Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Generation of Reactive Oxygen Species
4. Activation of the Immune Response
5. Problems
5.1. Soluble Factors and Extracellular Matrix
5.2. Cells
5.2.1. Myeloid-Derived Suppressor Cells
5.2.2. Other Immunosuppressive Cells
5.3. Other Immunosuppressive Factors
6. Latest Reports
7. Future Research Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zakhari, S. Chronic alcohol drinking: Liver and pancreatic cancer? Clin. Res. Hepatol. Gastroenterol. 2015, 39 (Suppl. S1), S86–S91. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, Y.; Jiang, X.; Wang, C.; Liu, M.; Yan, J.; Zhang, L.; Zhou, Y. Insight into the Prospects for Tumor Therapy Based on Photodynamic Immunotherapy. Pharmaceuticals 2022, 15, 1359. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Luo, T.; Nash, G.T.; Lin, W. Nanoscale Metal-Organic Frameworks for Cancer Immunotherapy. Acc. Chem. Res. 2020, 53, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tao, Y.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int. J. Cancer 2018, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiao, F.; Lu, C.; Wen, L. Multifunctional Nanosystems Powered Photodynamic Immunotherapy. Front. Pharmacol. 2022, 13, 905078. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Golab, J. Evaluation of the Antitumor Immune Response Following Photofrin-Based PDT in Combination with the Epigenetic Agent 5-Aza-2′-Deoxycytidine. Methods Mol. Biol. 2022, 2451, 559–567. [Google Scholar] [PubMed]
- Yu, X.; Zheng, H.; Chan, M.T.V.; Wu, W.K.K. Immune consequences induced by photodynamic therapy in non-melanoma skin cancers: A review. Environ. Sci. Pollut. Res. Int. 2018, 25, 20569–20574. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- Maeding, N.; Verwanger, T.; Krammer, B. Boosting Tumor-Specific Immunity Using PDT. Cancers 2016, 8, 91. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Demkow, U. Immunological aspects of antitumor photodynamic therapy outcome. Cent. Eur. J. Immunol. 2015, 40, 481–485. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, F.; Wu, W.; Qiu, W.X.; Zhang, L.; Li, R.; Zhuang, Z.N.; Yu, W.; Cheng, H.; Zhang, X.Z. Enzyme-Driven Membrane-Targeted Chimeric Peptide for Enhanced Tumor Photodynamic Immunotherapy. ACS Nano 2019, 13, 11249–11262. [Google Scholar] [CrossRef]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Wang, H. Targeting Antitumor Immune Response for Enhancing the Efficacy of Photodynamic Therapy of Cancer: Recent Advances and Future Perspectives. Oxid. Med. Cell Longev. 2016, 2016, 5274084. [Google Scholar] [CrossRef] [PubMed]
- Gellén, E.; Fidrus, E.; Péter, M.; Szegedi, A.; Emri, G.; Remenyik, É. Immunological effects of photodynamic therapy in the treatment of actinic keratosis and squamous cell carcinoma. Photodiagn. Photodyn. Ther. 2018, 24, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.Y.; Lo, P.C.; Ng, D.K.; Fong, W.P. Anti-tumor immunity of BAM-SiPc-mediated vascular photodynamic therapy in a BALB/c mouse model. Cell Mol. Immunol. 2017, 14, 223–234. [Google Scholar] [CrossRef]
- Nkune, N.W.; Simelane, N.W.N.; Montaseri, H.; Abrahamse, H. Photodynamic Therapy-Mediated Immune Responses in Three-Dimensional Tumor Models. Int. J. Mol. Sci. 2021, 22, 12618. [Google Scholar] [CrossRef] [PubMed]
- Weigelin, B.; Friedl, P. T cell-mediated additive cytotoxicity—Death by multiple bullets. Trends Cancer 2022, 8, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Bensussen, A.; Santana, M.A.; Rodríguez-Jorge, O. Metabolic alterations impair differentiation and effector functions of CD8+ T cells. Front. Immunol. 2022, 13, 945980. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Golab, J. Targeting Epigenetic Processes in Photodynamic Therapy-Induced Anticancer Immunity. Front. Oncol. 2015, 5, 176. [Google Scholar] [CrossRef] [PubMed]
