Proguanil Suppresses Breast Tumor Growth In Vitro and In Vivo by Inducing Apoptosis via Mitochondrial Dysfunction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Sulforhodamine B Assay (SRB)
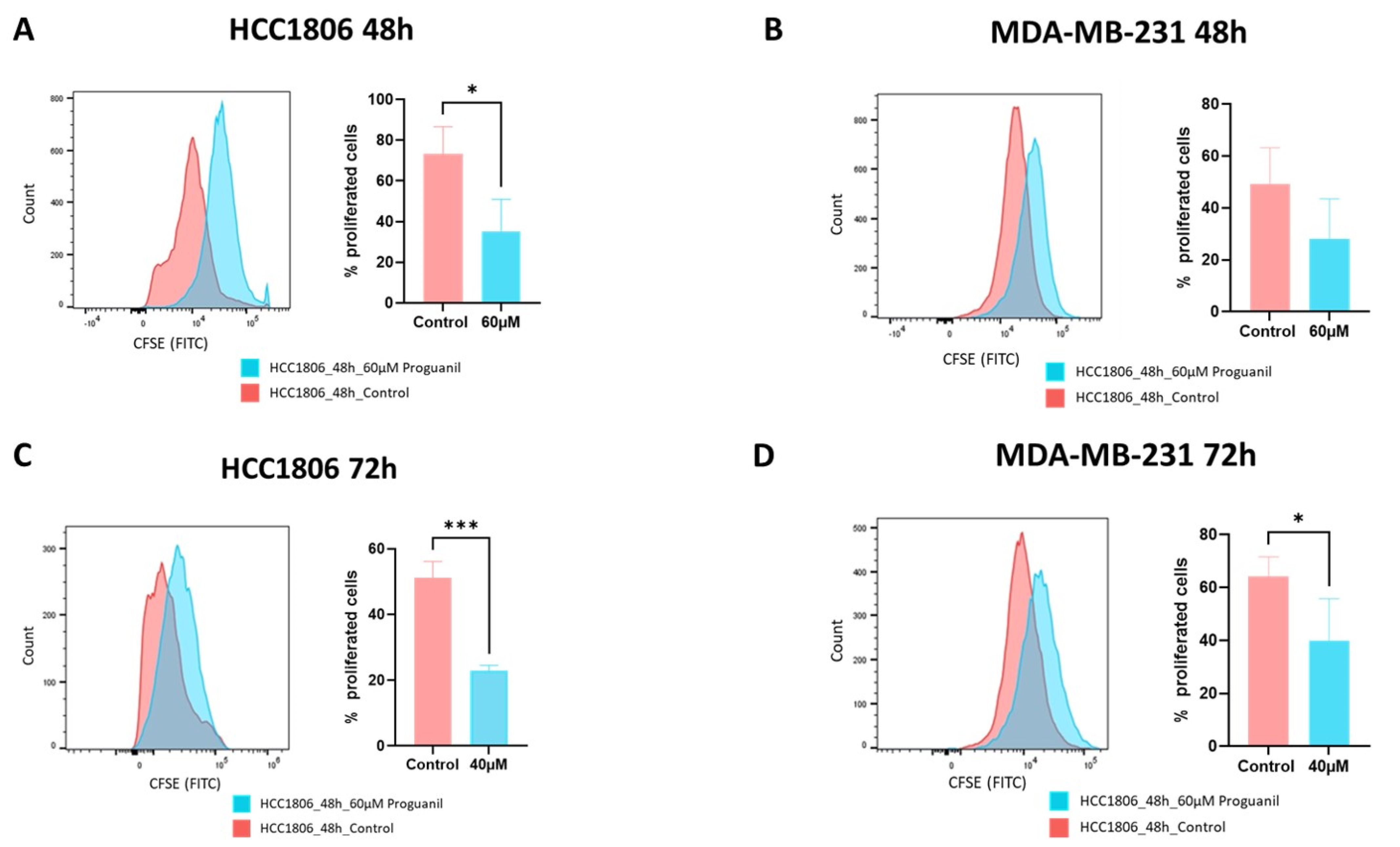
2.4. Proliferation Assay
2.5. Annexin V-FITC Apoptosis Assay
2.6. Generation of Reactive Oxygen Species
2.7. Fluorescence Microscopy
2.8. Mitochondrial Membrane Potential
2.9. Seahorse XFe-24 Metabolic Flux Analysis
2.10. Western Blot Analysis
2.11. In Vivo Tumor Model
2.12. Statistical Analysis
3. Results
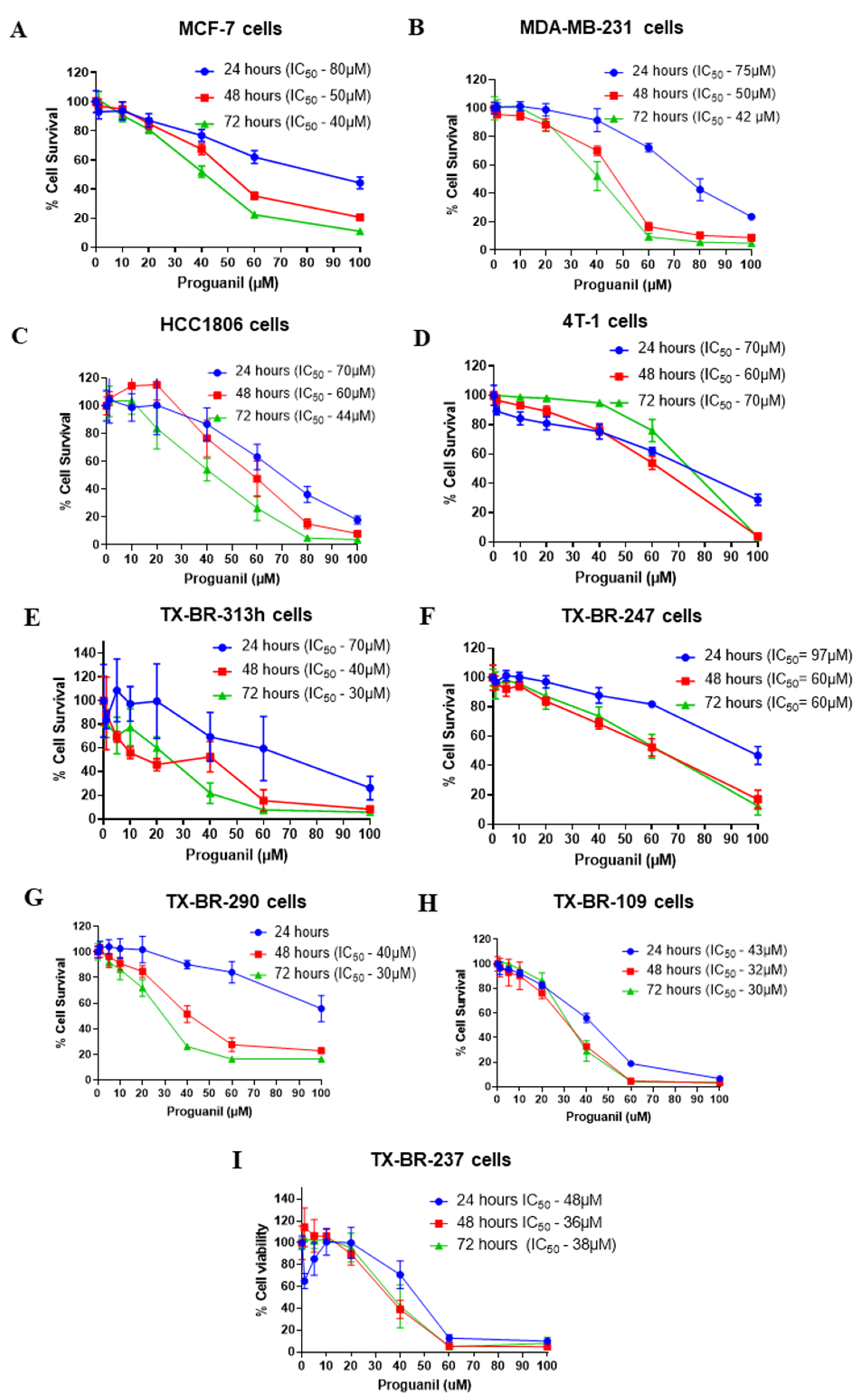
3.1. Proguanil Has Anti-Proliferative Effects on Breast Cancer Cells
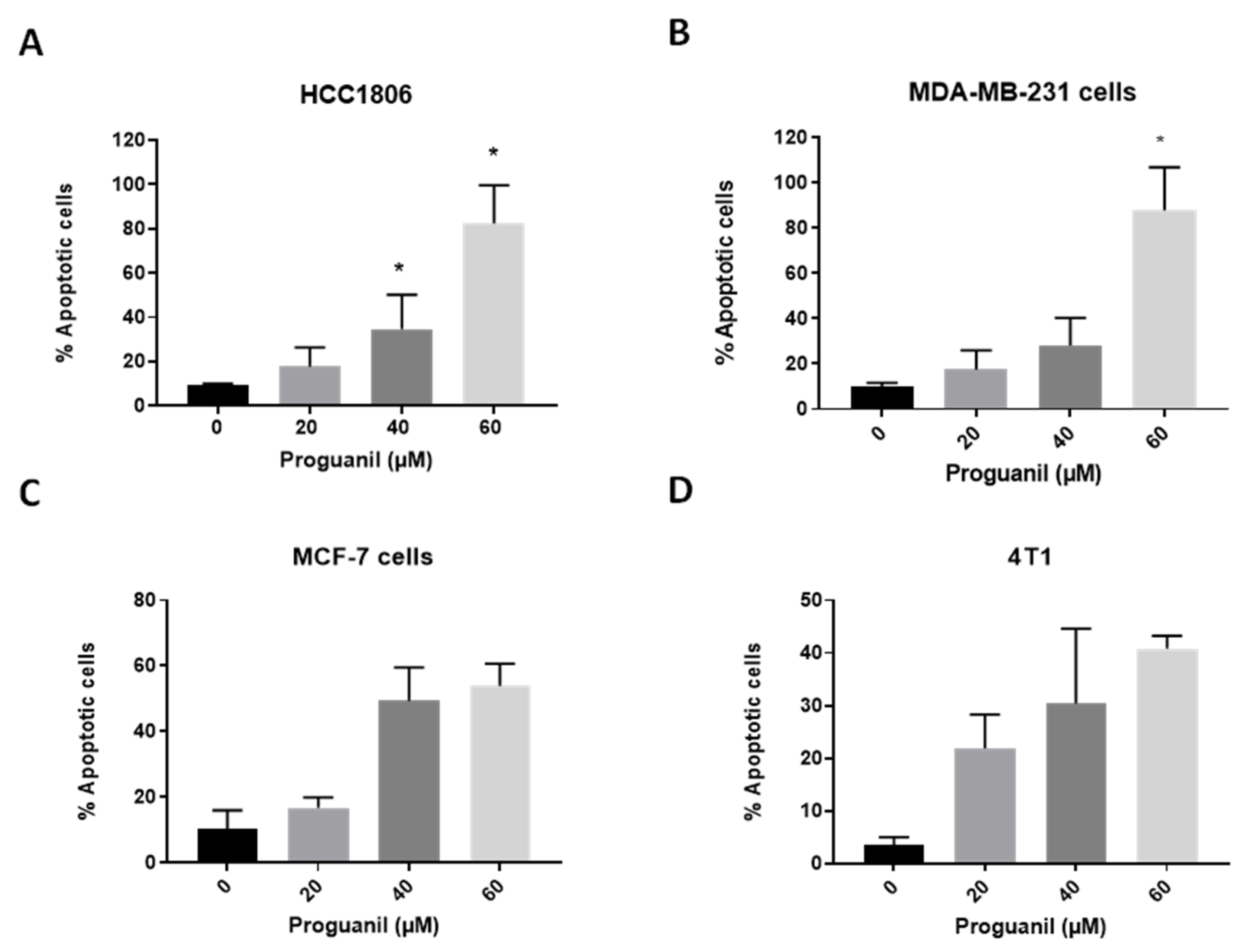
3.2. Proguanil Triggers Apoptosis in Breast Cancer Cells
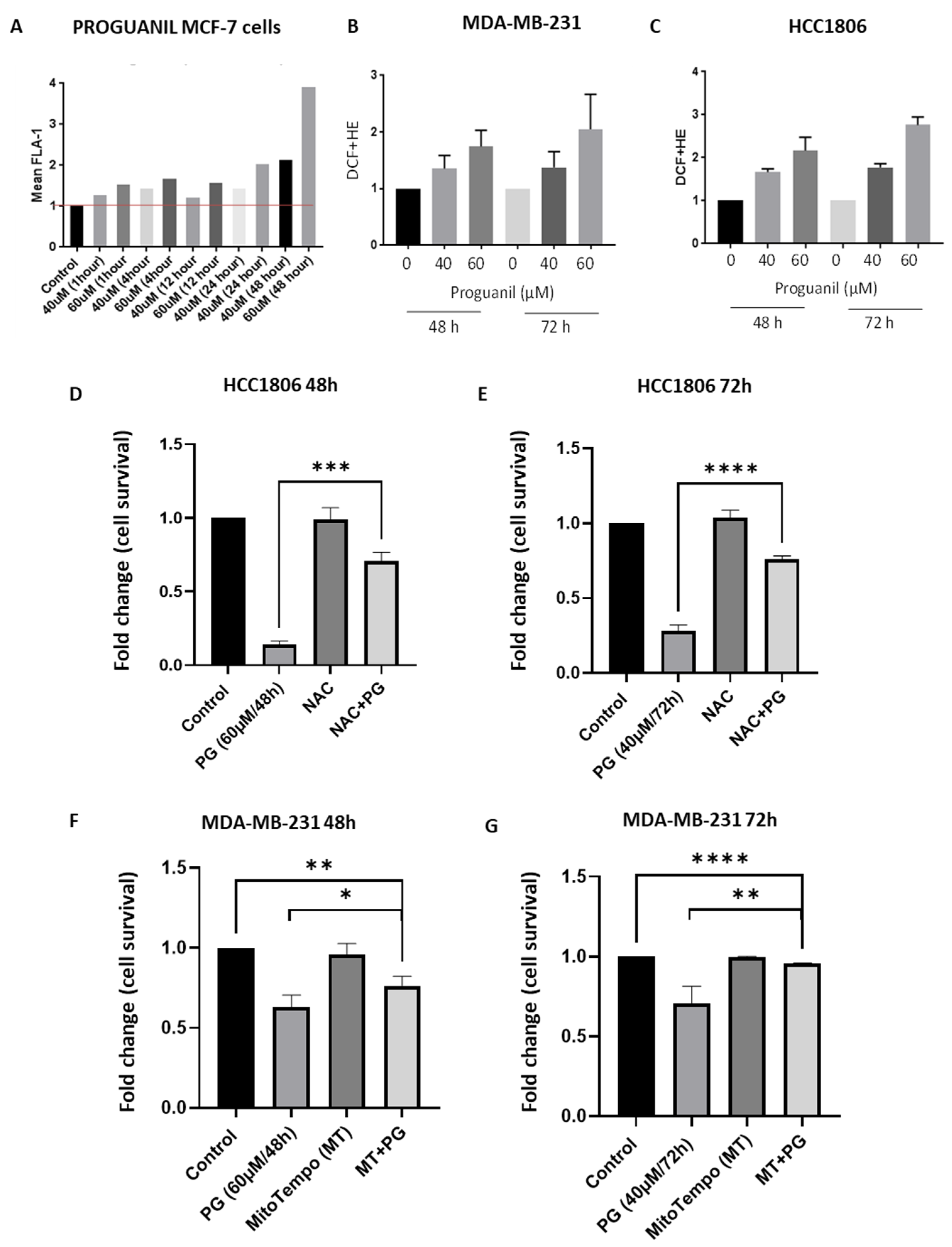
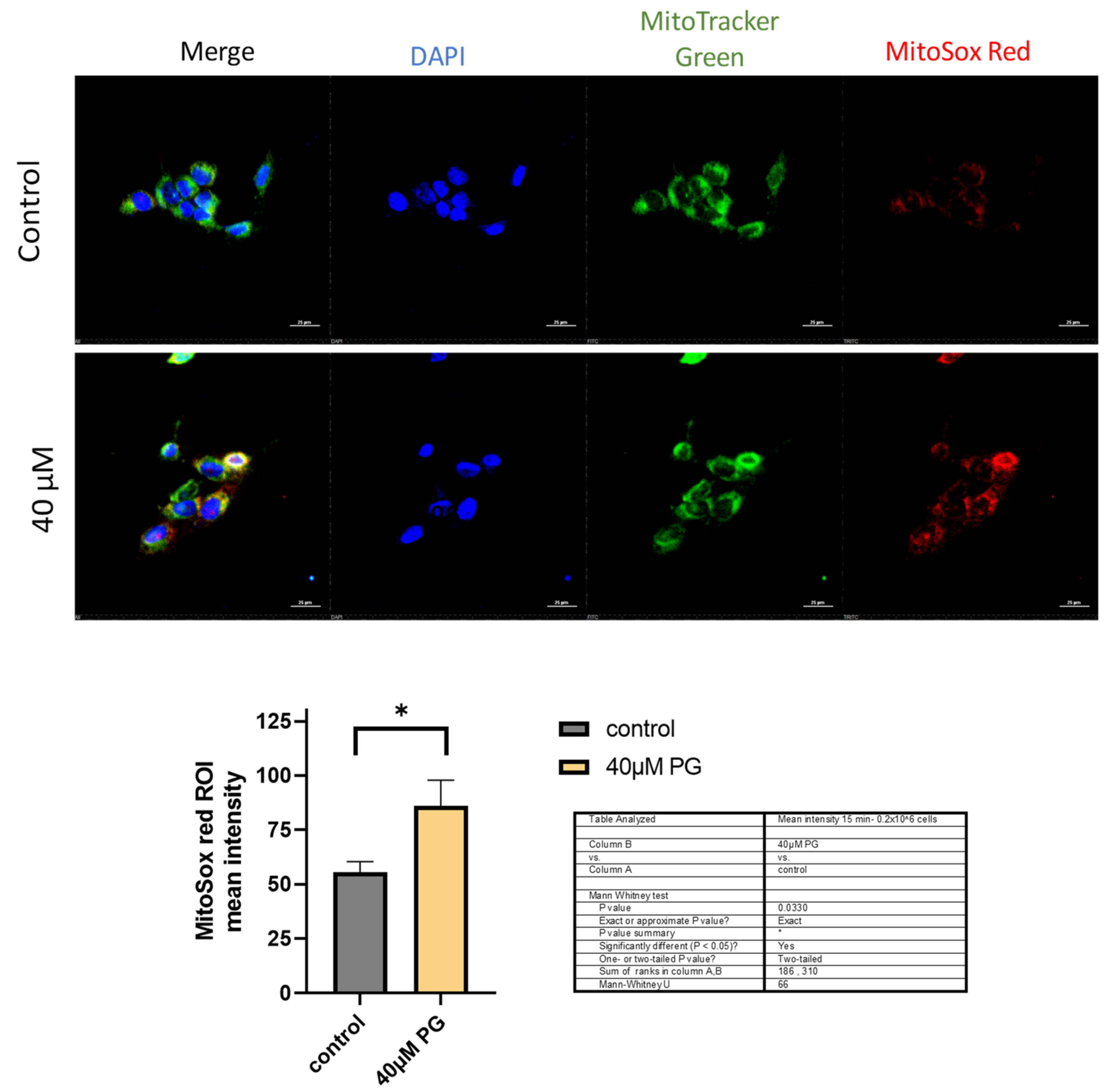
3.3. Proguanil Causes the Generation of Mitochondrial ROS in Breast Cancer Cells
3.4. Proguanil Disrupts Mitochondrial Membrane Potential in MDA-MB-231 and HCC1806 Cells
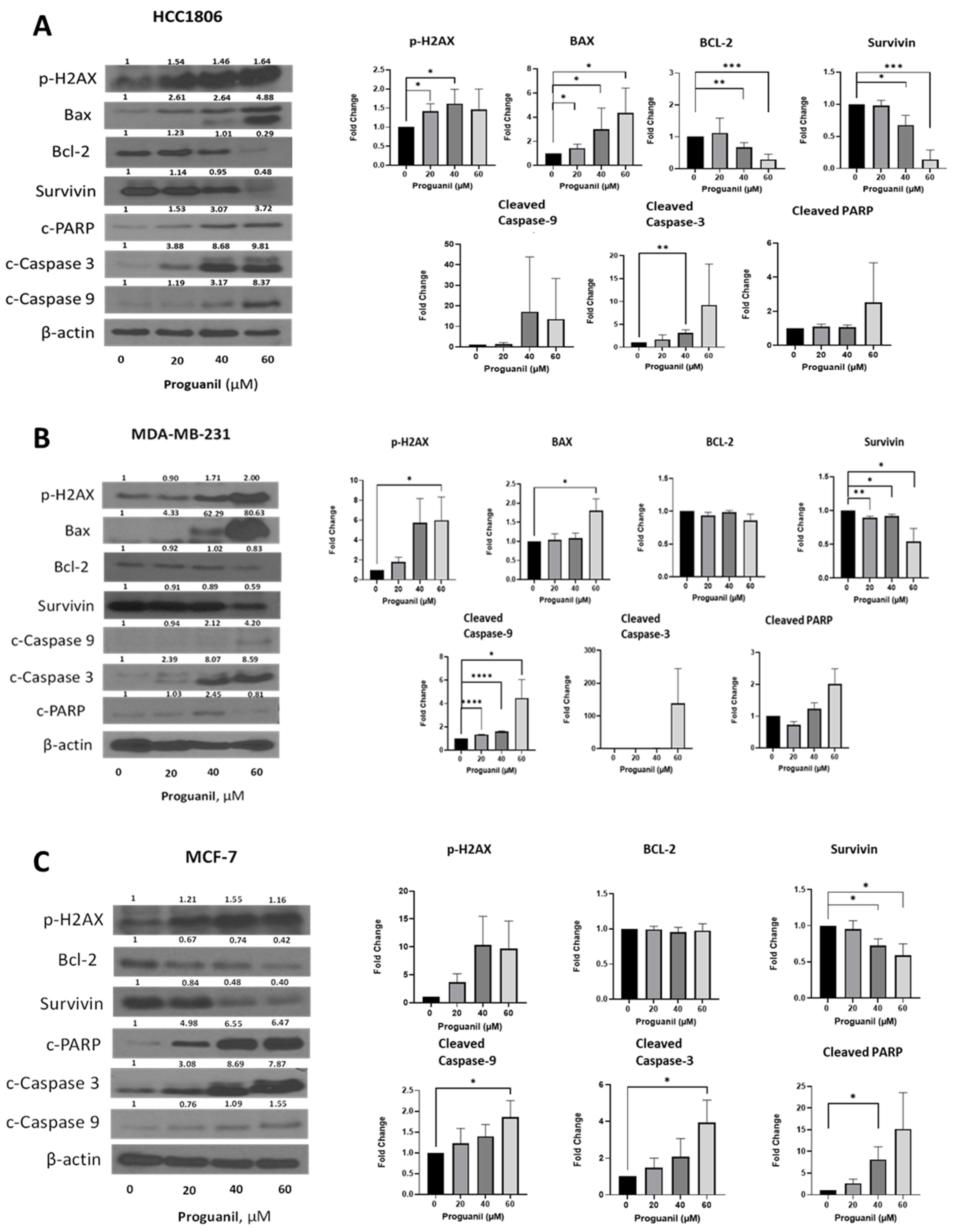
3.5. Proguanil Treatment Activates the Caspase-3 Cascade in Breast Cancer Cells
3.6. Proguanil Inhibits Oxidative Phosphorylation of Breast Cancer Cells
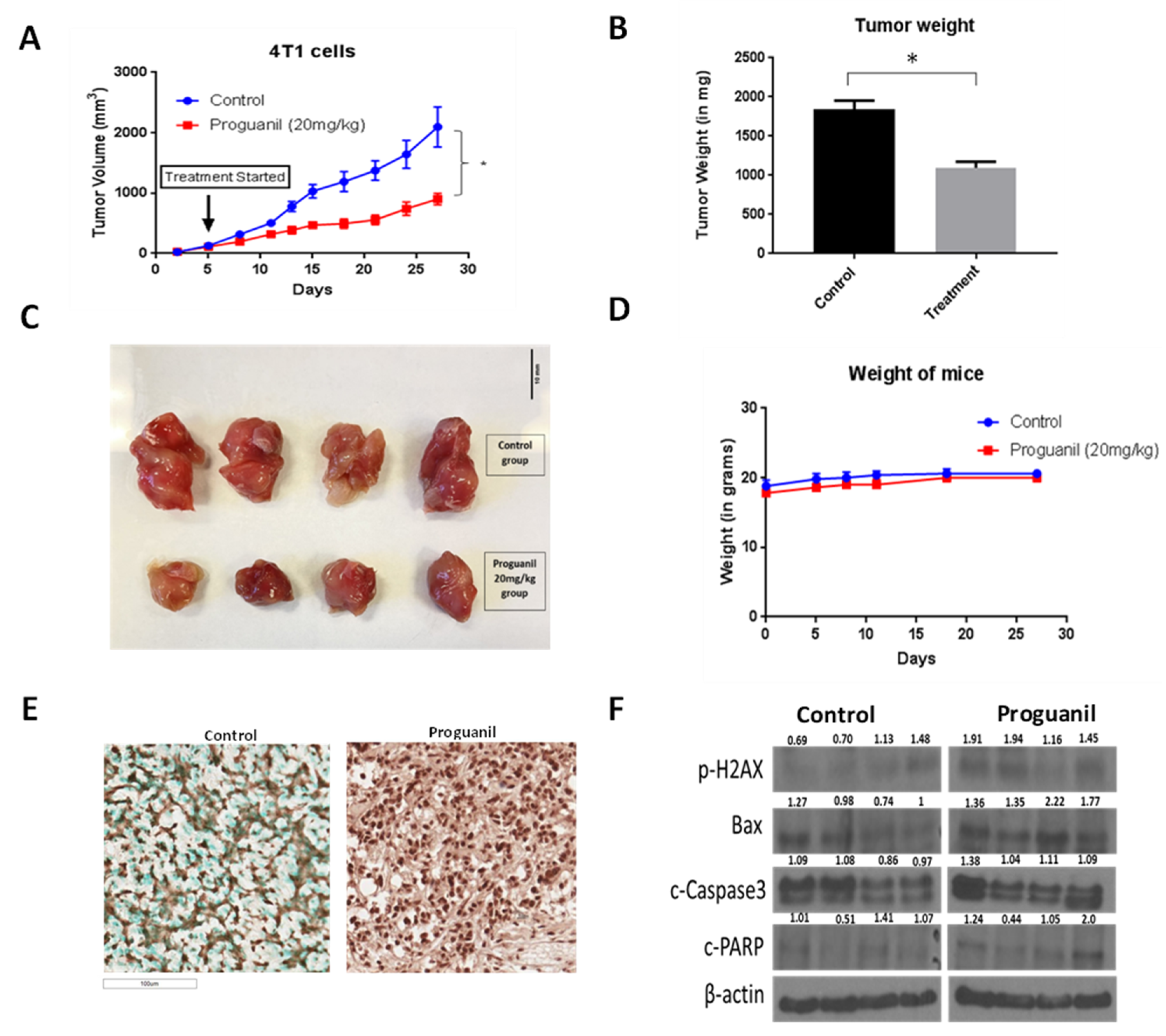
3.7. Proguanil Inhibits the Growth of Orthotopically Implanted 4T1 Breast Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| 4T1 | murine breast cancer cell line |
| ATCC | American Type Culture Collection |
| Bax | Bcl-2-associated X protein |
