The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Skin Physiology
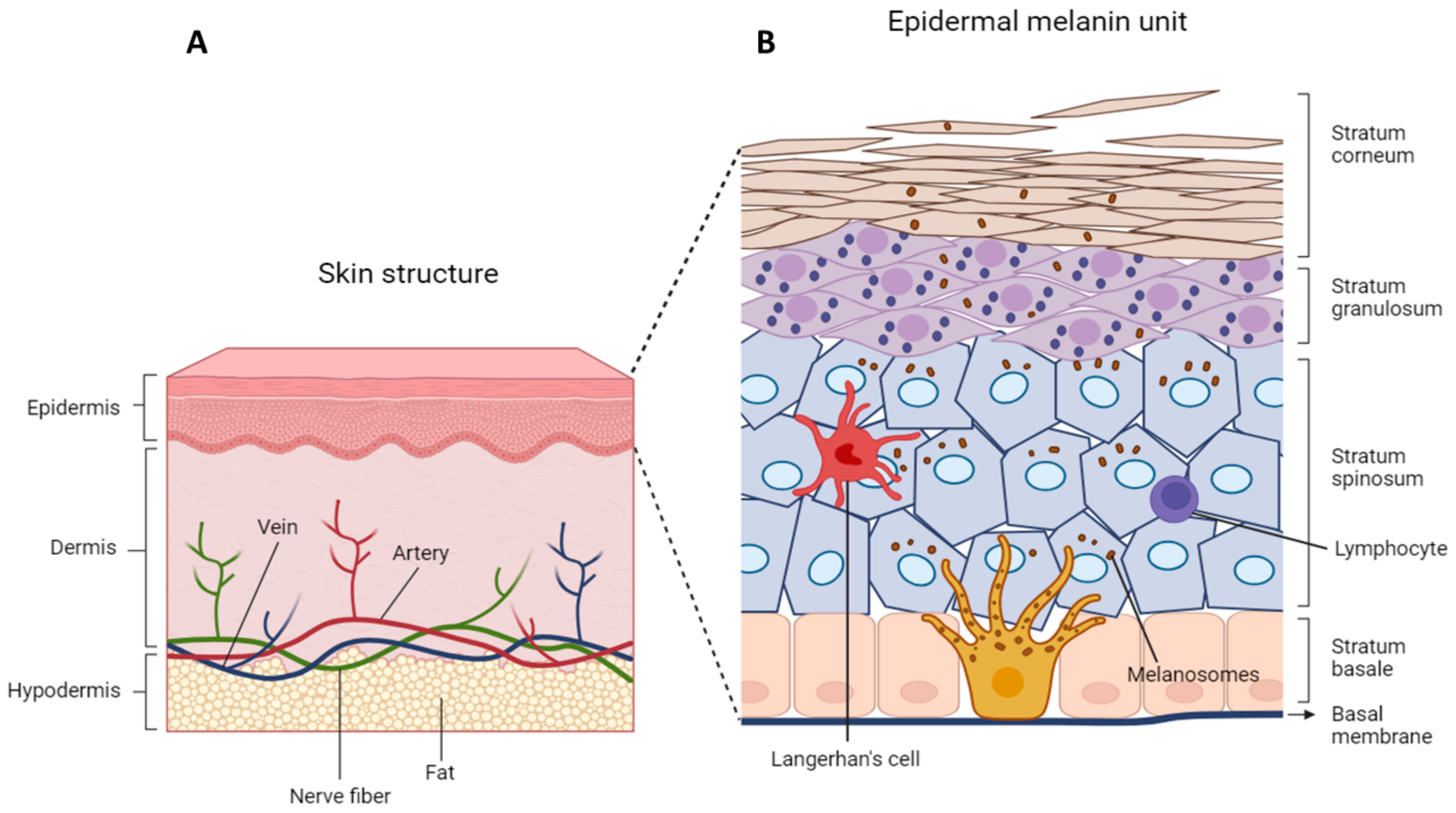
3.1. Skin Structure
3.2. Epidermal Organization
3.3. Epidermal–Melanin Units
3.4. Physiological Keratinocyte-Melanocyte Communication
3.4.1. Cell–Cell Contact in Melanocyte-Keratinocyte Interaction
3.4.2. Cross-Talk in the Epidermal–Melanin Unit
4. Remodeling of Epidermal Microenvironment during Melanoma Onset and Progression
4.1. Alteration of Melanocyte–Keratinocyte Physical Interaction during Melanoma Inception and Progression
4.2. Keratinocyte–Melanoma Cells Cross-Talk
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. Lancet 2023, 402, 485. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Karimkhani, C.; Green, A.C.; Nijsten, T.; Weinstock, M.A.; Dellavalle, R.P.; Naghavi, M.; Fitzmaurice, C. The Global Burden of Melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 2017, 177, 134. [Google Scholar] [CrossRef]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Nilsen, L.T.N.; Hannevik, M.; Veierød, M.B. Ultraviolet Exposure from Indoor Tanning Devices: A Systematic Review. Br. J. Dermatol. 2016, 174, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Boniol, M.; Autier, P.; Boyle, P.; Gandini, S. Cutaneous Melanoma Attributable to Sunbed use: Systematic Review and Meta-Analysis. BMJ 2012, 345, e4757. [Google Scholar] [CrossRef] [PubMed]
- Dzwierzynski, W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021, 48, 543–550. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Jin, S.; Padron, F.; Pfeifer, G.P. UVA Radiation, DNA Damage, and Melanoma. ACS Omega 2022, 7, 32936–32948. [Google Scholar] [CrossRef]
- Gorbunova, V.; Seluanov, A.; Mao, Z.; Hine, C. Changes in DNA Repair during Aging. Nucleic Acids Res. 2007, 35, 7466–7474. [Google Scholar] [CrossRef]
- Ren, P.; Dong, X.; Vijg, J. Age-Related Somatic Mutation Burden in Human Tissues. Front. Aging 2022, 3, 1018119. [Google Scholar] [CrossRef] [PubMed]
- Maslov, A.Y.; Vijg, J. Somatic Mutation Burden in Relation to Aging and Functional Life Span: Implications for Cellular Reprogramming and Rejuvenation. Curr. Opin. Genet. Dev. 2023, 83, 102132. [Google Scholar] [CrossRef] [PubMed]
- Wei, E.X.; Li, X.; Nan, H. Having a First-Degree Relative with Melanoma Increases Lifetime Risk of Melanoma, Squamous Cell Carcinoma, and Basal Cell Carcinoma. J. Am. Acad. Dermatol. 2019, 81, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.; Li, Y.; Navsaria, L.J.; Hinkston, C.L.; Margolis, D.J.; Wehner, M.R. Melanoma Risk in Skin of Color Patients with a History of a Keratinocyte Carcinoma. Br. J. Dermatol. 2023, 190, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Mercieca, L.; Aquilina, S.; Calleja, N.; Boffa, M.J. Cutaneous Melanoma More Likely to be Invasive in Fairer Skin Phototypes: A Retrospective Observational Study. Skinmed 2021, 19, 280–283. [Google Scholar] [PubMed]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.N.; Harland, M.; Bishop, D.T. The Genetics of Melanoma. Br. J. Hosp. Med. 2006, 67, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hemminki, K.; Kharazmi, E.; Ji, J.; Sundquist, K.; Fallah, M. Multiple Primary (Even in Situ) Melanomas in a Patient Pose Significant Risk to Family Members. Eur. J. Cancer 2014, 50, 2659–2667. [Google Scholar] [CrossRef]
- Hussussian, C.J.; Struewing, J.P.; Goldstein, A.M.; Higgins, P.A.T.; Ally, D.S.; Sheahan, M.D.; Clark, W.H.; Tucker, M.A.; Dracopoli, N.C. Germline p16 Mutations in Familial Melanoma. Nat. Genet. 1994, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Robles-Espinoza, C.D.; Harland, M.; Ramsay, A.J.; Aoude, L.G.; Quesada, V.; Ding, Z.; Pooley, K.A.; Pritchard, A.L.; Tiffen, J.C.; Petljak, M.; et al. POT1 Loss-of-Function Variants Predispose to Familial Melanoma. Nat. Genet. 2014, 46, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Puntervoll, H.E.; Yang, X.R.; Vetti, H.H.; Bachmann, I.M.; Avril, M.F.; Benfodda, M.; Catricalà, C.; Dalle, S.; Duval-Modeste, A.B.; Ghiorzo, P.; et al. Melanoma Prone Families withCDK4germline Mutation: Phenotypic Profile and Associations with MC1Rvariants. J. Med. Genet. 2013, 50, 264. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, L.; Bonelli, L.; Ghiorzo, P.; Queirolo, P.; Battistuzzi, L.; Balleari, E.; Nasti, S.; Gargiulo, S.; Gliori, S.; Savoia, P.; et al. CDKN2A Mutations and MC1R Variants in Italian Patients with Single or Multiple Primary Melanoma. Pigment Cell Melanoma Res. 2008, 21, 700–709. [Google Scholar] [CrossRef]
- O’Neill, C.H.; Scoggins, C.R. Melanoma. J. Surg. Oncol. 2019, 120, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Xiao, Y.; Sampson, J.; Zhu, B.; Rotunno, M.; Bennett, H.; Wen, Y.; Jones, K.; Vogt, A.; Burdette, L.; et al. Rare Germline Variants in Known Melanoma Susceptibility Genes in Familial Melanoma. Hum. Mol. Genet. 2017, 26, 4886–4895. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Fragoso, S.; Luís, R.; Pinto, F.; Brito, C.; Esteves, S.; Pataco, M.; Santos, S.; Machado, P.; Vicente, J.B.; et al. High-Throughput Sequencing Identifies 3 Novel Susceptibility Genes for Hereditary Melanoma. Genes 2020, 11, 403. [Google Scholar] [CrossRef]
- Castaneda-Garcia, C.; Iyer, V.; Nsengimana, J.; Trower, A.; Droop, A.; Brown, K.M.; Choi, J.; Zhang, T.; Harland, M.; Newton-Bishop, J.A.; et al. Defining Novel Causal SNPs and Linked Phenotypes at Melanoma-Associated Loci. Hum. Mol. Genet. 2022, 31, 2845–2856. [Google Scholar] [CrossRef]
- Bruno, W.; Dalmasso, B.; Barile, M.; Andreotti, V.; Elefanti, L.; Colombino, M.; Vanni, I.; Allavena, E.; Barbero, F.; Passoni, E.; et al. Predictors of Germline Status for Hereditary Melanoma: 5 Years of Multi-Gene Panel Testing within the Italian Melanoma Intergroup. ESMO Open 2022, 7, 100525. [Google Scholar] [CrossRef]
- Potjer, T.P.; Bollen, S.; Grimbergen, A.J.E.M.; van Doorn, R.; Gruis, N.A.; van Asperen, C.J.; Hes, F.J.; van der Stoep, N. Multigene Panel Sequencing of Established and Candidate Melanoma Susceptibility Genes in a Large Cohort of Dutch Non-CDKN2A/CDK4 Melanoma Families. Int. J. Cancer 2019, 144, 2453–2464. [Google Scholar] [CrossRef]
- Manganelli, M.; Guida, S.; Ferretta, A.; Pellacani, G.; Porcelli, L.; Azzariti, A.; Guida, G. Behind the Scene: Exploiting MC1R in Skin Cancer Risk and Prevention. Genes 2021, 12, 1093. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Gandini, S.; Bellocco, R.; Maisonneuve, P.; Newton-Bishop, J.; Polsky, D.; Lazovich, D.; Kanetsky, P.; Ghiorzo, P.; Gruis, N.; et al. MC1R Variants as Melanoma Risk Factors Independent of at-Risk Phenotypic Characteristics: A Pooled Analysis from the M-SKIP Project. CMAR 2018, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Swope, V.B.; Abdel-Malek, Z.A. MC1R: Front and Center in the Bright Side of Dark Eumelanin and DNA Repair. Int. J. Mol. Sci. 2018, 19, 2667. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.L. Genetics of Hair and Skin Color. Annu. Rev. Genet. 2003, 37, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; McQuillan, M.A.; Tishkoff, S.A. Evolutionary Genetics of Skin Pigmentation in African Populations. Hum. Mol. Genet. 2021, 30, R88–R97. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Picardo, M. A Framework of Major Tumor-Promoting Signal Transduction Pathways Implicated in Melanoma-Fibroblast Dialogue. Cancers 2020, 12, 3400. [Google Scholar] [CrossRef]
