Blood Vessel-Targeted Therapy in Colorectal Cancer: Current Strategies and Future Perspectives
Abstract
Simple Summary
Abstract
1. History and Development of Antiangiogenic Treatment for Cancer
2. Clinical Application of Antiangiogenic Treatment in Colorectal Cancer
2.1. Monoclonal Antibodies
2.1.1. Bevacizumab
2.1.2. Ramucirumab
2.1.3. Aflibercept
2.2. Tyrosine Kinase Inhibitors
2.2.1. Regorafenib
2.2.2. Fruquintinib
3. Challenges in Antiangiogenic Treatment of CRC
3.1. Dosing and Timing of Antiangiogenic Treatment
3.2. Combination of Antiangiogenic Treatment with Alternative Drugs
3.3. Heterogeneity of ECs According to Vessel Type, Organ, Disease, Patient, EC Hierarchy and Activation State
3.4. Tumor Microenvironment (TME)-Dependent Plasticity of ECs Involving Angiocrine Mediators
3.5. Induction of EC Anergy
3.6. Genomic Instability of TECs
3.7. Imbalance of Intracellular Signaling Molecules (ROS, Calcium)
3.8. Inadequate Preclinical Models and/or Limited Analysis
4. Conclusions and Future Opportunities for Antiangiogenic Tumor Therapy in CRC
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Colorectal Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 15 December 2023).
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for di-agnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 10–32. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Folkman, J. Antiangiogenesis Agents, in Cancer: Principles and Practice of Oncology; Vincent, S.H., De Vita, T., Jr., Rosenberg, S.A., Eds.; Lippincott Williams & Wilkins Publishers: Baltimore, MD, USA, 2001. [Google Scholar]
- Cao, Y.; Langer, R. A review of Judah Folkman’s remarkable achievements in biomedicine. Proc. Natl. Acad. Sci. USA 2008, 105, 13203–13205. [Google Scholar] [CrossRef]
- Ribatti, D. Judah Folkman, a pioneer in the study of angiogenesis. Angiogenesis 2008, 11, 3–10. [Google Scholar] [CrossRef]
- Hanson, F.R.; Eble, T.E. An Antiphage Agent Isolated from Aspergillus sp. J. Bacteriol. 1949, 58, 527–529. [Google Scholar] [CrossRef]
- Guruceaga, X.; Perez-Cuesta, U.; Cerio, A.D.D.; Gonzalez, O.; Rementeria, A. Fumagillin, a Mycotoxin of Aspergillus fumigatus: Biosynthesis, Biological Activities, Detection, and Ap-plications. Toxins 2019, 12, 7. [Google Scholar] [CrossRef]
- Ingber, D.; Fujita, T.; Kishimoto, S.; Sudo, K.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Holmgren, L.; Chen, C.; Folkman, J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat. Med. 1996, 2, 689–692. [Google Scholar] [CrossRef]
- Boehm, T.; Folkman, J.; Browder, T.; O’Reilly, M.S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997, 390, 404–407. [Google Scholar] [CrossRef]
- Kolata, G. Hope in the Lab: A Special Report; A Cautious Awe Greets Drugs That Eradicate Tumors in Mice. The New York Times, 3 May 1998. [Google Scholar]
- Watson, J.D. Opinion, High Hopes on Cancer, Letter to the Editor. The New York Times, 1998. [Google Scholar]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Hansen, T.F.; Qvortrup, C.; Pfeiffer, P. Angiogenesis Inhibitors for Colorectal Cancer. A Review of the Clinical Data. Cancers 2021, 13, 1031. [Google Scholar] [CrossRef]
- Passardi, A.; Nanni, O.; Tassinari, D.; Turci, D.; Cavanna, L.; Fontana, A.; Ruscelli, S.; Mucciarini, C.; Lorusso, V.; Ragazzini, A.; et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: Final results for first-line treatment from the ITACa randomized clinical trial. Ann. Oncol. 2015, 26, 1201–1207. [Google Scholar] [CrossRef]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients with Metastatic Colorectal Cancer Previously Treated With an Oxaliplatin-Based Regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Cutsem, E.V.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colo-rectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Dasari, A.; Lonardi, S.; Garcia-Carbonero, R.; Elez, E.; Yoshino, T.; Sobrero, A.; Yao, J.; García-Alfonso, P.; Kocsis, J.; Gracian, A.C.; et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): An interna-tional, multicentre, randomised, double-blind, phase 3 study. Lancet 2023, 402, 41–53. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Watanabe, T.; Itabashi, M.; Shimada, Y.; Tanaka, S.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hyodo, I.; Igarashi, M.; Ishida, H.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Chen, H.X.; Cleck, J.N. Adverse effects of anticancer agents that target the VEGF pathway. Nat. Rev. Clin. Oncol. 2009, 6, 465–477. [Google Scholar] [CrossRef]