- Redkin, T.S.; Sleptsova, E.E.; Turubanova, V.D.; Saviuk, M.O.; Lermontova, S.A.; Klapshina, L.G.; Peskova, N.N.; Balalaeva, I.V.; Krysko, O.; Mishchenko, T.A.; et al. Dendritic Cells Pulsed with Tumor Lysates Induced by Tetracyanotetra(aryl)porphyrazines-Based Photodynamic Therapy Effectively Trigger Anti-Tumor Immunity in an Orthotopic Mouse Glioma Model. Pharmaceutics 2023, 15, 2430. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci. Rep. 2021, 11, 7205. [Google Scholar] [CrossRef]
- Lian, G.; Gnanaprakasam, J.R.; Wang, T.; Wu, R.; Chen, X.; Liu, L.; Shen, Y.; Yang, M.; Yang, J.; Chen, Y.; et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. eLife 2018, 7, e36158. [Google Scholar] [CrossRef]
- Oberkampf, M.; Guillerey, C.; Mouriès, J.; Rosenbaum, P.; Fayolle, C.; Bobard, A.; Savina, A.; Ogier-Denis, E.; Enninga, J.; Amigorena, S.; et al. Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat. Commun. 2018, 9, 2241. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; Ossendorp, F. Measuring the Antitumor T-Cell Response in the Context of Photodynamic Therapy. Methods Mol. Biol. 2022, 2451, 579–588. [Google Scholar]
- Wang, J.; Liu, N.; Jiang, H.; Li, Q.; Xing, D. Reactive Oxygen Species in Anticancer Immunity: A Double-Edged Sword. Front. Bioeng. Biotechnol. 2021, 9, 784612. [Google Scholar] [CrossRef]
- Raber, P.L.; Thevenot, P.; Sierra, R.; Wyczechowska, D.; Halle, D.; Ramirez, M.E.; Ochoa, A.C.; Fletcher, M.; Velasco, C.; Wilk, A.; et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int. J. Cancer 2014, 134, 2853–2864. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade to Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef]
- Cramer, G.M.; Cengel, K.A.; Busch, T.M. Forging Forward in Photodynamic Therapy. Cancer Res. 2022, 82, 534–536. [Google Scholar] [CrossRef]
- Mishra, C.B.; Mongre, R.K.; Prakash, A.; Jeon, R.; Supuran, C.T.; Lee, M.S. Anti-breast cancer action of carbonic anhydrase IX inhibitor 4-[4-(4-Benzo[1,3]dioxol-5-ylmethyl-piperazin-1-yl)-benzylidene-hydrazinocarbonyl]-benzenesulfonamide (BSM-0004): In vitro and in vivo studies. J. Enzyme Inhib. Med. Chem. 2021, 36, 954–963. [Google Scholar] [CrossRef]
- Wang, X.; Ji, J.; Zhang, H.; Fan, Z.; Zhang, L.; Shi, L.; Zhou, F.; Chen, W.R.; Wang, H.; Wang, X. Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget 2015, 6, 44688–44702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, P.; Wang, X.; Shi, L.; Fan, Z.; Zhang, G.; Yang, D.; Bahavar, C.F.; Zhou, F.; Chen, W.R.; et al. Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice. Technol. Cancer Res. Treat. 2018, 17, 1533033818785275. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Govande, M.; Yasinchak, A.; Heusinkveld, L.; Shakya, S.; Fairchild, R.L.; Maytin, E.V. Painless Photodynamic Therapy Triggers Innate and Adaptive Immune Responses in a Murine Model of UV-induced Squamous Skin Pre-cancer. Photochem. Photobiol. 2021, 97, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Marangon, I.; Méreaux, J.; Nicolás-Boluda, A.; Lavieu, G.; Wilhelm, C.; Sarda-Mantel, L.; Silva, A.K.A.; Pocard, M.; Gazeau, F. Immune Reprogramming Precision Photodynamic Therapy of Peritoneal Metastasis by Scalable Stem-Cell-Derived Extracellular Vesicles. ACS Nano 2021, 15, 3251–3263. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Aragaki, M.; Bernards, N.; Chee, T.; Gregor, A.; Hiraishi, Y.; Ishiwata, T.; Leung, C.; Ding, L.; Kitazawa, S.; et al. Repeated photodynamic therapy mediates the abscopal effect through multiple innate and adaptive immune responses with and without immune checkpoint therapy. Biomaterials 2023, 292, 121918. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.J.; Mentucci, F.M.; Roselli, E.; Araya, P.; Rivarola, V.A.; Rumie Vittar, N.B.; Maccioni, M. Photodynamic Modulation of Type 1 Interferon Pathway on Melanoma Cells Promotes Dendritic Cell Activation. Front. Immunol. 2019, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Gabrysiak, M.; Muchowicz, A.; Bednarek, W.; Barankiewicz, J.; Rygiel, T.; Boon, L.; Mroz, P.; Hamblin, M.R.; Golab, J. 5-Aza-2′-deoxycytidine potentiates antitumour immune response induced by photodynamic therapy. Eur. J. Cancer 2014, 50, 1370–1381. [Google Scholar] [CrossRef]
