colorectal Cancer Surveillance of Patients with Lynch Syndrome—A Randomized Controlled Pilot Trial (CADLY). United Eur. Gastroenterol. J. 2023, 11, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Moriichi, K.; Mizukami, Y.; Fujiya, M.; Okumura, T. Artificial Intelligence–Assisted Detection of Colorectal Polyps in Lynch Syndrome. Gastrointest. Endosc. 2022, 95, 1276–1277. [Google Scholar] [CrossRef]
- Gupta, S.; Weiss, J.M.; Burke, C.A.; Chung, D.C.; Clayback, K.M.; Dallas, S.; Felder, S.; Giardiello, F.M.; Grady, W.; Hagemann, A.; et al. NCCN Guidelines Version 2.2023 Genetic/Familial High-Risk Assessment: Colorectal Continue. 2023. Available online: https://www.nccn.org/home/ (accessed on 20 December 2023).
- Kloor, M.; von Knebel Doeberitz, M. The Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer 2016, 2, 121–133. [Google Scholar] [CrossRef]
- Sæterdal, I.; Bjørheim, J.; Lislerud, K.; Gjertsen, M.K.; Bukholm, I.K.; Olsen, O.C.; Nesland, J.M.; Eriksen, J.A.; Møller, M.; Lindblom, A.; et al. Frameshift-Mutation-Derived Peptides as Tumor-Specific Antigens in Inherited and Spontaneous Colorectal Cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 13255–13260. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer Is Balanced by Multiple Counter-Inhibitory Checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef]
- Walkowska, J.; Kallemose, T.; Jönsson, G.; Jönsson, M.; Andersen, O.; Andersen, M.H.; Svane, I.M.; Langkilde, A.; Nilbert, M.; Therkildsen, C. Immunoprofiles of Colorectal Cancer from Lynch Syndrome. Oncoimmunology 2019, 8, e1515612. [Google Scholar] [CrossRef]
- Majumder, S.; Shah, R.; Elias, J.; Manoharan, M.; Shah, P.; Kumari, A.; Chakraborty, P.; Kode, V.; Mistry, Y.; Coral, K.; et al. A Cancer Vaccine Approach for Personalized Treatment of Lynch Syndrome. Sci. Rep. 2018, 8, 12122. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. New Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus Chemotherapy for Microsatellite Instability-High or Mismatch Repair-Deficient Metastatic Colorectal Cancer (KEYNOTE-177): Final Analysis of a Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- André, T.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Nivolumab plus Low-Dose Ipilimumab in Previously Treated Patients with Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: 4-Year Follow-up from CheckMate 142. Ann. Oncol. 2022, 33, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in Patients with Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Verschoor, Y.L.; van den Berg, J.; Sikorska, K.; Beets, G.; Lent, A.V.; Grootscholten, M.C.; Aalbers, A.; Buller, N.; Marsman, H.; et al. LBA7 Neoadjuvant Immune Checkpoint Inhibition in Locally Advanced MMR-Deficient Colon Cancer: The NICHE-2 Study. Ann. Oncol. 2022, 33, S1389. [Google Scholar] [CrossRef]
- Colle, R.; Lonardi, S.; Cachanado, M.; Overman, M.J.; Elez, E.; Fakih, M.; Corti, F.; Jayachandran, P.; Svrcek, M.; Dardenne, A.; et al. BRAF V600E/RAS Mutations and Lynch Syndrome in Patients With MSI-H/DMMR Metastatic Colorectal Cancer Treated with Immune Checkpoint Inhibitors. Oncologist 2023, 28, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Harrold, E.C.; Foote, M.B.; Rousseau, B.; Walch, H.; Kemel, Y.; Richards, A.L.; Keane, F.; Cercek, A.; Yaeger, R.; Rathkopf, D.; et al. Neoplasia Risk in Patients with Lynch Syndrome Treated with Immune Checkpoint Blockade. Nat. Med. 2023, 29, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Heudel, P.; Chabaud, S.; Perol, D.; Flechon, A.; Fayette, J.; Combemale, P.; Tredan, O.; Desseigne, F.; de la Fouchardiere, C.; Boyle, H.; et al. Immune Checkpoint Inhibitor Treatment of a First Cancer Is Associated with a Decreased Incidence of Second Primary Cancer. ESMO Open 2021, 6, 100044. [Google Scholar] [CrossRef] [PubMed]