| bcl-2 | B-cell lymphoma 2 protein |
| Balb/c | Balb/c mice |
| CFSE | carboxyfluorescein succinimidyl ester dye |
| DCFDA | 6-carboxy-2, 7-dichlorodihydrofluorescein diacetate |
| DHE | dihydroethidium |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | fetal bovine serum |
| HCC1806 | human breast carcinoma cell line |
| ITS | insulin–transferrin–selenous acid |
| MCF-7 | human breast carcinoma cell line |
| MDA-MB-231 | human breast carcinoma cell line |
| PSN | penicillin–streptomycin–neomycin |
| SRB | Sulforhodamine B assay |
| STR | short tandem repeat |
| TMRM | tetramethyl rhodamine |
| TX-BR | patient-derived cells |
| ROS | reactive oxygen species |
References
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Kabir, S.; Ahad, M.A. Parametric Study of Malignant Female Breast Tumor Size, Position and Orientation on EIM Parameters. In Proceedings of the 2020 SoutheastCon, Raleigh, NC, USA, 28–29 March 2020; pp. 1–5. [Google Scholar]
- Pantziarka, P.; Bouche, G.; Meheus, L.; Sukhatme, V.; Sukhatme, V.P.; Vikas, P. The Repurposing Drugs in Oncology (ReDO) Project. Ecancermedicalscience 2014, 8, 442. [Google Scholar] [CrossRef]
- de Sá Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The roles of ROS in cancer heterogeneity and therapy. Oxidative Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Mügge, A. The role of reactive oxygen species in atherosclerosis. Z. Fur Kardiol. 1998, 87, 851–864. [Google Scholar]