- Souglakos, J.; Ziras, N.; Kakolyris, S.; Boukovinas, I.; Kentepozidis, N.; Makrantonakis, P.; Xynogalos, S.; Christophyllakis, C.; Kouroussis, C.; Vamvakas, L.; et al. Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br. J. Cancer 2012, 106, 453–459. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Rivera, F.; Berry, S.; Kretzschmar, A.; Michael, M.; DiBartolomeo, M.; Mazier, M.-A.; Canon, J.-L.; Georgoulias, V.; Peeters, M.; et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study. Ann. Oncol. 2009, 20, 1842–1847. [Google Scholar] [CrossRef]
- Tampellini, M.; Sonetto, C.; Scagliotti, G.V. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin. Investig. Drugs 2016, 25, 507–520. [Google Scholar] [CrossRef]
- Kabbinavar, F. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003, 21, 60–65. [Google Scholar] [CrossRef]
- Cunningham, D.; Lang, I.; Marcuello, E.; Lorusso, V.; Ocvirk, J.; Shin, D.B.; Jonker, D.; Osborne, S.; Andre, N.; Waterkamp, D.; et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013, 14, 1077–1085. [Google Scholar] [CrossRef]
- Kabbinavar, F.F.; Schulz, J.; McCleod, M.; Patel, T.; Hamm, J.T.; Hecht, J.R.; Mass, R.; Perrou, B.; Nelson, B.; Novotny, W.F. Addition of Bevacizumab to Bolus Fluorouracil and Leucovorin in First-Line Metastatic Colorectal Cancer: Results of a Randomized Phase II Trial. J. Clin. Oncol. 2005, 23, 3697–3705. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination With Oxaliplatin-Based Chemotherapy As First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Tebbutt, N.C.; Wilson, K.; Gebski, V.J.; Cummins, M.M.; Zannino, D.; van Hazel, G.A.; Robinson, B.; Broad, A.; Ganju, V.; Ackland, S.P.; et al. Capecitabine, Bevacizumab, and Mitomycin in First-Line Treatment of Metastatic Colorectal Cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J. Clin. Oncol. 2010, 28, 3191–3198. [Google Scholar] [CrossRef]
- Baraniskin, A.; Buchberger, B.; Pox, C.; Graeven, U.; Holch, J.W.; Schmiegel, W.; Heinemann, V. Efficacy of bevacizumab in first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Eur. J. Cancer 2019, 106, 37–44. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients with KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.-L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (mFOLFOX6) or Bevacizumab Plus mFOLFOX6 in Patients with Previously Untreated, Unresectable, Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar]
- Holch, J.W.; Ricard, I.; Stintzing, S.; Modest, D.P.; Heinemann, V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur. J. Cancer 2016, 70, 87–98. [Google Scholar] [CrossRef]
- Loupakis, F.; Hurwitz, H.I.; Saltz, L.; Arnold, D.; Grothey, A.; Nguyen, Q.L.; Osborne, S.; Talbot, J.; Srock, S.; Lenz, H.-J. Impact of primary tumour location on efficacy of bevacizumab plus chemotherapy in metastatic colorectal cancer. Br. J. Cancer 2018, 119, 1451–1455. [Google Scholar] [CrossRef]
- Hegewisch-Becker, S.; Graeven, U.; A Lerchenmüller, C.; Killing, B.; Depenbusch, R.; Steffens, C.-C.; Al-Batran, S.-E.; Lange, T.; Dietrich, G.; Stoehlmacher, J.; et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 1355–1369. [Google Scholar] [CrossRef]
- Masi, G.; Salvatore, L.; Boni, L.; Loupakis, F.; Cremolini, C.; Fornaro, L.; Schirripa, M.; Cupini, S.; Barbara, C.; Safina, V.; et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: Final results of the randomized BEBYP trial. Ann. Oncol. 2015, 26, 724–730. [Google Scholar] [CrossRef]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; et al. Trifluridine–Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef]