- Sei, S.; Ahadova, A.; Keskin, D.B.; Bohaumilitzky, L.; Gebert, J.; von Knebel Doeberitz, M.; Lipkin, S.M.; Kloor, M. Lynch Syndrome Cancer Vaccines: A Roadmap for the Development of Precision Immunoprevention Strategies. Front. Oncol. 2023, 13, 1147590. [Google Scholar] [CrossRef] [PubMed]
- Kloor, M.; Reuschenbach, M.; Pauligk, C.; Karbach, J.; Rafiyan, M.-R.; Al-Batran, S.-E.; Tariverdian, M.; Jäger, E.; von Knebel Doeberitz, M. A Frameshift Peptide Neoantigen-Based Vaccine for Mismatch Repair-Deficient Cancers: A Phase I/IIa Clinical Trial. Clin. Cancer Res. 2020, 26, 4503–4510. [Google Scholar] [CrossRef]
- Gebert, J.; Gelincik, O.; Oezcan-Wahlbrink, M.; Marshall, J.D.; Hernandez-Sanchez, A.; Urban, K.; Long, M.; Cortes, E.; Tosti, E.; Katzenmaier, E.-M.; et al. Recurrent Frameshift Neoantigen Vaccine Elicits Protective Immunity with Reduced Tumor Burden and Improved Overall Survival in a Lynch Syndrome Mouse Model. Gastroenterology 2021, 161, 1288–1302.e13. [Google Scholar] [CrossRef]
- Serrano, D.; Patrignani, P.; Stigliano, V.; Turchetti, D.; Sciallero, S.; Roviello, F.; D’Arpino, A.; Grattagliano, I.; Testa, S.; Oliani, C.; et al. Aspirin Colorectal Cancer Prevention in Lynch Syndrome: Recommendations in the Era of Precision Medicine. Genes 2022, 13, 460. [Google Scholar] [CrossRef]
- Ait Ouakrim, D.; Dashti, S.G.; Chau, R.; Buchanan, D.D.; Clendenning, M.; Rosty, C.; Winship, I.M.; Young, J.P.; Giles, G.G.; Leggett, B.; et al. Aspirin, Ibuprofen, and the Risk of Colorectal Cancer in Lynch Syndrome. J. Natl. Cancer Inst. 2015, 107, djv170. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Elliott, A.; Xiu, J.; Wang, J.; Battaglin, F.; Kawanishi, N.; Soni, S.; Zhang, W.; Millstein, J.; Sohal, D.; et al. The Landscape of Alterations in DNA Damage Response Pathways in Colorectal Cancer. Clin. Cancer Res. 2021, 27, 3234–3242. [Google Scholar] [CrossRef]
- Catalano, F.; Borea, R.; Puglisi, S.; Boutros, A.; Gandini, A.; Cremante, M.; Martelli, V.; Sciallero, S.; Puccini, A. Targeting the DNA Damage Response Pathway as a Novel Therapeutic Strategy in Colorectal Cancer. Cancers 2022, 14, 1388. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Rump, A.; Widmann, T.J.; Heining, C.; Horak, P.; Hutter, B.; Paramasivam, N.; Uhrig, S.; Gieldon, L.; Drukewitz, S.; et al. Comprehensive Cancer Predisposition Testing within the Prospective MASTER Trial Identifies Hereditary Cancer Patients and Supports Treatment Decisions for Rare Cancers. Ann. Oncol. 2022, 33, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Pearlman, R.; Beightol, M.; Zhao, W.; Jones, D.; Frankel, W.L.; Goodfellow, P.J.; Yilmaz, A.; Miller, K.; Bacher, J.; et al. Assessment of Tumor Sequencing as a Replacement for Lynch Syndrome Screening and Current Molecular Tests for Patients with Colorectal Cancer. JAMA Oncol. 2018, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, N.; Navarro, M.; Sanjuán, X.; Ruiz, N.; Iglesias, S.; Matias-Guiu, X.; Guardiola, J.; Kreisler, E.; Biondo, S.; González, S.; et al. Lessons Learnt from the Implementation of a Colorectal Cancer Screening Programme for Lynch Syndrome in a Tertiary Public Hospital. Cancer Epidemiol. 2023, 82, 102291. [Google Scholar] [CrossRef] [PubMed]