- Chio, I.I.C.; Tuveson, D.A. ROS in cancer: The burning question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.-I. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Wu, W.-S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Toler, S.M.; Noe, D.; Sharma, A. Selective enhancement of cellular oxidative stress by chloroquine: Implications for the treatment of glioblastoma multiforme. Neurosurg. Focus 2006, 21, 1–4. [Google Scholar] [CrossRef]
- Johar, R.; Sharma, R.; Kaur, A.; K Mukherjee, T. Role of reactive oxygen species in estrogen dependant breast cancer complication. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2016, 16, 190–199. [Google Scholar] [CrossRef]
- Jones, R., Jr.; Pullman, T.N.; Whorton, C.M.; Craige, B.; Alving, A.S.; Eichelberger, L. The therapeutic effectiveness of large doses of paludrine in acute attacks of sporozoite-induced vivax malaria, Chesson strain. J. Clin. Investig. 1948, 27, 51–55. [Google Scholar] [CrossRef]
- Kaneko, A.; Bergqvist, Y.; Takechi, M.; Kalkoa, M.; Kaneko, O.; Kobayakawa, T.; Ishizaki, T.; Bjorkman, A. Intrinsic efficacy of proguanil against falciparum and vivax malaria independent of the metabolite cycloguanil. J. Infect. Dis. 1999, 179, 974–979. [Google Scholar] [CrossRef][Green Version]
- Crowther, A.; Levi, A. Proguanil—The isolation of a metabolite with high antimalarial activity. Br. J. Pharmacol. Chemother. 1953, 8, 93. [Google Scholar] [CrossRef]
- Fidock, D.A.; Nomura, T.; Wellems, T.E. Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol. Pharmacol. 1998, 54, 1140–1147. [Google Scholar] [CrossRef]