- Cerdido, S.; Sánchez-Beltrán, J.; Lambertos, A.; Abrisqueta, M.; Padilla, L.; Herraiz, C.; Olivares, C.; Jiménez-Cervantes, C.; García-Borrón, J.C. A Side-by-Side Comparison of Wildtype and Variant Melanocortin 1 Receptor Signaling with Emphasis on Protection Against Oxidative Damage to DNA. Int. J. Mol. Sci. 2023, 24, 14381. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.A.; Ruwe, A.; Kavanagh-Starner, R.; Kadekaro, A.L.; Swope, V.; Haskell-Luevano, C.; Koikov, L.; Knittel, J.J. A-MSH Tripeptide Analogs Activate the Melanocortin 1 Receptor and Reduce UV-Induced DNA Damage in Human Melanocytes. Pigment Cell Melanoma Res. 2009, 22, 635–644. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Chen, J.; Yang, J.; Chen, S.; Jameson, J.; Swope, V.B.; Cheng, T.; Kadakia, M.; Abdel-Malek, Z. Alpha-Melanocyte–Stimulating Hormone Suppresses Oxidative Stress through a p53-Mediated Signaling Pathway in Human Melanocytes. Mol. Cancer Res. 2012, 10, 778–786. [Google Scholar] [CrossRef]
- Herraiz, C.; Journé, F.; Abdel-Malek, Z.; Ghanem, G.; Jiménez-Cervantes, C.; García-Borrón, J.C. Signaling from the Human Melanocortin 1 Receptor to ERK1 and ERK2 Mitogen-Activated Protein Kinases Involves Transactivation of cKIT. Mol. Endocrinol. 2011, 25, 138–156. [Google Scholar] [CrossRef]
- Herraiz, C.; Martínez-Vicente, I.; Maresca, V. The A-Melanocyte-Stimulating Hormone/Melanocortin-1 Receptor Interaction: A Driver of Pleiotropic Effects Beyond Pigmentation. Pigment Cell Melanoma Res. 2021, 34, 748–761. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, M. Novel Approaches to the Design of Bioavailable Melanotropins. Expert Opin. Drug Discov. 2017, 12, 1023–1030. [Google Scholar] [CrossRef]
- Caini, S.; Gandini, S.; Botta, F.; Tagliabue, E.; Raimondi, S.; Nagore, E.; Zanna, I.; Maisonneuve, P.; Newton-Bishop, J.; Polsky, D.; et al. MC1R Variants and Cutaneous Melanoma Risk According to Histological Type, Body Site, and Breslow Thickness: A Pooled Analysis from the M-SKIP Project. Melanoma Res. 2020, 30, 500–510. [Google Scholar] [CrossRef]
- Latreille, J.; Ezzedine, K.; Elfakir, A.; Ambroisine, L.; Jdid, R.; Galan, P.; Hercberg, S.; Gruber, F.; Malvy, D.; Tschachler, E.; et al. MC1R Polymorphisms and Facial Photoaging. Ann. Dermatol. Venereol. 2011, 138, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; André, M.; Adhikari, K.; Blin, M.; Bonfante, B.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Palmal, S.; Chacón-Duque, J.C.; Hurtado, M.; et al. A Genome-Wide Association Study Identifies Novel Gene Associations with Facial Skin Wrinkling and Mole Count in Latin Americans. Br. J. Dermatol. 2021, 185, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Ciardo, S.; De Pace, B.; De Carvalho, N.; Peccerillo, F.; Manfredini, M.; Farnetani, F.; Chester, J.; Kaleci, S.; Manganelli, M.; et al. The Influence of MC1R On dermal Morphological Features of Photo-Exposed Skin in Women Revealed by Reflectance Confocal Microscopy and Optical Coherence Tomography. Exp. Dermatol. 2019, 28, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Law, M.H.; Medland, S.E.; Zhu, G.; Yazar, S.; Viñuela, A.; Wallace, L.; Shekar, S.N.; Duffy, D.L.; Bataille, V.; Glass, D.; et al. Genome-Wide Association shows that Pigmentation Genes Play a Role in Skin Aging. J. Investig. Dermatol. 2017, 137, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.D.; Wong, T.; Morris, M.K.; Di Germanio, C.; Ma, Z.; Stone, M.; Ball, E.; Fritts, L.; Rustagi, A.; Simmons, G.; et al. Administration of Vaccine-Boosted COVID-19 Convalescent Plasma to SARS-CoV-2 Infected Hamsters Decreases Virus Replication in Lungs and Hastens Resolution of the Infection Despite Transiently Enhancing Disease and Lung Pathology. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cirri, P.; Chiarugi, P. Cancer Associated Fibroblasts: The Dark Side of the Coin. Am. J. Cancer Res. 2011, 1, 482–497. [Google Scholar] [PubMed]
- Bellei, B.; Picardo, M. Premature Cell Senescence in Human Skin: Dual Face in Chronic Acquired Pigmentary Disorders. Ageing Res. Rev. 2020, 57, 100981. [Google Scholar] [CrossRef] [PubMed]
- Swope, V.B.; Starner, R.J.; Rauck, C.; Abdel-Malek, Z.A. Endothelin-1 and A-Melanocortin have Redundant Effects on Global Genome Repair in UV-Irradiated Human Melanocytes Despite Distinct Signaling Pathways. Pigment Cell Melanoma Res. 2020, 33, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Sturm, R.A.; Smith, A.G. MC1R and NR4A Receptors in Cellular Stress and DNA Repair: Implications for UVR Protection. Exp. Dermatol. 2014, 23, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Castejón-Griñán, M.; Herraiz, C.; Olivares, C.; Jiménez-Cervantes, C.; García-Borrón, J.C. cAMP-Independent Non-Pigmentary Actions of Variant Melanocortin 1 Receptor: AKT-Mediated Activation of Protective Responses to Oxidative DNA Damage. Oncogene 2018, 37, 3631–3646. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.T.; Huurne, J.A.C.t.; Kielich, C.; Gruis, N.A.; Westendorp, R.G.J.; Vermeer, B.J.; Bavinck, J.N.B. Melanocortin-1 Receptor Gene Variants Determine the Risk of Nonmelanoma Skin Cancer Independently of Fair Skin and Red Hair. Am. J. Hum. Genet. 2001, 68, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Miao, Y.; Cao, L.; Guo, L.; Cui, Y.; Yan, C.; Zeng, Z.; Xu, M.; Han, T. Activation of Melanocortin-1 Receptor Signaling in Melanoma Cells Impairs T Cell Infiltration to Dampen Antitumor Immunity. Nat. Commun. 2023, 14, 5740. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Strippoli, S.; Ferretta, A.; Bartolomeo, N.; Porcelli, L.; Maida, I.; Azzariti, A.; Tommasi, S.; Grieco, C.; Guida, S.; et al. Detrimental Effects of Melanocortin-1 Receptor (MC1R) Variants on the Clinical Outcomes of BRAF V600 Metastatic Melanoma Patients Treated with BRAF Inhibitors. Pigment Cell Melanoma Res. 2016, 29, 679–687. [Google Scholar] [CrossRef]
- Su, D.; Djureinovic, D.; Schoenfeld, D.; Marquez-Nostra, B.; Olino, K.; Jilaveanu, L.; Kluger, H. Melanocortin 1 Receptor (MC1R) Expression as a Marker of Progression in Melanoma. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Sarasin, A.; Kauffmann, A. Overexpression of DNA Repair Genes is Associated with Metastasis: A New Hypothesis. Mutat. Res. 2008, 659, 49–55. [Google Scholar] [CrossRef]