- Otsu, S.; Hironaka, S. Current Status of Angiogenesis Inhibitors as Second-Line Treatment for Unresectable Colorectal Cancer. Cancers 2023, 15, 4564. [Google Scholar] [CrossRef]
- Hashimoto, T.; Otsu, S.; Hironaka, S.; Takashima, A.; Mizusawa, J.; Kataoka, T.; Fukuda, H.; Tsukamoto, S.; Hamaguchi, T.; Kanemitsu, Y. Phase II biomarker identification study of anti-VEGF agents with FOLFIRI for pretreated metastatic colorectal cancer. Future Oncol. 2023, 19, 1593–1600. [Google Scholar] [CrossRef]
- Kumar, R.; Crouthamel, M.-C.; Rominger, D.H.; Gontarek, R.R.; Tummino, P.J.; A Levin, R.; King, A.G. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br. J. Cancer 2009, 101, 1717–1723. [Google Scholar] [CrossRef]
- Cao, Y.; Langer, R.; Ferrara, N. Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug Discov. 2023, 22, 476–495. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.Y.; Wang, Z.; Wang, Y. Fruquintinib: A novel antivascular endothelial growth factor receptor tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. Cancer Manag. Res. 2019, 11, 7787–7803. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.H.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.D.; Zhong, H.; et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients with Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018, 319, 2486–2496. [Google Scholar] [CrossRef]
- Shirley, M. Fruquintinib: First Global Approval. Drugs 2018, 78, 1757–1761. [Google Scholar] [CrossRef]
- Siu, L.L.; Shapiro, J.D.; Jonker, D.J.; Karapetis, C.S.; Zalcberg, J.R.; Simes, J.; Couture, F.; Moore, M.J.; Price, T.J.; Siddiqui, J.; et al. Phase III Randomized, Placebo-Controlled Study of Cetuximab Plus Brivanib Alaninate Versus Cetuximab Plus Placebo in Patients With Metastatic, Chemotherapy-Refractory, Wild-Type K-RAS Colorectal Carcinoma: The NCIC Clinical Trials Group and AGITG CO.20 Trial. J. Clin. Oncol. 2013, 31, 2477–2484. [Google Scholar]
- Cunningham, D.; Wong, R.P.; D’Haens, G.; Douillard, J.Y.; Robertson, J.; Stone, A.M.; Van Cutsem, E. Cediranib with mFOLFOX6 vs bevacizumab with mFOLFOX6 in previously treated metastatic colo-rectal cancer. Br. J. Cancer 2013, 108, 493–502. [Google Scholar] [CrossRef]
- Hoff, P.M.; Hochhaus, A.; Pestalozzi, B.C.; Tebbutt, N.C.; Li, J.; Kim, T.W.; Koynov, K.D.; Kurteva, G.; Pintér, T.; Cheng, Y.; et al. Cediranib Plus FOLFOX/CAPOX Versus Placebo Plus FOLFOX/CAPOX in Patients with Previously Untreated Metastatic Colorectal Cancer: A Randomized, Double-Blind, Phase III Study (HORIZON II). J. Clin. Oncol. 2012, 30, 3596–3603. [Google Scholar] [CrossRef]
- Schmoll, H.-J.; Cunningham, D.; Sobrero, A.; Karapetis, C.S.; Rougier, P.; Koski, S.L.; Kocakova, I.; Bondarenko, I.; Bodoky, G.; Mainwaring, P.; et al. Cediranib With mFOLFOX6 Versus Bevacizumab With mFOLFOX6 As First-Line Treatment for Patients with Advanced Colorectal Cancer: A Double-Blind, Randomized Phase III Study (HORIZON III). J. Clin. Oncol. 2012, 30, 3588–3595. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Cainap, C.; Van Cutsem, E.; Gorbunova, V.; Karapetis, C.S.; Berlin, J.; Goldberg, R.M.; Qin, Q.; Qian, J.; Ricker, J.L.; et al. Randomized Phase II Open-Label Study of mFOLFOX6 in Combination with Linifanib or Bevacizumab for Metastatic Colorectal Cancer. Clin. Color. Cancer 2014, 13, 156–163.e2. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; Kiss, I.; Bridgewater, J.; Eskens, F.A.; Sasse, C.; Vossen, S.; Chen, J.; Van Sant, C.; Ball, H.A.; Keating, A.; et al. BATON-CRC: A Phase II Randomized Trial Comparing Tivozanib Plus mFOLFOX6 with Bevacizumab Plus mFOLFOX6 in Stage IV Metastatic Colorectal Cancer. Clin. Cancer Res. 2016, 22, 5058–5067. [Google Scholar] [CrossRef]
- Yang, T.S.; Oh, D.Y.; Guimbaud, R.; Szanto, J.; Salek, T.; Thurzo, L.; Vieitez, J.M.; Pover, G.M.; Kim, T.W. Vandetanib plus mFOLFOX6 in patients with advanced colorectal cancer (CRC): A randomized, double-blind, placebo-controlled phase II study. J. Clin. Oncol. 2009, 27, 4084. [Google Scholar] [CrossRef]