- Skinner-Adams, T.S.; Fisher, G.M.; Riches, A.G.; Hutt, O.E.; Jarvis, K.E.; Wilson, T.; von Itzstein, M.; Chopra, P.; Antonova-Koch, Y.; Meister, S. Cyclization-blocked proguanil as a strategy to improve the antimalarial activity of atovaquone. Commun. Biol. 2019, 2, 166. [Google Scholar] [CrossRef]
- Looareesuwan, S.; Chulay, J.D.; Canfield, C.J.; Hutchinson, D. Malarone (atovaquone and proguanil hydrochloride): A review of its clinical development for treatment of malaria. Malarone Clinical Trials Study Group. Am. J. Trop. Med. Hyg. 1999, 60, 533–541. [Google Scholar] [CrossRef]
- Marra, F.; Salzman, J.R.; Ensom, M.H. Atovaquone–proguanil for prophylaxis and treatment of malaria. Ann. Pharmacother. 2003, 37, 1266–1275. [Google Scholar] [CrossRef]
- Boggild, A.K.; Parise, M.E.; Lewis, L.S.; Kain, K.C. Atovaquone-proguanil: Report from the CDC expert meeting on malaria chemoprophylaxis (II). Am. J. Trop. Med. Hyg. 2007, 76, 208–223. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, T.; Hu, X.; Xu, S.; Xiao, D.; Wu, J.; Yan, X.; Yang, X.; Li, G. Proguanil synergistically sensitizes ovarian cancer cells to olaparib by increasing DNA damage and inducing apoptosis. Int. J. Med. Sci. 2022, 19, 233. [Google Scholar] [CrossRef]
- Srivastava, I.K.; Vaidya, A.B. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob. Agents Chemother. 1999, 43, 1334–1339. [Google Scholar] [CrossRef]