- Zhang, G.; Herlyn, M. Human Nevi: No Longer Precursors of Melanomas? J. Investig. Dermatol. 2012, 132, 2133–2134. [Google Scholar] [CrossRef]
- Tsao, H.; Bevona, C.; Goggins, W.; Quinn, T. The Transformation Rate of Moles (Melanocytic Nevi) into Cutaneous Melanoma: A Population-Based Estimate. Arch. Dermatol. (1960) 2003, 139, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Shreberk-Hassidim, R.; Ostrowski, S.M.; Fisher, D.E. The Complex Interplay between Nevi and Melanoma: Risk Factors and Precursors. Int. J. Mol. Sci. 2023, 24, 3541. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.; et al. High Frequency of BRAF Mutations in Nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Requena, C.; Manrique, E.; Nagore, E. Update on Lentigo Maligna: Diagnostic Signs and Treatment. Actas Dermosifiliogr. 2023, 114, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Brînzea, A.; Nedelcu, R.I.; Ion, D.A.; Turcu, G.; Antohe, M.; Hodorogea, A.; Călinescu, A.; Pirici, D.; Popescu, R.; Popescu, C.M.; et al. Matrix Metalloproteinases Expression in Lentigo Maligna∕lentigo Maligna Melanoma—A Review of the Literature and Personal Experience. Rom. J. Morphol. Embryol. 2019, 60, 1091–1095. [Google Scholar] [PubMed]
- DeWane, M.E.; Kelsey, A.; Oliviero, M.; Rabinovitz, H.; Grant-Kels, J.M. Melanoma on Chronically Sun-Damaged Skin: Lentigo Maligna and Desmoplastic Melanoma. J. Am. Acad. Dermatol. 2019, 81, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Flanagan, T.W.; Hassan, S.; Shalaby, H.; Khabaz, M.; Hassan, S.; Megahed, M.; Haikel, Y.; Santourlidis, S.; Hassan, M. Tumor Microenvironment as a Therapeutic Target in Melanoma Treatment. Cancers 2023, 15, 3147. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, P.; Xia, P.; Song, H.; Guo, Z.; Hu, X.; Guo, Y.; Yuan, X.; Song, Y.; Shen, R.; et al. Identification and Targeting of Cancer-Associated Fibroblast Signature Genes for Prognosis and Therapy in Cutaneous Melanoma. Comput. Biol. Med. 2023, 167, 107597. [Google Scholar] [CrossRef]
- Anderson-Crannage, M.; Ascensión, A.M.; Ibanez-Solé, O.; Zhu, H.; Schaefer, E.; Ottomanelli, D.; Hochberg, B.; Pan, J.; Luo, W.; Tian, M.; et al. Inflammation-Mediated Fibroblast Activation and Immune Dysregulation in Collagen VII-Deficient Skin. Front. Immunol. 2023, 14, 1211505. [Google Scholar] [CrossRef]
- Wu, B.; Sodji, Q.H.; Oyelere, A.K. Inflammation, Fibrosis and Cancer: Mechanisms, Therapeutic Options and Challenges. Cancers 2022, 14, 552. [Google Scholar] [CrossRef]
- Bottazzi, B.; Riboli, E.; Mantovani, A. Aging, Inflammation and Cancer. Semin. Immunol. 2018, 40, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The Role of Cellular Senescence in Skin Aging and Age-Related Skin Pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef] [PubMed]
- D’Arino, A.; Caputo, S.; Eibenschutz, L.; Piemonte, P.; Buccini, P.; Frascione, P.; Bellei, B. Skin Cancer Microenvironment: What we can Learn from Skin Aging? Int. J. Mol. Sci. 2023, 24, 14043. [Google Scholar] [CrossRef]
- Chatsirisupachai, K.; Lagger, C.; de Magalhães, J.P. Age-Associated Differences in the Cancer Molecular Landscape. Trends Cancer 2022, 8, 962–971. [Google Scholar] [CrossRef]
- Nicolas, E.; Golemis, E.A.; Arora, S. POLD1: Central Mediator of DNA Replication and Repair, and Implication in Cancer and Other Pathologies. Gene 2016, 590, 128–141. [Google Scholar] [CrossRef]
- Council, M.L.; Sheinbein, D.M. Common Skin Cancers in Older Adults Approach to Diagnosis and Management. Clin. Geriatr. Med. 2024, 40, 25–36. [Google Scholar] [CrossRef]
- Papaccio, F.; Kovacs, D.; Bellei, B.; Caputo, S.; Migliano, E.; Cota, C.; Picardo, M. Profiling Cancer-Associated Fibroblasts in Melanoma. Int. J. Mol. Sci. 2021, 22, 7255. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, R.; Zhang, Y.; Jia, S.; He, Y.; Liu, J. The Cross Talk between Cellular Senescence and Melanoma: From Molecular Pathogenesis to Target Therapies. Cancers 2023, 15, 2640. [Google Scholar] [CrossRef]
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L. Dermal Extracellular Matrix Molecules in Skin Development, Homeostasis, Wound Regeneration and Diseases. Semin. Cell Dev. Biol. 2022, 128, 137–144. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Tsepkolenko, A.; Tsepkolenko, V.; Dash, S.; Mishra, A.; Bader, A.; Melerzanov, A.; Giri, S. The Regenerative Potential of Skin and the Immune System. Clin. Cosmet. Investig. Dermatol. 2019, 12, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Bruckner-Tuderman, L. Matrix Molecules and Skin Biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Juarez, C.F.; Plikus, M.V. Emerging Nonmetabolic Functions of Skin Fat. Nat. Rev. Endocrinol. 2018, 14, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Nicu, C.; O’Sullivan, J.D.B.; Ramos, R.; Timperi, L.; Lai, T.; Farjo, N.; Farjo, B.; Pople, J.; Bhogal, R.; Hardman, J.A.; et al. Dermal Adipose Tissue Secretes HGF to Promote Human Hair Growth and Pigmentation. J. Investig. Dermatol. 2021, 141, 1633–1645.e13. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Chen, J.; Ye, Y.; Tseng, H.; Lai, F.; Tay, K.H.; Jin, L.; Guo, S.T.; Jiang, C.C.; Zhang, X.D. Adipocytes Contribute to Resistance of Human Melanoma Cells to Chemotherapy and Targeted Therapy. Curr. Med. Chem. 2014, 21, 1255–1267. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Fu, X.; Liu, B.; Chao, X.; Chan, C.L.; Cao, H.; Su, T.; Tse, A.K.W.; Fong, W.F.; Yu, Z. Subcutaneous Adipocytes Promote Melanoma Cell Growth by Activating the Akt Signaling Pathway: Role of Palmitic Acid. J. Biol. Chem. 2014, 289, 30525–30537. [Google Scholar] [CrossRef]
- Zhang, M.; Di Martino, J.S.; Bowman, R.L.; Campbell, N.R.; Baksh, S.C.; Simon-Vermot, T.; Kim, I.S.; Haldeman, P.; Mondal, C.; Yong-Gonzales, V.; et al. Adipocyte-Derived Lipids Mediate Melanoma Progression Via FATP Proteins. Cancer Discov. 2018, 8, 1006–1025. [Google Scholar] [CrossRef]
- Okumura, T.; Ohuchida, K.; Kibe, S.; Iwamoto, C.; Ando, Y.; Takesue, S.; Nakayama, H.; Abe, T.; Endo, S.; Koikawa, K.; et al. Adipose Tissue-Derived Stromal Cells are Sources of Cancer-Associated Fibroblasts and Enhance Tumor Progression by Dense Collagen Matrix. Int. J. Cancer 2019, 144, 1401–1413. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Lee, Y.K.; Koo, J.S. Expression of Cancer-Associated Fibroblast-Related Proteins in Adipose Stroma of Breast Cancer. Tumour Biol. 2015, 36, 8685–8695. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuédé, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-Derived Fibroblasts Promote Tumor Progression and Contribute to the Desmoplastic Reaction in Breast Cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef]