- Peterse, E.F.P.; Naber, S.K.; Daly, C.; Pollett, A.; Paszat, L.F.; Spaander, M.C.W.; Aronson, M.; Gryfe, R.; Rabeneck, L.; Lansdorp-Vogelaar, I.; et al. Cost-Effectiveness of Active Identification and Subsequent Colonoscopy Surveillance of Lynch Syndrome Cases. Clin. Gastroenterol. Hepatol. 2020, 18, 2760–2767.e12. [Google Scholar] [CrossRef]
- Kang, Y.J.; Caruana, M.; McLoughlin, K.; Killen, J.; Simms, K.; Taylor, N.; Frayling, I.M.; Coupé, V.M.H.; Boussioutas, A.; Trainer, A.H.; et al. The Predicted Effect and Cost-Effectiveness of Tailoring Colonoscopic Surveillance According to Mismatch Repair Gene in Patients with Lynch Syndrome. Genet. Med. 2022, 24, 1831–1846. [Google Scholar] [CrossRef]
| Cancer Type | MLH1 | MSH2/ EPCAM | MSH6 | PMS2 | General Population |
|---|---|---|---|---|---|
| Colorectal cancer | 46–61% | 33–52% | 10–44% | 8.7–20% | 4.2% |
| Endometrial cancer | 34–54% | 21–57% | 16.49% | 13–26% | 2.7% |
| Ovarian cancer | 4–20% | 8–28% | ≤1–13% | 1.3–3% | 1.3% |
| Ureteral cancer | 0.2–5% | 2.2–28% | 0.7–5.5% | ≤1–3.7% | <1% |
| Gastric cancer | 5–7% | 0.2–9% | ≤1–7.9% | Not known | 0.9% |
| Pancreatic cancer | 6.2% | 0.5–1.6% | 1.4–1.6% | ≤1–1.6% | 1.6% |
| Prostate cancer | 4.4–13.8% | 3.9–23.8% | 2.5–11.6% | 4.6–11.6% | 11.6% |
| Cancer Type | MLH1 | MSH2 | MSH6 | PMS2 |
|---|---|---|---|---|
| Colorectal cancer | Colonoscopy every 1–2 years, starting at 20–25 y | Colonoscopy every 1–2 years, starting at 20–25 y | Colonoscopy every 1–3 years, starting at 30–35 y | Colonoscopy every 1–3 years, starting at 30–35 y |
| Endometrial and ovarian cancers * | Pelvic ultrasound and/or endometrial biopsy every 1–2 years, starting at 30–35 y | Pelvic ultrasound and/or endometrial biopsy every 1–2 years, starting at 30–35 y | Pelvic ultrasound and/or endometrial biopsy every 1–2 years, starting at 30–35 y | Pelvic ultrasound and/or endometrial biopsy every 1–2 years, starting at 30–35 y |
| Ureteral cancer | Urinalysis, urine cytology, and abdominal ultrasound every 1–2 years, starting at 40–45 y | Urinalysis, urine cytology, and abdominal ultrasound every 1–2 years, starting at 40–45 y | Urinalysis, urine cytology, and abdominal ultrasound every 1–2 years, starting at 40–45 y | Urinalysis, urine cytology, and abdominal ultrasound every 1–2 years, starting at 40–45 y |
| Gastric and duodenal cancers | EGD every 3–5 years, starting at 30–35 y | EGD every 3–5 years, starting at 30–35 y | EGD every 3–5 years, starting at 30–35 y | EGD every 3–5 years, starting at 30–35 y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Buono, A.; Puccini, A.; Franchellucci, G.; Airoldi, M.; Bartolini, M.; Bianchi, P.; Santoro, A.; Repici, A.; Hassan, C. Lynch Syndrome: From Multidisciplinary Management to Precision Prevention. Cancers 2024, 16, 849. https://doi.org/10.3390/cancers16050849
Dal Buono A, Puccini A, Franchellucci G, Airoldi M, Bartolini M, Bianchi P, Santoro A, Repici A, Hassan C. Lynch Syndrome: From Multidisciplinary Management to Precision Prevention. Cancers. 2024; 16(5):849. https://doi.org/10.3390/cancers16050849
Chicago/Turabian StyleDal Buono, Arianna, Alberto Puccini, Gianluca Franchellucci, Marco Airoldi, Michela Bartolini, Paolo Bianchi, Armando Santoro, Alessandro Repici, and Cesare Hassan. 2024. "Lynch Syndrome: From Multidisciplinary Management to Precision Prevention" Cancers 16, no. 5: 849. https://doi.org/10.3390/cancers16050849
APA StyleDal Buono, A., Puccini, A., Franchellucci, G., Airoldi, M., Bartolini, M., Bianchi, P., Santoro, A., Repici, A., & Hassan, C. (2024). Lynch Syndrome: From Multidisciplinary Management to Precision Prevention. Cancers, 16(5), 849. https://doi.org/10.3390/cancers16050849