- Mounkoro, P.; Michel, T.; Meunier, B. Revisiting the mode of action of the antimalarial proguanil using the yeast model. Biochem. Biophys. Res. Commun. 2021, 534, 94–98. [Google Scholar] [CrossRef]
- Bridges, H.R.; Sirviö, V.A.; Agip, A.-N.A.; Hirst, J. Molecular features of biguanides required for targeting of mitochondrial respiratory complex I and activation of AMP-kinase. BMC Biol. 2016, 14, 65. [Google Scholar] [CrossRef]
- Lea, M.A.; Kim, H. Effects of Biguanides on Growth and Glycolysis of Bladder and Colon Cancer Cells. Anticancer Res. 2018, 38, 5003–5011. [Google Scholar] [CrossRef]
- Barbieri, F.; Wurth, R.; Pattarozzi, A.; Verduci, I.; Mazzola, C.; Cattaneo, M.G.; Tonelli, M.; Solari, A.; Bajetto, A.; Daga, A.; et al. Inhibition of Chloride Intracellular Channel 1 (CLIC1) as Biguanide Class-Effect to Impair Human Glioblastoma Stem Cell Viability. Front. Pharmacol. 2018, 9, 899. [Google Scholar] [CrossRef]
- Xiao, D.; Hu, X.; Peng, M.; Deng, J.; Zhou, S.; Xu, S.; Wu, J.; Yang, X. Inhibitory role of proguanil on the growth of bladder cancer via enhancing EGFR degradation and inhibiting its downstream signaling pathway to induce autophagy. Cell Death Dis. 2022, 13, 499. [Google Scholar] [CrossRef]
- Scholar, E. Proguanil. In xPharm: The Comprehensive Pharmacology Reference; S. J. Enna, D.B.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–4. [Google Scholar]
- Gupta, N.; Srivastava, S.K. Atovaquone: An Antiprotozoal Drug Suppresses Primary and Resistant Breast Tumor Growth by Inhibiting HER2/beta-Catenin Signaling. Mol. Cancer Ther. 2019, 18, 1708–1720. [Google Scholar] [CrossRef]