- Hecht, J.R.; Trarbach, T.; Hainsworth, J.D.; Major, P.; Jäger, E.; Wolff, R.A.; Lloyd-Salvant, K.; Bodoky, G.; Pendergrass, K.; Berg, W.; et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J. Clin. Oncol. 2011, 29, 1997–2003. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bajetta, E.; Valle, J.; Köhne, C.H.; Hecht, J.R.; Moore, M.; Germond, C.; Berg, W.; Chen, B.L.; Jalava, T.; et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J. Clin. Oncol. 2011, 29, 2004–2010. [Google Scholar]
- Xu, R.-H.; Shen, L.; Wang, K.-M.; Wu, G.; Shi, C.-M.; Ding, K.-F.; Lin, L.-Z.; Wang, J.-W.; Xiong, J.-P.; Wu, C.-P.; et al. Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: A multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin. J. Cancer 2017, 36, 97. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Prenen, H.; D’Haens, G.; Bennouna, J.; Carrato, A.; Ducreux, M.; Bouché, O.; Sobrero, A.; Latini, L.; Staines, H.; et al. A phase I/II, open-label, randomised study of nintedanib plus mFOLFOX6 versus bevacizumab plus mFOLFOX6 in first-line metastatic colorectal cancer patients. Ann. Oncol. 2015, 26, 2085–2091. [Google Scholar] [CrossRef]
- Ettrich, T.J.; Perkhofer, L.; Decker, T.; Hofheinz, R.D.; Heinemann, V.; Hoffmann, T.; Hebart, H.F.; Herrmann, T.; Hannig, C.V.; Büchner-Steudel, P.; et al. Nintedanib plus mFOLFOX6 as second-line treatment of metastatic, chemorefractory colorectal cancer: The randomised, placebo-controlled, phase II TRICC-C study (AIO-KRK-0111). Int. J. Cancer 2021, 148, 1428–1437. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Yoshino, T.; Lenz, H.; Lonardi, S.; Falcone, A.; Limón, M.; Saunders, M.; Sobrero, A.; Park, Y.; Ferreiro, R.; et al. Nintedanib for the treatment of patients with refractory metastatic colorectal cancer (LUME-Colon 1): A phase III, international, randomized, placebo-controlled study. Ann. Oncol. 2018, 29, 1955–1963. [Google Scholar] [CrossRef]
- Infante, J.R.; Reid, T.R.; Cohn, A.L.; Edenfield, W.J.; Cescon, T.P.; Hamm, J.T.; Malik, I.A.; Rado, T.A.; McGee, P.J.; Richards, D.A.; et al. Axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for metastatic colorectal cancer: A randomized phase 2 study. Cancer 2013, 119, 2555–2563. [Google Scholar] [CrossRef]
- Grávalos, C.; Carrato, A.; Tobeña, M.; Rodriguez-Garrote, M.; Soler, G.; Vieitez, J.M.; Robles, L.; Valladares-Ayerbes, M.; Polo, E.; Limón, M.L.; et al. A Randomized Phase II Study of Axitinib as Maintenance Therapy After First-line Treatment for Metastatic Colorectal Cancer. Clin. Color. Cancer 2018, 17, e323–e329. [Google Scholar] [CrossRef]
- Bendell, J.C.; Tournigand, C.; Swieboda-Sadlej, A.; Barone, C.; Wainberg, Z.A.; Kim, J.G.; Pericay, C.; Pastorelli, D.; Tarazi, J.; Rosbrook, B.; et al. Axitinib or Bevacizumab Plus FOLFIRI or Modified FOLFOX-6 After Failure of First-Line Therapy for Metastatic Colorectal Cancer: A Randomized Phase II Study. Clin. Color. Cancer 2013, 12, 239–247. [Google Scholar] [CrossRef]
- García-Carbonero, R.; van Cutsem, E.; Rivera, F.; Jassem, J.; Gore, I., Jr.; Tebbutt, N.; Braiteh, F.; Argiles, G.; Wainberg, Z.; Funke, R.; et al. Randomized Phase II Trial of Parsatuzumab (Anti-EGFL7) or Placebo in Combination with FOLFOX and Bevacizumab for First-Line Metastatic Colorectal Cancer. Oncologist 2017, 22, 1281. [Google Scholar] [CrossRef][Green Version]
- Bendell, J.C.; Sauri, T.; Gracián, A.C.; Alvarez, R.; Lopez, C.L.; García-Alfonso, P.; Hussein, M.; Miron, M.L.; Cervantes, A.; Montagut, C.; et al. The McCAVE Trial: Vanucizumab plus mFOLFOX-6 Versus Bevacizumab plus mFOLFOX-6 in Patients with Previously Untreated Metastatic Colorectal Carcinoma (mCRC). Oncologist 2019, 25, e451–e459. [Google Scholar] [CrossRef]
- Peeters, M.; Strickland, A.H.; Lichinitser, M.; Suresh, A.V.S.; Manikhas, G.; Shapiro, J.; Rogowski, W.; Huang, X.; Wu, B.; Warner, D.; et al. A randomised, double-blind, placebo-controlled phase 2 study of trebananib (AMG 386) in combination with FOLFIRI in patients with previously treated metastatic colorectal carcinoma. Br. J. Cancer 2013, 108, 503–511. [Google Scholar] [CrossRef]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.-S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar]