- Menon, G.K. Skin Basics; Structure and Function. In Lipids and Skin Health; Springer International Publishing: Cham, Switzerland, 2014; pp. 9–23. [Google Scholar]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gdula, M.R.; Poterlowicz, K.; Mardaryev, A.N.; Sharov, A.A.; Peng, Y.; Fessing, M.Y.; Botchkarev, V.A. Remodeling of Three-Dimensional Organization of the Nucleus during Terminal Keratinocyte Differentiation in the Epidermis. J. Investig. Dermatol. 2013, 133, 2191–2201. [Google Scholar] [CrossRef]
- Monteleon, C.L.; Agnihotri, T.; Dahal, A.; Liu, M.; Rebecca, V.W.; Beatty, G.L.; Amaravadi, R.K.; Ridky, T.W. Lysosomes Support the Degradation, Signaling, and Mitochondrial Metabolism Necessary for Human Epidermal Differentiation. J. Investig. Dermatol. 2018, 138, 1945–1954. [Google Scholar] [CrossRef]
- Murata, T.; Honda, T.; Mostafa, A.; Kabashima, K. Stratum Corneum as Polymer Sheet: Concept and Cornification Processes. Trends Mol. Med. 2022, 28, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Venus, M.; Waterman, J.; McNab, I. Basic Physiology of the Skin. Surgery 2010, 28, 469–472. [Google Scholar]
- Proksch, E.; Brandner, J.M.; Jensen, J. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.R.; Kupper, T.S. Immunity at the Surface: Homeostatic Mechanisms of the Skin Immune System. Life Sci. 1996, 58, 1485–1507. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin Barrier Immunity and Ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef]
- Morizane, S.; Mukai, T.; Sunagawa, K.; Tachibana, K.; Kawakami, Y.; Ouchida, M. “Input/Output Cytokines” in Epidermal Keratinocytes and the Involvement in Inflammatory Skin Diseases. Front. Immunol. 2023, 14, 1239598. [Google Scholar] [CrossRef]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular Mechanisms Regulating Human Melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Casalou, C.; Moreiras, H.; Mayatra, J.M.; Fabre, A.; Tobin, D.J. Loss of ‘Epidermal Melanin Unit’ Integrity in Human Skin during Melanoma-Genesis. Front. Oncol. 2022, 12, 878336. [Google Scholar] [CrossRef]
- Naik, P.P.; Farrukh, S.N. Influence of Ethnicities and Skin Color Variations in Different Populations: A Review. Skin. Pharmacol. Physiol. 2022, 35, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.L.; Gaylor, J.; Everett, M.A. Skin Color, Melanin, and Erythema. Arch. Dermatol. (1960) 1973, 108, 541–544. [Google Scholar] [CrossRef]
- Montagna, W.; Carlisle, K. The Architecture of Black and White Facial Skin. J. Am. Acad. Dermatol. 1991, 24, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of Tyrosinase as the Determinant of Pigmentation in Cultured Human Melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef]
- Markiewicz, E.; Karaman-Jurukovska, N.; Mammone, T.; Idowu, O.C. Post-Inflammatory Hyperpigmentation in Dark Skin: Molecular Mechanism and Skincare Implications. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2555–2565. [Google Scholar] [CrossRef]
- Van Den Bossche, K.; Naeyaert, J.; Lambert, J. The Quest for the Mechanism of Melanin Transfer. Traffic 2006, 7, 769–778. [Google Scholar] [CrossRef]
- Bento-Lopes, L.; Cabaço, L.C.; Charneca, J.; Neto, M.V.; Seabra, M.C.; Barral, D.C. Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. Int. J. Mol. Sci. 2023, 24, 11289. [Google Scholar] [CrossRef]
- Costin, G.; Hearing, V.J. Human Skin Pigmentation: Melanocytes Modulate Skin Color in Response to Stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin Melanocytes: Biology and Development. Postep. Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Nordlund, J.J. The Lives of Pigment Cells. Dermatol. Clin. 1986, 4, 407–418. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Blog, F.B.; Szabo, G. Effects of Aging and Chronic Sun Exposure on Melanocytes in Human Skin. J. Investig. Dermatol. 1979, 73, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current Challenges in Understanding Melanogenesis: Bridging Chemistry, Biological Control, Morphology, and Function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef]
- Land, E.J.; Riley, P.A. Spontaneous Redox Reactions of Dopaquinone and the Balance between the Eumelanic and Phaeomelanic Pathways. Pigment Cell Res. 2000, 13, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Hertzman Johansson, C.; Azimi, A.; Frostvik Stolt, M.; Shojaee, S.; Wiberg, H.; Grafström, E.; Hansson, J.; Egyházi Brage, S. Association of MITF and Other Melanosome-Related Proteins with Chemoresistance in Melanoma Tumors and Cell Lines. Melanoma Res. 2013, 23, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Juntti-Berggren, L.; Lindh, U.; Berggren, P.O. Starvation is Associated with Changes in the Elemental Composition of the Pancreatic Beta-Cell. Biosci. Rep. 1991, 11, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Fisher, D.E. The Master Role of Microphthalmia-Associated Transcription Factor in Melanocyte and Melanoma Biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Czyz, M. MITF in Melanoma: Mechanisms Behind its Expression and Activity. Cell. Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Migliano, E.; Muscardin, L.; Silipo, V.; Catricalà, C.; Picardo, M.; Bellei, B. The Role of Wnt/Β-Catenin Signaling Pathway in Melanoma Epithelial-to-Mesenchymal-Like Switching: Evidences from Patients-Derived Cell Lines. Oncotarget 2016, 7, 43295–43314. [Google Scholar] [CrossRef]
- Hossain, S.M.; Eccles, M.R. Phenotype Switching and the Melanoma Microenvironment; Impact on Immunotherapy and Drug Resistance. Int. J. Mol. Sci. 2023, 24, 1601. [Google Scholar] [CrossRef]
- Jimbow, K.; Quevedo, W.C.; Fitzpatrick, T.B.; Szabo, G. Some Aspects of Melanin Biology: 1950–1975. J. Investig. Dermatol. 1976, 67, 72–89. [Google Scholar] [CrossRef]
- Hardman, M.J.; Liu, K.; Avilion, A.A.; Merritt, A.; Brennan, K.; Garrod, D.R.; Byrne, C. Desmosomal Cadherin Misexpression Alters Beta-Catenin Stability and Epidermal Differentiation. Mol. Cell. Biol. 2005, 25, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Charest, J.L.; Jennings, J.M.; King, W.P.; Kowalczyk, A.P.; García, A.J. Cadherin-Mediated Cell-Cell Contact Regulates Keratinocyte Differentiation. J. Investig. Dermatol. 2009, 129, 564–572. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, C.; Kiel, C. Cell Adhesion Molecules in Normal Skin and Melanoma. Biomolecules 2021, 11, 1213. [Google Scholar] [CrossRef]
- Tang, A.; Eller, M.S.; Hara, M.; Yaar, M.; Hirohashi, S.; Gilchrest, B.A. E-Cadherin is the Major Mediator of Human Melanocyte Adhesion to Keratinocytes In Vitro. J. Cell Sci. 1994, 107 Pt 4, 983–992. [Google Scholar] [CrossRef]