- Baggish, A.L.; Hill, D.R. Antiparasitic agent atovaquone. Antimicrob. Agents Chemother. 2002, 46, 1163–1173. [Google Scholar] [CrossRef]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Sci. Rep. 2019, 9, 13045. [Google Scholar] [CrossRef]
- Olelewe, C.; Kim, J.H.; Ofori, S.; Mertens, R.T.; Gukathasan, S.; Awuah, S.G.J.I. Gold (III)-P-chirogenic complex induces mitochondrial dysfunction in triple-negative breast cancer. Iscience 2022, 25, 104340. [Google Scholar] [CrossRef]
- Verma, K.; Gupta, N.; Zang, T.; Wangtrakluldee, P.; Srivastava, S.K.; Penning, T.M.; Trippier, P.C. AKR1C3 Inhibitor KV-37 Exhibits Antineoplastic Effects and Potentiates Enzalutamide in Combination Therapy in Prostate Adenocarcinoma CellsAKR1C3 Inhibitor for CRPC Treatment. Mol. Cancer Ther. 2018, 17, 1833–1845. [Google Scholar] [CrossRef]
- Trnka, J.; Blaikie, F.H.; Smith, R.A.; Murphy, M.P. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic. Biol. Med. 2008, 44, 1406–1419. [Google Scholar] [CrossRef]
- Bridges, H.R.; Jones, A.J.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014, 462, 475–487. [Google Scholar] [CrossRef]
- Choi, A.R.; Kim, J.H.; Woo, Y.H.; Kim, H.S.; Yoon, S. Anti-malarial Drugs Primaquine and Chloroquine Have Different Sensitization Effects with Anti-mitotic Drugs in Resistant Cancer Cells. Anticancer Res. 2016, 36, 1641–1648. [Google Scholar] [CrossRef]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, A.R.; Kim, Y.K.; Yoon, S. Co-treatment with the anti-malarial drugs mefloquine and primaquine highly sensitizes drug-resistant cancer cells by increasing P-gp inhibition. Biochem. Biophys. Res. Commun. 