- Helfrich, I.; Scheffrahn, I.; Bartling, S.; Weis, J.; von Felbert, V.; Middleton, M.; Kato, M.; Ergün, S.; Augustin, H.G.; Schadendorf, D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010, 207, 491–503. [Google Scholar] [CrossRef]
- Frentzas, S.; Simoneau, E.; Bridgeman, V.L.; Vermeulen, P.B.; Foo, S.; Kostaras, E.; Nathan, M.R.; Wotherspoon, A.; Gao, Z.-H.; Shi, Y.; et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 2016, 22, 1294–1302. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, B.-S.; Guo, P.; Lee, S.B.; Butler, J.M.; Casey, S.C.; Simons, M.; Tam, W.; Felsher, D.W.; Shido, K.; et al. Angiocrine Factors Deployed by Tumor Vascular Niche Induce B Cell Lymphoma Invasiveness and Chemoresistance. Cancer Cell 2014, 25, 350–365. [Google Scholar] [CrossRef]
- Kerbel, R.S.; Shaked, Y. The potential clinical promise of ‘multimodality’ metronomic chemotherapy revealed by preclinical studies of metastatic disease. Cancer Lett. 2017, 400, 293–304. [Google Scholar] [CrossRef]
- Cremolini, C.; Marmorino, F.; Bergamo, F.; Aprile, G.; Salvatore, L.; Masi, G.; Dell’aquila, E.; Antoniotti, C.; Murgioni, S.; Allegrini, G.; et al. Phase II randomised study of maintenance treatment with bevacizumab or bevacizumab plus metronomic chemotherapy after first-line induction with FOLFOXIRI plus Bevacizumab for metastatic colorectal cancer patients: The MOMA trial. Eur. J. Cancer 2019, 109, 175–182. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Jia, W.; Deng, H.; Li, G.; Deng, W.; Chen, J.; Kim, B.Y.; Jiang, W.; Liu, Q.; et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020, 26, 1712–1724. [Google Scholar] [CrossRef]
- Mettu, N.B.; Ou, F.S.; Zemla, T.J.; Halfdanarson, T.R.; Lenz, H.J.; Breakstone, R.A.; Boland, P.M.; Crysler, O.V.; Wu, C.; Nixon, A.B. Assessment of Capecitabine and Bevacizumab with or Without Atezolizumab for the Treatment of Refractory Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2149040. [Google Scholar] [CrossRef]
- Lamplugh, Z.; Fan, Y. Vascular Microenvironment, Tumor Immunity and Immunotherapy. Front. Immunol. 2021, 12, 811485. [Google Scholar] [CrossRef]
- Schmittnaegel, M.; Rigamonti, N.; Kadioglu, E.; Cassará, A.; Rmili, C.W.; Kiialainen, A.; Kienast, Y.; Mueller, H.-J.; Ooi, C.-H.; Laoui, D.; et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 2017, 9, eaak9670. [Google Scholar] [CrossRef]
- Di Tacchio, M.; Macas, J.; Weissenberger, J.; Sommer, K.; Bahr, O.; Steinbach, J.P.; Senft, C.; Seifert, V.; Glas, M.; Herrlinger, U.; et al. Tumor Vessel Normalization, Immunostimulatory Reprogramming, and Improved Survival in Glio-blastoma with Combined Inhibition of PD-1, Angiopoietin-2, and VEGF. Cancer Immunol. Res. 2019, 7, 1910–1927. [Google Scholar] [CrossRef]
- Shigeta, K.; Datta, M.; Hato, T.; Kitahara, S.; Chen, I.X.; Matsui, A.; Kikuchi, H.; Mamessier, E.; Aoki, S.; Ramjiawan, R.R.; et al. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology 2019, 71, 1247–1261. [Google Scholar] [CrossRef]
- Weiss, A.; Ding, X.; van Beijnum, J.R.; Wong, I.; Wong, T.J.; Berndsen, R.H.; Dormond, O.; Dallinga, M.; Shen, L.; Schlingemann, R.O.; et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis 2015, 18, 233–244. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Cao, Z.; Ji, H.; Yang, X.; Iwamoto, H.; Wahlberg, E.; Länne, T.; Sun, B.; Cao, Y. Anti-VEGF– and anti-VEGF receptor–induced vascular alteration in mouse healthy tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 12018–12023. [Google Scholar] [CrossRef]
- Huang, X.; Bai, X.; Cao, Y.; Wu, J.; Huang, M.; Tang, D.; Tao, S.; Zhu, T.; Liu, Y.; Yang, Y.; et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by me-diating immune evasion. J. Exp. Med. 2010, 207, 505–520. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Nolan, D.J.; Ginsberg, M.; Israely, E.; Palikuqi, B.; Poulos, M.G.; James, D.; Ding, B.-S.; Schachterle, W.; Liu, Y.; Rosenwaks, Z.; et al. Molecular Signatures of Tissue-Specific Microvascular Endothelial Cell Heterogeneity in Organ Maintenance and Regeneration. Dev. Cell 2013, 26, 204–219. [Google Scholar] [CrossRef]