- Hung, C.; Chiang, H.; Lo, H.; Jian, J.; Wu, W. E-Cadherin and its Downstream Catenins are Proteolytically Cleaved in Human HaCaT Keratinocytes Exposed to UVB. Exp. Dermatol. 2006, 15, 315–321. [Google Scholar] [CrossRef]
- Gambichler, T.; Rotterdam, S.; Tigges, C.; Altmeyer, P.; Bechara, F.G. Impact of Ultraviolet Radiation on the Expression of Marker Proteins of Gap and Adhesion Junctions in Human Epidermis. Photodermatol. Photoimmunol. Photomed. 2008, 24, 318–321. [Google Scholar] [CrossRef]
- Jamal, S.; Schneider, R.J. UV-Induction of Keratinocyte Endothelin-1 Downregulates E-Cadherin in Melanocytes and Melanoma Cells. J. Clin. Investig. 2002, 110, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Jiang, S.; Miao, F.; Lei, T. sPmel17 Secreted by Ultraviolet B-Exposed Melanocytes Alters the Intercellular Adhesion of Keratinocytes. Oxid. Med. Cell. Longev. 2022, 2022, 1856830. [Google Scholar] [CrossRef]
- Shain, A.H.; Bastian, B.C. From Melanocytes to Melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Huber, O.; Bierkamp, C.; Kemler, R. Cadherins and Catenins in Development. Curr. Opin. Cell Biol. 1996, 8, 685–691. [Google Scholar] [CrossRef]
- Ramani, V.; Teshima, T.; Tamura, K.; Chung, J.; Kobayashi, M.; Cruz, P.D.; Ariizumi, K. Melanoma-Derived Soluble DC-HIL/GPNMB Promotes Metastasis by Excluding T-Lymphocytes from the Pre-Metastatic Niches. J. Investig. Dermatol. 2018, 138, 2443–2451. [Google Scholar] [CrossRef]
- Tomihari, M.; Hwang, S.; Chung, J.; Cruz, P.D.; Ariizumi, K. Gpnmb is a Melanosome-Associated Glycoprotein that Contributes to Melanocyte/Keratinocyte Adhesion in a RGD-Dependent Fashion. Exp. Dermatol. 2009, 18, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.B.; Takahashi, A.; Mizutani, Y.; Takayama, S.; Ishitsuka, A.; Yang, L.; Yang, F.; Iddamalgoda, A.; Katayama, I.; Inoue, S. GPNMB is Expressed in Human Epidermal Keratinocytes but Disappears in the Vitiligo Lesional Skin. Sci. Rep. 2020, 10, 4930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kuroda, Y.; Yang, L.; Lai, S.; Mizutani, Y.; Iddamalgoda, A.; Guo, J.; Yamamoto, A.; Murase, D.; Takahashi, Y.; et al. GPNMB Extracellular Fragment Protects Melanocytes from Oxidative Stress by Inhibiting AKT Phosphorylation Independent of CD44. Int. J. Mol. Sci. 2021, 22, 10843. [Google Scholar] [CrossRef]
- Arnette, C.R.; Roth-Carter, Q.R.; Koetsier, J.L.; Broussard, J.A.; Burks, H.E.; Cheng, K.; Amadi, C.; Gerami, P.; Johnson, J.L.; Green, K.J. Keratinocyte Cadherin Desmoglein 1 Controls Melanocyte Behavior through Paracrine Signaling. Pigment Cell Melanoma Res. 2020, 33, 305–317. [Google Scholar] [CrossRef]
- Hsu, M.; Andl, T.; Li, G.; Meinkoth, J.L.; Herlyn, M. Cadherin Repertoire Determines Partner-Specific Gap Junctional Communication during Melanoma Progression. J. Cell Sci. 2000, 113 Pt 9, 1535–1542. [Google Scholar] [CrossRef]
- Haass, N.K.; Wladykowski, E.; Kief, S.; Moll, I.; Brandner, J.M. Differential Induction of Connexins 26 and 30 in Skin Tumors and their Adjacent Epidermis. J. Histochem. Cytochem. 2006, 54, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Jiang, J.X. Gap Junction and Hemichannel-Independent Actions of Connexins on Cell and Tissue Functions—An Update. FEBS Lett. 2014, 588, 1186–1192. [Google Scholar] [CrossRef]
- Bellei, B.; Mastrofrancesco, A.; Briganti, S.; Aspite, N.; Ale-Agha, N.; Sies, H.; Picardo, M. Ultraviolet A Induced Modulation of Gap Junctional Intercellular Communication by P38 MAPK Activation in Human Keratinocytes. Exp. Dermatol. 2008, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.L.; Walicke, P.; Garovoy, M. Anti-Adhesion Antibodies Efalizumab, a Humanized Anti-CD11a Monoclonal Antibody. Transpl. Immunol. 2002, 9, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.R.; Ho, T.; Abdel-Malek, Z.A. Participation of Keratinocyte- and Fibroblast-Derived Factors in Melanocyte Homeostasis, the Response to UV, and Pigmentary Disorders. Pigment Cell Melanoma Res. 2021, 34, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Fukunaga-Kalabis, M.; Herlyn, M. Crosstalk in Skin: Melanocytes, Keratinocytes, Stem Cells, and Melanoma. J. Cell Commun. Signal. 2016, 10, 191–196. [Google Scholar] [CrossRef]
- Hirobe, T. Keratinocytes Regulate the Function of Melanocytes. Dermatol. Sin. 2014, 32, 200–204. [Google Scholar] [CrossRef]
- Haass, N.K.; Smalley, K.S.M.; Li, L.; Herlyn, M. Adhesion, Migration and Communication in Melanocytes and Melanoma. Pigment Cell Res. 2005, 18, 150–159. [Google Scholar] [CrossRef]
- Imokawa, G. Autocrine and Paracrine Regulation of Melanocytes in Human Skin and in Pigmentary Disorders. Pigment Cell Res. 2004, 17, 96–110. [Google Scholar] [CrossRef]
- Schiller, M.; Brzoska, T.; Böhm, M.; Metze, D.; Scholzen, T.E.; Rougier, A.; Luger, T.A. Solar-Simulated Ultraviolet Radiation-Induced Upregulation of the Melanocortin-1 Receptor, Proopiomelanocortin, and Alpha-Melanocyte-Stimulating Hormone in Human Epidermis In Vivo. J. Investig. Dermatol. 2004, 122, 468–476. [Google Scholar]
- Swope, V.B.; Medrano, E.E.; Smalara, D.; Abdel-Malek, Z.A. Long-Term Proliferation of Human Melanocytes is Supported by the Physiologic Mitogens Alpha-Melanotropin, Endothelin-1, and Basic Fibroblast Growth Factor. Exp. Cell Res. 1995, 217, 453–459. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Amano, Y.; Ohuchi, A.; Kitahara, T.; Takema, Y. The Essential Role of p53 in Hyperpigmentation of the Skin Via Regulation of Paracrine Melanogenic Cytokine Receptor Signaling. J. Biol. Chem. 2009, 284, 4343–4353. [Google Scholar] [CrossRef]
- Lübbe, J.; Reichel, M.; Burg, G.; Kleihues, P. Absence of p53 Gene Mutations in Cutaneous Melanoma. J. Investig. Dermatol. 1994, 102, 819–821. [Google Scholar] [CrossRef]
- Choi, S.; Bin, B.; Kim, W.; Lee, E.; Lee, T.R.; Cho, E. Exposure of Human Melanocytes to UVB Twice and Subsequent Incubation Leads to Cellular Senescence and Senescence-Associated Pigmentation through the Prolonged p53 Expression. J. Dermatol. Sci. 2018, 90, 303–312. [Google Scholar] [CrossRef]
- Wu, C.; Lan, C.E.; Chiou, M.; Yu, H. Basic Fibroblast Growth Factor Promotes Melanocyte Migration Via Increased Expression of p125(FAK) on Melanocytes. Acta Derm. Venereol. 2006, 86, 498–502. [Google Scholar] [CrossRef]
- Shi, H.; Lin, B.; Huang, Y.; Wu, J.; Zhang, H.; Lin, C.; Wang, Z.; Zhu, J.; Zhao, Y.; Fu, X.; et al. Basic Fibroblast Growth Factor Promotes Melanocyte Migration Via Activating PI3K/Akt-Rac1-FAK-JNK and ERK Signaling Pathways. IUBMB Life 2016, 68, 735–747. [Google Scholar] [CrossRef]
- Halaban, R.; Rubin, J.S.; Funasaka, Y.; Cobb, M.; Boulton, T.; Faletto, D.; Rosen, E.; Chan, A.; Yoko, K.; White, W. Met and Hepatocyte Growth Factor/Scatter Factor Signal Transduction in Normal Melanocytes and Melanoma Cells. Oncogene 1992, 7, 2195–2206. [Google Scholar]