2013, 441, 655–660. [Google Scholar] [CrossRef]
- Xie, F.; Gong, J.; Tan, H.; Zhang, H.; Ma, J. Preclinical evidence of synergism between atovaquone and chemotherapy by AMPK-dependent mitochondrial dysfunction. Eur. J. Pharmacol. 2021, 907, 174256. [Google Scholar] [CrossRef]
- Mudassar, F.; Shen, H.; O’Neill, G.; Hau, E. Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas. J. Exp. Clin. Cancer Res. 2020, 39, 208. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Gurer-Orhan, H.; Ince, E.; Konyar, D.; Saso, L.; Suzen, S. The Role of Oxidative Stress Modulators in Breast Cancer. Curr. Med. Chem. 2018, 25, 4084–4101. [Google Scholar] [CrossRef]
- Brown, N.S.; Bicknell, R. Hypoxia and oxidative stress in breast cancer Oxidative stress-its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001, 3, 323. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Liu, Y.; Holbrook, N.J. The cellular response to oxidative stress: Influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998, 333, 291–300. [Google Scholar] [CrossRef]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17. [Google Scholar] [CrossRef]
- Yokomizo, A.; Ono, M.; Nanri, H.; Makino, Y.; Ohga, T.; Wada, M.; Okamoto, T.; Yodoi, J.; Kuwano, M.; Kohno, K. Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res. 1995, 55, 4293–4296. [Google Scholar]