- Zhao, Q.; Eichten, A.; Parveen, A.; Adler, C.; Huang, Y.; Wang, W.; Ding, Y.; Adler, A.; Nevins, T.; Ni, M.; et al. Single-Cell Transcriptome Analyses Reveal Endothelial Cell Heterogeneity in Tumors and Changes following Antiangiogenic Treatment. Cancer Res. 2018, 78, 2370–2382. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Nowak-Sliwinska, P.; Huijbers, E.J.; Thijssen, V.L.; Griffioen, A.W. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol. Rev. 2015, 67, 441–461. [Google Scholar] [CrossRef]
- Bridgeman, V.L.; Vermeulen, P.B.; Foo, S.; Bilecz, A.; Daley, F.; Kostaras, E.; Nathan, M.R.; Wan, E.; Frentzas, S.; Schweiger, T.; et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J. Pathol. 2016, 241, 362–374. [Google Scholar] [CrossRef]
- Schellerer, V.S.; Croner, R.S.; Weinländer, K.; Hohenberger, W.; Stürzl, M.; Naschberger, E. Endothelial cells of human colorectal cancer and healthy colon reveal phenotypic differences in culture. Lab Investig. 2007, 87, 1159–1170. [Google Scholar] [CrossRef][Green Version]
- Naschberger, E.; Fuchs, M.; Dickel, N.; Kunz, M.; Popp, B.; Anchang, C.G.; Demmler, R.; Lyu, Y.; Uebe, S.; Ekici, A.B.; et al. Tumor microenvironment-dependent epigenetic imprinting in the vasculature predicts colon cancer outcome. Cancer Commun. 2023, 43, 1280–1285. [Google Scholar] [CrossRef]
- Wang, Q.; He, Z.; Huang, M.; Liu, T.; Wang, Y.; Xu, H.; Duan, H.; Ma, P.; Zhang, L.; Zamvil, S.S.; et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 2018, 9, 559. [Google Scholar] [CrossRef]
- Naschberger, E.; Liebl, A.; Schellerer, V.S.; Schütz, M.; Britzen-Laurent, N.; Kölbel, P.; Schaal, U.; Haep, L.; Regensburger, D.; Wittmann, T.; et al. Matricellular protein SPARCL1 regulates tumor microenvironment-dependent endothelial cell heterogeneity in colorectal carcinoma. J. Clin. Investig. 2016, 126, 4187–4204. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Peinado, H.; Mori, H.; Matei, I.R.; Evason, K.J.; Brazier, H.; Almeida, D.; Koller, A.; Hajjar, K.A.; Stainier, D.Y.; et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013, 15, 807–817. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, H.; Ge, W.; Liu, X.; Loera, S.; Chu, P.; Chen, H.; Peng, J.; Zhou, L.; Yu, S.; et al. Secreted protein acidic and rich in cysteines-like 1 suppresses aggressiveness and predicts better survival in colorectal cancers. Clin. Cancer Res. 2012, 18, 5438–5448. [Google Scholar] [CrossRef]
- Khan, A.K.; Wu, F.T.; Cruz-Munoz, W.; Kerbel, R.S. Ang2 inhibitors and Tie2 activators: Potential therapeutics in perioperative treatment of early stage cancer. EMBO Mol. Med. 2021, 13, e08253. [Google Scholar] [CrossRef]
- Hendrikx, S.; Coso, S.; Prat-Luri, B.; Wetterwald, L.; Sabine, A.; Franco, C.A.; Nassiri, S.; Zangger, N.; Gerhardt, H.; Delorenzi, M.; et al. Endothelial Calcineurin Signaling Restrains Metastatic Outgrowth by Regulating Bmp2. Cell Rep. 2019, 26, 1227–1241.e6. [Google Scholar] [CrossRef]
- Thomas, H. Colorectal cancer: CRC endothelial regulation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 682. [Google Scholar]
- Dirkx, A.E.M.; Egbrink, M.G.A.O.; E Kuijpers, M.J.; Van Der Niet, S.T.; Heijnen, V.V.T.; Steege, J.C.A.B.-T.; Wagstaff, J.; Griffioen, A.W. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003, 63, 2322–2329. [Google Scholar]
- Nambiar, D.K.; Aguilera, T.; Cao, H.; Kwok, S.; Kong, C.; Bloomstein, J.; Wang, Z.; Rangan, V.S.; Jiang, D.; von Eyben, R.; et al. Galectin-1–driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Investig. 2019, 129, 5553–5567. [Google Scholar] [CrossRef]
- Motz, G.T.; Santoro, S.P.; Wang, L.-P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef]
- Streubel, B.; Chott, A.; Huber, D.; Exner, M.; Jäger, U.; Wagner, O.; Schwarzinger, I. Lymphoma-Specific Genetic Aberrations in Microvascular Endothelial Cells in B-Cell Lymphomas. N. Engl. J. Med. 2004, 351, 250–259. [Google Scholar] [CrossRef]