- Weidner, K.M.; Di Cesare, S.; Sachs, M.; Brinkmann, V.; Behrens, J.; Birchmeier, W. Interaction between Gab1 and the C-Met Receptor Tyrosine Kinase is Responsible for Epithelial Morphogenesis. Nature 1996, 384, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Ponzetto, C.; Bardelli, A.; Zhen, Z.; Maina, F.; dalla Zonca, P.; Giordano, S.; Graziani, A.; Panayotou, G.; Comoglio, P.M. A Multifunctional Docking Site Mediates Signaling and Transformation by the Hepatocyte Growth Factor/Scatter Factor Receptor Family. Cell 1994, 77, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Mildner, M.; Mlitz, V.; Gruber, F.; Wojta, J.; Tschachler, E. Hepatocyte Growth Factor Establishes Autocrine and Paracrine Feedback Loops for the Protection of Skin Cells After UV Irradiation. J. Investig. Dermatol. 2007, 127, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Grichnik, J.M.; Burch, J.A.; Burchette, J.; Shea, C.R. The SCF/KIT Pathway Plays a Critical Role in the Control of Normal Human Melanocyte Homeostasis. J. Investig. Dermatol. 1998, 111, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Ewing, J.; Ryan, D.; Abboud, C. Stem Cell Factor Regulates Human Melanocyte-Matrix Interactions. Pigment Cell Res. 1994, 7, 44–51. [Google Scholar] [CrossRef]
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins Secreted from Human Keratinocytes are Intrinsic Mitogens for Human Melanocytes. J. Biol. Chem. 1992, 267, 24675–24680. [Google Scholar] [CrossRef]
- Imokawa, G.; Yada, Y.; Kimura, M. Signalling Mechanisms of Endothelin-Induced Mitogenesis and Melanogenesis in Human Melanocytes. Biochem. J. 1996, 314 Pt 1, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ujfaludi, Z.; Tuzesi, A.; Majoros, H.; Rothler, B.; Pankotai, T.; Boros, I.M. Coordinated Activation of a Cluster of MMP Genes in Response to UVB Radiation. Sci. Rep. 2018, 8, 2660. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Norisugi, O.; Matsunaga, K.; Nishihira, J.; Shimizu, T. Involvement of MIF in Basement Membrane Damage in Chronically UVB-Exposed Skin in Mice. PLoS ONE 2014, 9, e89569. [Google Scholar] [CrossRef] [PubMed]
- Purwar, R.; Kraus, M.; Werfel, T.; Wittmann, M. Modulation of Keratinocyte-Derived MMP-9 by IL-13: A Possible Role for the Pathogenesis of Epidermal Inflammation. J. Investig. Dermatol. 2008, 128, 59–66. [Google Scholar] [CrossRef]
- Wang, X.; Bi, Z.; Chu, W.; Wan, Y. IL-1 Receptor Antagonist Attenuates MAP Kinase/AP-1 Activation and MMP1 Expression in UVA-Irradiated Human Fibroblasts Induced by Culture Medium from UVB-Irradiated Human Skin Keratinocytes. Int. J. Mol. Med. 2005, 16, 1117–1124. [Google Scholar] [CrossRef]
- Lee, M.J.; Oh, J.; Park, C.; Kim, K.H.; Lee, D.H.; Chung, J.H. Galanin Contributes to Ultraviolet Irradiation-Induced Inflammation in Human Skin. Exp. Dermatol. 2017, 26, 744–747. [Google Scholar] [CrossRef]
- Harsha, A.; Stojadinovic, O.; Brem, H.; Sehara-Fujisawa, A.; Wewer, U.; Loomis, C.A.; Blobel, C.P.; Tomic-Canic, M. ADAM12: A Potential Target for the Treatment of Chronic Wounds. J. Mol. Med. 2008, 86, 961–969. [Google Scholar] [CrossRef]
- Oh, S.T.; Schramme, A.; Stark, A.; Tilgen, W.; Gutwein, P.; Reichrath, J. Overexpression of ADAM 10 and ADAM 12 in Lesional Psoriatic Skin. Br. J. Dermatol. 2008, 158, 1371–1373. [Google Scholar] [CrossRef]
- Abbes, A.; Zayani, Y.; Zidi, W.; Hammami, M.B.; Mebazaa, A.; El Euch, D.; Ben Ammar, A.; Sanhaji, H.; El May, M.V.; Mokni, M.; et al. Matrix Metalloproteinase-7 could be a Predictor for Acute Inflammation in Psoriatic Patients. Cytokine 2020, 134, 155195. [Google Scholar] [CrossRef]
- Suomela, S.; Kariniemi, A.L.; Snellman, E.; Saarialho-Kere, U. Metalloelastase (MMP-12) and 92-kDa Gelatinase (MMP-9) as Well as their Inhibitors, TIMP-1 and -3, are Expressed in Psoriatic Lesions. Exp. Dermatol. 2001, 10, 175–183. [Google Scholar] [CrossRef]
- Boukhedouni, N.; Martins, C.; Darrigade, A.; Drullion, C.; Rambert, J.; Barrault, C.; Garnier, J.; Jacquemin, C.; Thiolat, D.; Lucchese, F.; et al. Type-1 Cytokines Regulate MMP-9 Production and E-Cadherin Disruption to Promote Melanocyte Loss in Vitiligo. JCI Insight 2020, 5, e133772. [Google Scholar]
- Su, M.; Miao, F.; Jiang, S.; Shi, Y.; Luo, L.; He, X.; Wan, J.; Xu, S.; Lei, T. Role of the p53-TRPM1/miR-211-MMP9 Axis in UVB-induced Human Melanocyte Migration and its Potential in Repigmentation. Int. J. Mol. Med. 2020, 45, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Parsad, D.; Kanwar, A.J.; Kaul, D. Altered Levels of Ets-1 Transcription Factor and Matrix Metalloproteinases in Melanocytes from Patients with Vitiligo. Br. J. Dermatol. 2011, 165, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Valyi-Nagy, I.T.; Hirka, G.; Jensen, P.J.; Shih, I.M.; Juhasz, I.; Herlyn, M. Undifferentiated Keratinocytes Control Growth, Morphology, and Antigen Expression of Normal Melanocytes through Cell-Cell Contact. Lab. Investig. 1993, 69, 152–159. [Google Scholar]
- Le Varlet, B.; Chaudagne, C.; Saunois, A.; Barré, P.; Sauvage, C.; Berthouloux, B.; Meybeck, A.; Dumas, M.; Bonté, F. Age-Related Functional and Structural Changes in Human Dermo-Epidermal Junction Components. J. Investig. Dermatol. Symp. Proc. 1998, 3, 172–179. [Google Scholar] [CrossRef]
- Craven, N.M.; Watson, R.E.; Jones, C.J.; Shuttleworth, C.A.; Kielty, C.M.; Griffiths, C.E. Clinical Features of Photodamaged Human Skin are Associated with a Reduction in Collagen VII. Br. J. Dermatol. 1997, 137, 344–350. [Google Scholar] [CrossRef]
- Bosset, S.; Bonnet-Duquennoy, M.; Barré, P.; Chalon, A.; Lazou, K.; Kurfurst, R.; Bonté, F.; Schnébert, S.; Disant, F.; Le Varlet, B.; et al. Decreased Expression of Keratinocyte Beta1 Integrins in Chronically Sun-Exposed Skin In Vivo. Br. J. Dermatol. 2003, 148, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting Against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Miskolczi, Z.; Smith, M.P.; Rowling, E.J.; Ferguson, J.; Barriuso, J.; Wellbrock, C. Collagen Abundance Controls Melanoma Phenotypes through Lineage-Specific Microenvironment Sensing. Oncogene 2018, 37, 3166–3182. [Google Scholar] [CrossRef]
- Kirkpatrick, S.J.; Wang, R.K.; Duncan, D.D.; Kulesz-Martin, M.; Lee, K. Imaging the Mechanical Stiffness of Skin Lesions by in Vivo Acousto-Optical Elastography. Opt. Express 2006, 14, 9770–9779. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.B.; Sherratt, M.J. Molecular Aspects of Skin Ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Taloni, A.; Alemi, A.A.; Ciusani, E.; Sethna, J.P.; Zapperi, S.; La Porta, C.A.M. Mechanical Properties of Growing Melanocytic Nevi and the Progression to Melanoma. PLoS ONE 2014, 9, e94229. [Google Scholar] [CrossRef] [PubMed]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Bella, V.D.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional Roles of Matrix Metalloproteinases and their Inhibitors in Melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Beck, I.M.; Gadesmann, J.; Karschuk, N.; Paschen, A.; Proksch, E.; Djonov, V.; Reiss, K.; Sedlacek, R. MMP19 is Upregulated during Melanoma Progression and Increases Invasion of Melanoma Cells. Mod. Pathol. 2010, 23, 511–521. [Google Scholar] [CrossRef]