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE 2011, 6, e20151. [Google Scholar] [CrossRef]
- Wen, J.-J.; Garg, N.J. Mitochondrial complex III defects contribute to inefficient respiration and ATP synthesis in the myocardium of Trypanosoma cruzi–infected mice. Antioxid. Redox Signal. 2010, 12, 27–37. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Renschler, M.F. The emerging role of reactive oxygen species in cancer therapy. Eur. J. Cancer 2004, 40, 1934–1940. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Curcic, M.; Srivastava, S.K. Proguanil Suppresses Breast Tumor Growth In Vitro and In Vivo by Inducing Apoptosis via Mitochondrial Dysfunction. Cancers 2024, 16, 872. https://doi.org/10.3390/cancers16050872
Gupta N, Curcic M, Srivastava SK. Proguanil Suppresses Breast Tumor Growth In Vitro and In Vivo by Inducing Apoptosis via Mitochondrial Dysfunction. Cancers. 2024; 16(5):872. https://doi.org/10.3390/cancers16050872
Chicago/Turabian StyleGupta, Nehal, Marina Curcic, and Sanjay K. Srivastava. 2024. "Proguanil Suppresses Breast Tumor Growth In Vitro and In Vivo by Inducing Apoptosis via Mitochondrial Dysfunction" Cancers 16, no. 5: 872. https://doi.org/10.3390/cancers16050872
APA StyleGupta, N., Curcic, M., & Srivastava, S. K. (2024). Proguanil Suppresses Breast Tumor Growth In Vitro and In Vivo by Inducing Apoptosis via Mitochondrial Dysfunction. Cancers, 16(5), 872. https://doi.org/10.3390/cancers16050872