- Dunleavey, J.M.; Dudley, A.C. Vascular Mimicry: Concepts and Implications for Anti-Angiogenic Therapy. Curr. Angiogenesis 2012, 1, 133–138. [Google Scholar] [CrossRef]
- Van der Schaft, D.W.; Seftor, R.E.; Seftor, E.A.; Hess, A.R.; Gruman, L.M.; Kirschmann, D.A.; Yokoyama, Y.; Griffioen, A.W.; Hendrix, M.J. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J. Natl. Cancer Inst. 2004, 96, 1473–1477. [Google Scholar] [CrossRef]
- Zhao, C.; Gomez, G.A.; Zhao, Y.; Yang, Y.; Cao, D.; Lu, J.; Yang, H.; Lin, S. ETV2 mediates endothelial transdifferentiation of glioblastoma. Signal Transduct. Target. Ther. 2018, 3, 4. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumor vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef]
- Wang, R.; Chadalavada, K.; Wilshire, J.; Kowalik, U.; Hovinga, K.E.; Geber, A.; Fligelman, B.; Leversha, M.; Brennan, C.; Tabar, V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010, 468, 829–833. [Google Scholar] [CrossRef]
- Akino, T.; Hida, K.; Hida, Y.; Tsuchiya, K.; Freedman, D.; Muraki, C.; Ohga, N.; Matsuda, K.; Akiyama, K.; Harabayashi, T.; et al. Cytogenetic Abnormalities of Tumor-Associated Endothelial Cells in Human Malignant Tumors. Am. J. Pathol. 2009, 175, 2657–2667. [Google Scholar] [CrossRef]
- Ohga, N.; Ishikawa, S.; Maishi, N.; Akiyama, K.; Hida, Y.; Kawamoto, T.; Sadamoto, Y.; Osawa, T.; Yamamoto, K.; Kondoh, M.; et al. Heterogeneity of tumor endothelial cells: Comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am. J. Pathol. 2012, 180, 1294–1307. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, C.-L.; Zhang, W.-R.; Cheng, H.-P.; Liu, J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006, 27, 821–826. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, R.; Cai, J.; Yang, N.; Wen, Z.; Zhang, Z.; Sun, H.; Huang, G.; Guan, Y.; Huang, N.; et al. Matrix Stiffness Triggers Lipid Metabolic Cross-talk between Tumor and Stromal Cells to Mediate Bevacizumab Resistance in Colorectal Cancer Liver Metastases. Cancer Res. 2023, 83, 3577–3592. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, F.; Yan, C.; Su, L.; Luo, J.; Chi, W.; Zhang, S. Mitophagy Protects the Retina Against Anti-Vascular Endothelial Growth Factor Therapy-Driven Hypoxia via Hypoxia-Inducible Factor-1α Signaling. Front. Cell Dev. Biol. 2021, 9, 727822. [Google Scholar] [CrossRef]
- Suleyman, H.; Kocaturk, H.; Bedir, F.; Turangezli, O.; Arslan, R.; Coban, T.; Altuner, D. Effect of adenosine triphosphate, benidipine and their combinations on bevacizumab-induced kidney damage in rats. Adv. Clin. Exp. Med. 2021, 30, 1175–1183. [Google Scholar] [CrossRef]
- Jackstadt, R.; van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e7. [Google Scholar] [CrossRef]
- Varga, J.; Nicolas, A.; Petrocelli, V.; Pesic, M.; Mahmoud, A.; Michels, B.E.; Etlioglu, E.; Yepes, D.; Häupl, B.; Ziegler, P.K.; et al. AKT-dependent NOTCH3 activation drives tumor progression in a model of mesenchymal colorectal cancer. J. Exp. Med. 2020, 217, e20191515. [Google Scholar] [CrossRef]
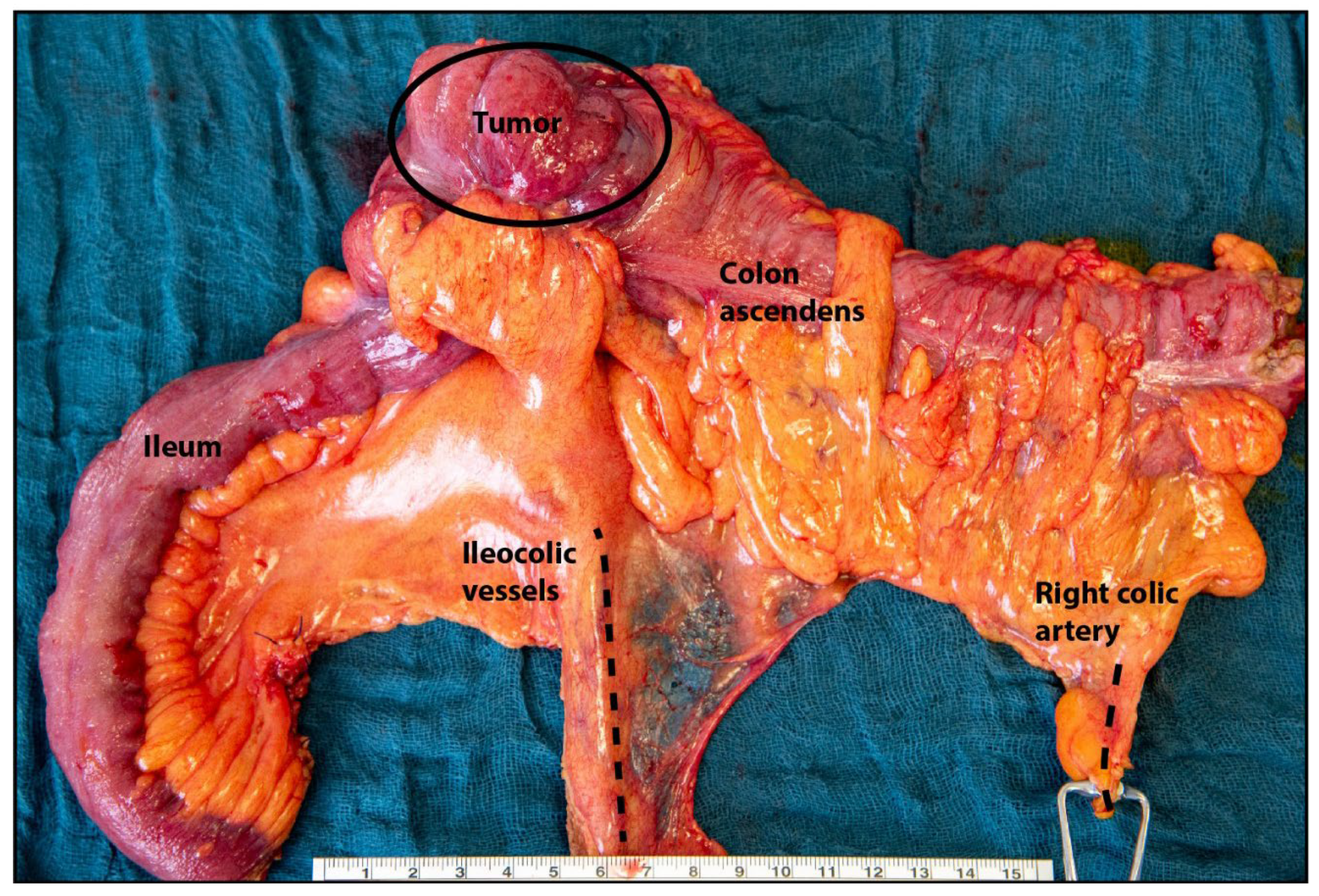
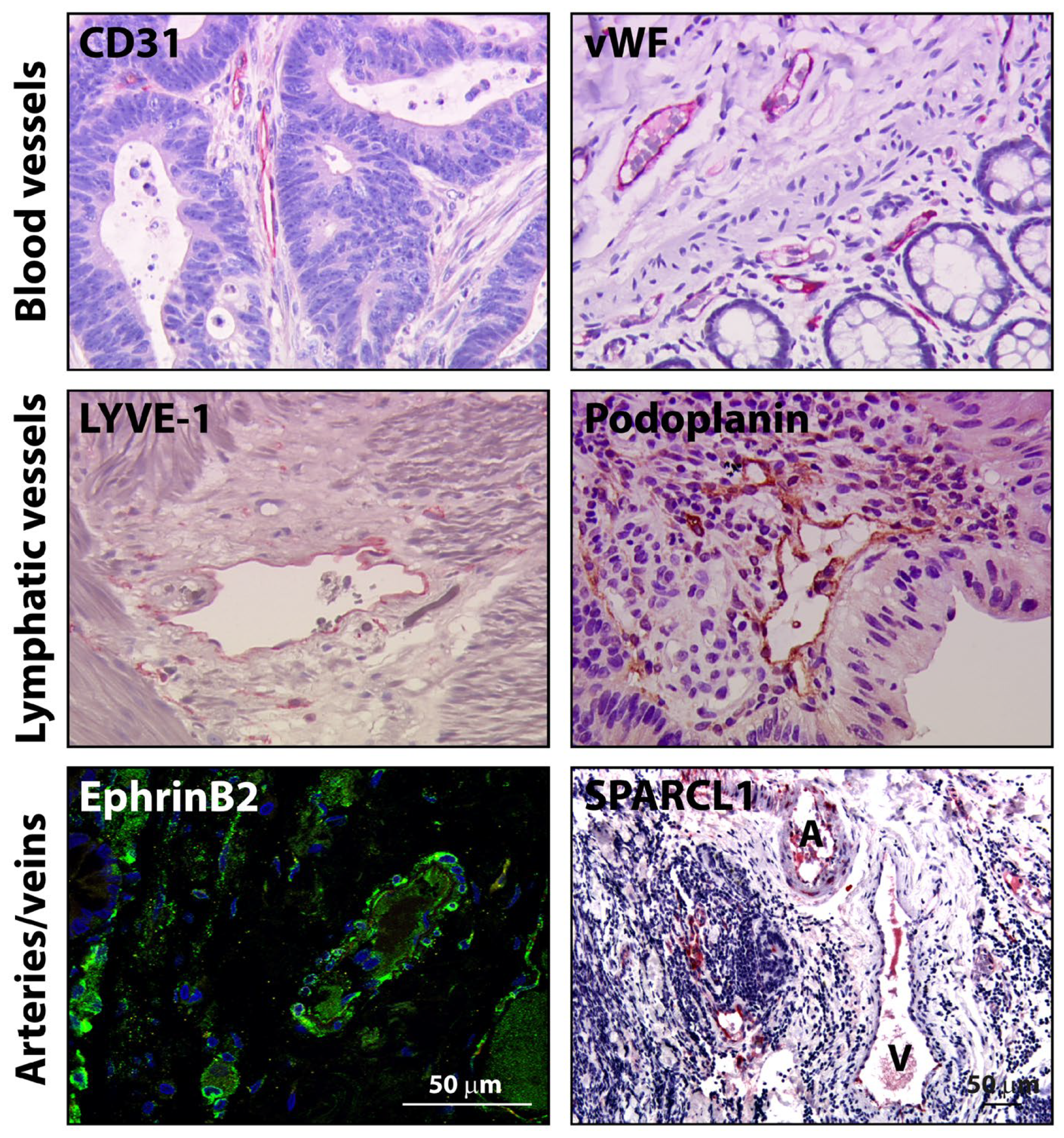
| Clinical Trial | Treatment | Indication | mOS, Months (95%CI) | mPFS, Months (95% CI) | ORR, % | HR (OS) (95%CI) | Ref. |
|---|---|---|---|---|---|---|---|
| AVF2107g | Beva + IFL Beva + placebo | 1st line | 20.3 (n.r.) 15.6 (n.r.) | 10.6 (n.r.) 6.2 (n.r.) | 44.8 34.8 | 0.66 (n.r.), p < 0.001 | [19] |
| ITACa | Beva + FOLFOX/FOLFIRI FOLFOX/FOLFIRI | 1st line | 20.8 (15.9–23.2) 21.3 (19.9–24.1) | 9.6 (8.2–10.3) 8.4 (7.2–9.0) | 50.6 50 | 1.13 (0.89–1.43), p = 0.304 | [21] |
| ML18147 | Beva+FOLFOX/FOLFIRI FOLFOX/FOLFIRI | 2nd line | 11.2 (10.4–12.2) 9.8 (8.9–10.7) | 5.7 (5.2–6.2) 4.1 (3.7–4.4) | 5 4 | 0.81 (0.69–0.94), p = 0.0062) | [22] |
| VELOUR | Aflibercept + FOLFIRI FOLFIRI | 2nd line | 13.5 (12.52–14.95) 12.6 (11.07–13.11) | 6.9 (6.51–7.2) 4.67 (4.21–5.36) | 19.8 11.1 | 0.817 (0.713–0.937), p = 0.0032) | [23] |
| RAISE | Ramucirumab + FOLFIRI FOLFIRI | 2nd line | 13.3 (12.4–14.5) 11.7 (10.8–12–7) | 5.7 (5.5–6.2) 4.5 (4.2–5.4) | 13.4 12.5 | 0.844 (0.73–0.976), p = 0.0219 | [24] |