- Hofmann, U.B.; Westphal, J.R.; Zendman, A.J.; Becker, J.C.; Ruiter, D.J.; van Muijen, G.N. Expression and Activation of Matrix Metalloproteinase-2 (MMP-2) and its Co-Localization with Membrane-Type 1 Matrix Metalloproteinase (MT1-MMP) Correlate with Melanoma Progression. J. Pathol. 2000, 191, 245–256. [Google Scholar] [CrossRef]
- Salemi, R.; Falzone, L.; Madonna, G.; Polesel, J.; Cinà, D.; Mallardo, D.; Ascierto, P.A.; Libra, M.; Candido, S. MMP-9 as a Candidate Marker of Response to BRAF Inhibitors in Melanoma Patients with BRAFV600E Mutation Detected in Circulating-Free DNA. Front. Pharmacol. 2018, 9, 856. [Google Scholar] [CrossRef]
- Guarneri, C.; Bevelacqua, V.; Polesel, J.; Falzone, L.; Cannavò, P.S.; Spandidos, D.A.; Malaponte, G.; Libra, M. NF-κB Inhibition is Associated with OPN/MMP-9 Downregulation in Cutaneous Melanoma. Oncol. Rep. 2017, 37, 737–746. [Google Scholar] [CrossRef]
- Frank, A.; David, V.; Aurelie, T.; Florent, G.; William, H.; Philippe, B. Regulation of MMPs during Melanoma Progression: From Genetic to Epigenetic. Anticancer Agents Med. Chem. 2012, 12, 773–782. [Google Scholar] [CrossRef]
- Moustakas, A. TGF-Beta Targets PAX3 to Control Melanocyte Differentiation. Dev. Cell 2008, 15, 797–799. [Google Scholar] [CrossRef]
- Brenner, M.; Degitz, K.; Besch, R.; Berking, C. Differential Expression of Melanoma-Associated Growth Factors in Keratinocytes and Fibroblasts by Ultraviolet A and Ultraviolet B Radiation. Br. J. Dermatol. 2005, 153, 733–739. [Google Scholar] [CrossRef]
- Lee, S.B.; Schramme, A.; Doberstein, K.; Dummer, R.; Abdel-Bakky, M.S.; Keller, S.; Altevogt, P.; Oh, S.T.; Reichrath, J.; Oxmann, D.; et al. ADAM10 is Upregulated in Melanoma Metastasis Compared with Primary Melanoma. J. Investig. Dermatol. 2010, 130, 763–773. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Hearing, V.J. The Roles of ADAMs Family Proteinases in Skin Diseases. Enzym. Res. 2011, 2011, 482498. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, J.; Simiczyjew, A.; Dratkiewicz, E.; Kot, M.; Pietraszek-Gremplewicz, K.; Wilk, D.; Ziętek, M.; Matkowski, R.; Nowak, D. Melanoma Stimulates the Proteolytic Activity of HaCaT Keratinocytes. Cell Commun. Signal. 2022, 20, 146. [Google Scholar] [CrossRef]
- Loh, C.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Karim, R.; Tse, G.; Putti, T.; Scolyer, R.; Lee, S. The Significance of the Wnt Pathway in the Pathology of Human Cancers. Pathology 2004, 36, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Dorsky, R.I.; Raible, D.W.; Moon, R.T. Direct Regulation of Nacre, a Zebrafish MITF Homolog Required for Pigment Cell Formation, by the Wnt Pathway. Genes Dev. 2000, 14, 158–162. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Catricalà, C.; Larue, L.; Picardo, M. Wnt/Β-Catenin Signaling is Stimulated by A-Melanocyte-Stimulating Hormone in Melanoma and Melanocyte Cells: Implication in Cell Differentiation. Pigment Cell Melanoma Res. 2011, 24, 309–325. [Google Scholar] [CrossRef]
- Gallagher, S.J.; Rambow, F.; Kumasaka, M.; Champeval, D.; Bellacosa, A.; Delmas, V.; Larue, L. Beta-Catenin Inhibits Melanocyte Migration but Induces Melanoma Metastasis. Oncogene 2013, 32, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Yasumoto, K.; Takeda, K.; Takahashi, K.; Yamamoto, H.; Shibahara, S. Microphthalmia-Associated Transcription Factor in the Wnt Signaling Pathway. Pigment Cell Res. 2003, 16, 261–265. [Google Scholar] [CrossRef]
- Untiveros, G.; Dezi, L.; Gillette, M.; Sidor, J.; Strizzi, L. Normal Skin Cells Increase Aggressiveness of Cutaneous Melanoma by Promoting Epithelial-to-Mesenchymal Transition Via Nodal and Wnt Activity. Int. J. Mol. Sci. 2021, 22, 11719. [Google Scholar] [CrossRef]
- Chien, A.J.; Moore, E.C.; Lonsdorf, A.S.; Kulikauskas, R.M.; Rothberg, B.G.; Berger, A.J.; Major, M.B.; Hwang, S.T.; Rimm, D.L.; Moon, R.T. Activated Wnt/Beta-Catenin Signaling in Melanoma is Associated with Decreased Proliferation in Patient Tumors and a Murine Melanoma Model. Proc. Natl. Acad. Sci. USA 2009, 106, 1193–1198. [Google Scholar] [CrossRef]
- Widlund, H.R.; Horstmann, M.A.; Price, E.R.; Cui, J.; Lessnick, S.L.; Wu, M.; He, X.; Fisher, D.E. Beta-Catenin-Induced Melanoma Growth Requires the Downstream Target Microphthalmia-Associated Transcription Factor. J. Cell Biol. 2002, 158, 1079–1087. [Google Scholar] [CrossRef]
- Arozarena, I.; Bischof, H.; Gilby, D.; Belloni, B.; Dummer, R.; Wellbrock, C. In Melanoma, Beta-Catenin is a Suppressor of Invasion. Oncogene 2011, 30, 4531–4543. [Google Scholar] [CrossRef]
- Bachmann, I.M.; Straume, O.; Puntervoll, H.E.; Kalvenes, M.B.; Akslen, L.A. Importance of P-Cadherin, Beta-Catenin, and Wnt5a/Frizzled for Progression of Melanocytic Tumors and Prognosis in Cutaneous Melanoma. Clin. Cancer Res. 2005, 11, 8606–8614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Pang, D.; Wang, Y.; Bai, J.; Tian, F.; Han, D.; Shi, S.; Hu, L. Nomogram Incorporating the WNT/Β-Catenin Signaling Pathway for Predicting the Survival of Cutaneous Melanoma. Int. J. Gen. Med. 2021, 14, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Koetsier, J.L.; Sirico, A.; Agidi, A.T.; Antonini, D.; Missero, C.; Green, K.J. The Desmosomal Protein Desmoglein 1 Aids Recovery of Epidermal Differentiation After Acute UV Light Exposure. J. Investig. Dermatol. 2014, 134, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- El Kharbili, M.; Cario, M.; Béchetoille, N.; Pain, C.; Boucheix, C.; Degoul, F.; Masse, I.; Berthier-Vergnes, O. Tspan8 Drives Melanoma Dermal Invasion by Promoting ProMMP-9 Activation and Basement Membrane Proteolysis in a Keratinocyte-Dependent Manner. Cancers 2020, 12, 1297. [Google Scholar] [CrossRef]
- Berthier-Vergnes, O.; Barbollat-Boutrand, L.; Pommier, R.M.; de la Fouchardière, A.; Combemale, P.; Grimont, M.; Lopez-Ramirez, N.; Caramel, J.; Dalle, S.; Perrot, J.; et al. Tetraspanin8 Expression Predicts an Increased Metastatic Risk and is Associated with Cancer-Related Death in Human Cutaneous Melanoma. Mol. Cancer 2021, 20, 127. [Google Scholar] [CrossRef]
- Navarrete, M.; Salazar-Onfray, F.; Tittarelli, A. Flow Cytometry Evaluation of Gap Junction-Mediated Intercellular Communication between Cytotoxic T Cells and Target Tumor Cells. Methods Mol. Biol. 2021, 2346, 225–236. [Google Scholar]