| CORRECT | Regorafenib Placebo | refractory | 6.4 (CI n.r.) 5.0 (CI n.r.) | 1.9 (CI n.r.) 1.7 (CI n.r.) | 1 0.4 | 0.77 (0.64–0.94), p = 0.0052 | [25] |
| FRESCO II | Fruquintinib Placebo | 3rd/later line | 7.4 (6.7–8.2) 4.8 (4.0–5.8) | 3.7 (3.5–3.8) 1.8 (1.8–1.9) | 5 0 | 0.66 (0.55–0.80), p < 0.0001 | [26] |
| Drug | Target | Regimen | Phase | Indication | Results in CRC | Ref. |
|---|---|---|---|---|---|---|
| TKI | ||||||
| Brivanib | VEGFR-2, -3, FGFR-1, -2, -3 | Brivanib/cetuximab Placebo/cetuximab | III | Refractory | No improvement of OS, significant improvement of ORR and PFS, increased toxicity | [55] |
| Cediranib | VEGFR-1, -2, -3, PDGFRβ, KIT | Cediranib/FOLFOX Beva/FOLFOX | II | 2nd line | No improvement of PFS or OS | [56] |
| Cediranib/FOLFOX or CAPOX Placebo/FOLFOX or CAPOX | III | 1st line | Modest PFS prolongation, no impact on OS | [57] | ||
| Cediranib/FOLFOX Beva/FOLFOX | II/III | 1st line | PFS and OS comparable to those of beva, less favorable profile of adverse events | [58] | ||
| Linifanib | VEGFR-1, -2, -3, PDGFRβ | Linifanib/FOLFOX Beva/FOLFOX | II | 2nd line | PFS and OS comparable to those of beva, more adverse events | [59] |
| Tivozanib | VEGFR-1, -2, -3, KIT, PDGFRβ | Tivozanib/FOLFOX Beva/FOLFOX | II | 1st line | Efficacy comparable to that of beva | [60] |
| Vandetanib | EGFR, VEGFR-2, RET, BRK, TIE-2 | Vandetanib/FOLFOX Placebo/FOLFOX | II | 2nd line | No efficacy | [61] |
| Vatalanib | VEGFR-1, -2, -3 | Vatalanib/FOLFOX Placebo/FOLFOX | III | 1st line | No efficacy in OS, PFS, ORR | [62] |
| Vatalanib/FOLFOX Placebo/FOLFOX | III | 2nd line | Improvement of PFS, but not OS | [63] | ||
| Famitinib | VEGFR-2, -3, KIT, PDGFR, RET | Famitinib Placebo | II | 3rd or later line | Prolongation of PFS, no improvement of OS | [64] |
| Nintedanib | VEGFR-1, -2, -3, FGFR-1, -2, -3, PDGFRα/β | Nintedanib/FOLFOX Beva/FOLFOX | I/II | 1st line | Similar PFS | [65] |
| Nintedanib/FOLFOX Placebo/FOLFOX | II | 2nd line | Nonsignificant trend for improved PFS, OS, DCR | [66] | ||
| Nintedanib Placebo | III | Refractory | No improvement of OS, modest increase of PFS | [67] | ||
| Monoclonal antibodies | ||||||
| Axitinib | VEGFR-1, -2, -3 | Axitinib/FOLFOX Beva/FOLFO Axitinib/Beva/FOLFOX | II | 1st line | No improvement of ORR, PFS or OS by addition of axitinib or combination with beva | [68] |
| Axitinib vs. placebo | II | Maintenance | Significantly longer PFS with axitinib | [69] | ||
| Axitinib/FOLFOX Beva/FOLFOX Axitinib/FOLFIRI Beva/FOLFIRI | II | 2nd line | No improvement of PFS and OR, but more adverse events with axitinib | [70] | ||
| Parsatuzumab | EGFL7 | Parsatuzumab/FOLFOX/beva Placebo/FOLFOX/beva | II | 1st line | No improvement of ORR, PFS, OS | [71] |
| Vanucizumab | VEGF-A, Ang-2 | Vanucizumab/FOLFOX Placebo/FOLFOX | II | 1st line | No improvement of PFS, increased toxicity | [72] |
| Peptibody | ||||||
| Trebananib | Ang-1, -2 | Trebananib/FOLFIRI Placebo/FOLFIRI | II | 2nd line | No improvement of OS or PFS | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobsen, A.; Siebler, J.; Grützmann, R.; Stürzl, M.; Naschberger, E. Blood Vessel-Targeted Therapy in Colorectal Cancer: Current Strategies and Future Perspectives. Cancers 2024, 16, 890. https://doi.org/10.3390/cancers16050890
Jacobsen A, Siebler J, Grützmann R, Stürzl M, Naschberger E. Blood Vessel-Targeted Therapy in Colorectal Cancer: Current Strategies and Future Perspectives. Cancers. 2024; 16(5):890. https://doi.org/10.3390/cancers16050890
Chicago/Turabian StyleJacobsen, Anne, Jürgen Siebler, Robert Grützmann, Michael Stürzl, and Elisabeth Naschberger. 2024. "Blood Vessel-Targeted Therapy in Colorectal Cancer: Current Strategies and Future Perspectives" Cancers 16, no. 5: 890. https://doi.org/10.3390/cancers16050890
APA StyleJacobsen, A., Siebler, J., Grützmann, R., Stürzl, M., & Naschberger, E. (2024). Blood Vessel-Targeted Therapy in Colorectal Cancer: Current Strategies and Future Perspectives. Cancers, 16(5), 890. https://doi.org/10.3390/cancers16050890