- Tittarelli, A.; Mendoza-Naranjo, A.; Farías, M.; Guerrero, I.; Ihara, F.; Wennerberg, E.; Riquelme, S.; Gleisner, A.; Kalergis, A.; Lundqvist, A.; et al. Gap Junction Intercellular Communications Regulate NK Cell Activation and Modulate NK Cytotoxic Capacity. J. Immunol. 2014, 192, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Naranjo, A.; Cormie, P.; Serrano, A.E.; Wang, C.M.; Thrasivoulou, C.; Sutcliffe, J.E.S.; Gilmartin, D.J.; Tsui, J.; Serena, T.E.; Phillips, A.R.J.; et al. Overexpression of the Gap Junction Protein Cx43 as found in Diabetic Foot Ulcers can Retard Fibroblast Migration. Cell Biol. Int. 2012, 36, 661–667. [Google Scholar] [CrossRef]
- Trosko, J.E.; Ruch, R.J. Cell-Cell Communication in Carcinogenesis. Front. Biosci. 1998, 3, 208. [Google Scholar] [CrossRef]
- Tittarelli, A.; Guerrero, I.; Tempio, F.; Gleisner, M.A.; Avalos, I.; Sabanegh, S.; Ortíz, C.; Michea, L.; López, M.N.; Mendoza-Naranjo, A.; et al. Overexpression of Connexin 43 Reduces Melanoma Proliferative and Metastatic Capacity. Br. J. Cancer 2015, 113, 259–267. [Google Scholar] [CrossRef]
- Scatolini, M.; Patel, A.; Grosso, E.; Mello-Grand, M.; Ostano, P.; Coppo, R.; Vitiello, M.; Venesio, T.; Zaccagna, A.; Pisacane, A.; et al. GJB5 Association with BRAF Mutation and Survival in Cutaneous Malignant Melanoma. Br. J. Dermatol. 2022, 186, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Borsig, L. Selectins Promote Tumor Metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef]
- Shih, I.M.; Elder, D.E.; Hsu, M.Y.; Herlyn, M. Regulation of Mel-CAM/MUC18 Expression on Melanocytes of Different Stages of Tumor Progression by Normal Keratinocytes. Am. J. Pathol. 1994, 145, 837–845. [Google Scholar]
- Brose, M.S.; Volpe, P.; Feldman, M.; Kumar, M.; Rishi, I.; Gerrero, R.; Einhorn, E.; Herlyn, M.; Minna, J.; Nicholson, A.; et al. BRAF and RAS Mutations in Human Lung Cancer and Melanoma. Cancer Res. 2002, 62, 6997–7000. [Google Scholar]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Stefanato, C.M.; Yaar, M.; Bhawan, J.; Phillips, T.J.; Kosmadaki, M.G.; Botchkarev, V.; Gilchrest, B.A. Modulations of Nerve Growth Factor and Bcl-2 in Ultraviolet-Irradiated Human Epidermis. J. Cutan. Pathol. 2003, 30, 351–357. [Google Scholar] [CrossRef]
- Li, G.; Schaider, H.; Satyamoorthy, K.; Hanakawa, Y.; Hashimoto, K.; Herlyn, M. Downregulation of E-Cadherin and Desmoglein 1 by Autocrine Hepatocyte Growth Factor during Melanoma Development. Oncogene 2001, 20, 8125–8135. [Google Scholar] [CrossRef] [PubMed]
- Noujarède, J.; Carrié, L.; Garcia, V.; Grimont, M.; Eberhardt, A.; Mucher, E.; Genais, M.; Schreuder, A.; Carpentier, S.; Ségui, B.; et al. Sphingolipid Paracrine Signaling Impairs Keratinocyte Adhesion to Promote Melanoma Invasion. Cell Rep. 2023, 42, 113586. [Google Scholar] [CrossRef]
- Albinet, V.; Bats, M.-L.; Huwiler, A.; Rochaix, P.; Chevreau, C.; Ségui, B.; Levade, T.; Andrieu-Abadie, N. Dual Role of Sphingosine Kinase-1 in Promoting the Differentiation of Dermal Fibroblasts and the Dissemination of Melanoma Cells. Oncogene 2014, 33, 3364–3373. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, M.L.; Herlyn, M.; Weil, D.; Jambrosic, J.; Rodeck, U.; Becker, D.; Diamond, L.; Clark, W.H.; Koprowski, H. Growth and Phenotypic Characteristics of Human Nevus Cells in Culture. J. Investig. Dermatol. 1988, 90, 134–141. [Google Scholar] [CrossRef]
- Michaloglou, C.; Vredeveld, L.C.W.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; van der Horst, C.M.A.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-Associated Senescence-Like Cell Cycle Arrest of Human Naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef]
- Sadangi, S.; Milosavljevic, K.; Castro-Perez, E.; Lares, M.; Singh, M.; Altameemi, S.; Beebe, D.J.; Ayuso, J.M.; Setaluri, V. Role of the Skin Microenvironment in Melanomagenesis: Epidermal Keratinocytes and Dermal Fibroblasts Promote BRAF Oncogene-Induced Senescence Escape in Melanocytes. Cancers 2022, 14, 1233. [Google Scholar] [CrossRef] [PubMed]
- Tagore, M.; Hergenreder, E.; Perlee, S.C.; Cruz, N.M.; Menocal, L.; Suresh, S.; Chan, E.; Baron, M.; Melendez, S.; Dave, A.; et al. GABA Regulates Electrical Activity and Tumor Initiation in Melanoma. Cancer Discov. 2023, 13, 2270–2291. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Messer, A.R.; Amitai-Lange, A.; Melamed, Z.; Ohana, R.; Bell, R.E.; Kapitansky, O.; Lerman, G.; Greenberger, S.; Khaled, M.; et al. Interactions of Melanoma Cells with Distal Keratinocytes Trigger Metastasis Via Notch Signaling Inhibition of MITF. Mol. Cell 2015, 59, 664–676. [Google Scholar] [CrossRef]
- Hou, J.; Karin, M.; Sun, B. Targeting Cancer-Promoting Inflammation—Have Anti-Inflammatory Therapies Come of Age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef]
- Sheng, Y.; Liu, J.; Zhang, M.; Zheng, S. Unveiling the Link between Inflammasomes and Skin Cutaneous Melanoma: Insights into Expression Patterns and Immunotherapy Response Prediction. Math. Biosci. Eng. 2023, 20, 19912–19928. [Google Scholar] [CrossRef] [PubMed]
- Kučera, J.; Strnadová, K.; Dvořánková, B.; Lacina, L.; Krajsová, I.; Štork, J.; Kovářová, H.; Skalníková, H.K.; Vodička, P.; Motlík, J.; et al. Serum Proteomic Analysis of Melanoma Patients with Immunohistochemical Profiling of Primary Melanomas and Cultured Cells: Pilot Study. Oncol. Rep. 2019, 42, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Smatlik, N.; Drexler, S.K.; Burian, M.; Röcken, M.; Yazdi, A.S. ASC Speck Formation After Inflammasome Activation in Primary Human Keratinocytes. Oxid. Med. Cell. Longev. 2021, 2021, 7914829. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Awoyemi, A.A.; Fahy, K.E.; Thapa, P.; Borchers, C.; Wu, B.Y.; McGlone, C.L.; Schmeusser, B.; Sattouf, Z.; Rohan, C.A.; et al. Keratinocyte-Derived Microvesicle Particles Mediate Ultraviolet B Radiation-Induced Systemic Immunosuppression. J. Clin. Investig. 2021, 131, e144963. [Google Scholar] [CrossRef]
- Katiyar, S.K. UV-Induced Immune Suppression and Photocarcinogenesis: Chemoprevention by Dietary Botanical Agents. Cancer Lett. 2007, 255, 1–11. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrapodi, R.; Bellei, B. The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment. Cancers 2024, 16, 913. https://doi.org/10.3390/cancers16050913
Marrapodi R, Bellei B. The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment. Cancers. 2024; 16(5):913. https://doi.org/10.3390/cancers16050913
Chicago/Turabian StyleMarrapodi, Ramona, and Barbara Bellei. 2024. "The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment" Cancers 16, no. 5: 913. https://doi.org/10.3390/cancers16050913
APA StyleMarrapodi, R., & Bellei, B. (2024). The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment. Cancers, 16(5), 913. https://doi.org/10.3390/cancers16050913