- Wu, D.; Wang, S.; Zuo, M.; Zhang, M.; Li, D.; Chen, W.; Sha, M.; Yuan, L.; Qin, N. Er: YAG laser assisted photodynamic therapy for the management of severe oral epithelial dysplasia with innate and adaptive immune responses. Photodiagn. Photodyn. Ther. 2023, 42, 103565. [Google Scholar] [CrossRef]
- López-Soto, A.; Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L.; Gonzalez, S. Involvement of autophagy in NK cell development and function. Autophagy 2017, 13, 633–636. [Google Scholar] [CrossRef]
- Martin-Antonio, B.; Najjar, A.; Robinson, S.N.; Chew, C.; Li, S.; Yvon, E.; Thomas, M.W.; Mc Niece, I.; Orlowski, R.; Muñoz-Pinedo, C.; et al. Transmissible cytotoxicity of multiple myeloma cells by cord blood-derived NK cells is mediated by vesicle trafficking. Cell Death Differ. 2015, 22, 96–107. [Google Scholar] [CrossRef]
- Ma, J.; Wei, K.; Zhang, H.; Tang, K.; Li, F.; Zhang, T.; Liu, J.; Xu, P.; Yu, Y.; Sun, W.; et al. Mechanisms by Which Dendritic Cells Present Tumor Microparticle Antigens to CD8+ T Cells. Cancer Immunol. Res. 2018, 6, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Chong, G.; Dong, H.; Gu, J.; Zang, J.; He, R.; Sun, J.; Zhang, T.; Zhao, Y.; Zheng, X.; et al. Nanovaccine biomineralization for cancer immunotherapy: A NADPH oxidase-inspired strategy for improving antigen cross-presentation via lipid peroxidation. Biomaterials 2021, 277, 121089. [Google Scholar] [CrossRef] [PubMed]
- Dingjan, I.; Verboogen, D.R.; Paardekooper, L.M.; Revelo, N.H.; Sittig, S.P.; Visser, L.J.; Mollard, G.F.; Henriet, S.S.; Figdor, C.G.; Ter Beest, M.; et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 2016, 6, 22064. [Google Scholar] [CrossRef]
- Singel, K.L.; Segal, B.H. NOX2-dependent regulation of inflammation. Clin. Sci. 2016, 130, 479–490. [Google Scholar] [CrossRef]
- Canton, J.; Blees, H.; Henry, C.M.; Buck, M.D.; Schulz, O.; Rogers, N.C.; Childs, E.; Zelenay, S.; Rhys, H.; Domart, M.C.; et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat. Immunol. 2021, 22, 140–153, Erratum in Nat. Immunol. 2021, 22, 391. [Google Scholar] [CrossRef]
- Marrache, S.; Tundup, S.; Harn, D.A.; Dhar, S. Ex vivo generation of functional immune cells by mitochondria-targeted photosensitization of cancer cells. Methods Mol. Biol. 2015, 1265, 113–122. [Google Scholar] [PubMed]
- Im, S.; Lee, J.; Park, D.; Park, A.; Kim, Y.M.; Kim, W.J. Hypoxia-Triggered Transforming Immunomodulator for Cancer Immunotherapy via Photodynamically Enhanced Antigen Presentation of Dendritic Cell. ACS Nano 2019, 13, 476–488. [Google Scholar] [CrossRef]
- Nunes-Hasler, P.; Maschalidi, S.; Lippens, C.; Castelbou, C.; Bouvet, S.; Guido, D.; Bermont, F.; Bassoy, E.Y.; Page, N.; Merkler, D.; et al. STIM1 promotes migration, phagosomal maturation and antigen cross-presentation in dendritic cells. Nat. Commun. 2017, 8, 1852. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926, Erratum in J. Immunother. Cancer 2021, 9, e001926corr1. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Ma, R.; Ji, T.; Zhang, H.; Dong, W.; Chen, X.; Xu, P.; Chen, D.; Liang, X.; Yin, X.; Liu, Y.; et al. A Pck1-directed glycogen metabolic program regulates formation and maintenance of memory CD8+ T cells. Nat. Cell Biol. 2018, 20, 21–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheung, Y.K.; Ng, D.K.P.; Fong, W.P. Enhancement of innate and adaptive anti-tumor immunity by serum obtained from vascular photodynamic therapy-cured BALB/c mouse. Cancer Immunol. Immunother. 2021, 70, 3217–3233. [Google Scholar] [CrossRef]
- Kue, C.S.; Kamkaew, A.; Voon, S.H.; Kiew, L.V.; Chung, L.Y.; Burgess, K.; Lee, H.B. Tropomyosin Receptor Kinase C Targeted Delivery of a Peptidomimetic Ligand-Photosensitizer Conjugate Induces Antitumor Immune Responses Following Photodynamic Therapy. Sci. Rep. 2016, 6, 37209. [Google Scholar] [CrossRef]
- Rashida Gnanaprakasam, J.N.; Wu, R.; Wang, R. Metabolic Reprogramming in Modulating T Cell Reactive Oxygen Species Generation and Antioxidant Capacity. Front. Immunol. 2018, 9, 1075. [Google Scholar] [CrossRef]
- Bassoy, E.Y.; Walch, M.; Martinvalet, D. Reactive Oxygen Species: Do They Play a Role in Adaptive Immunity? Front. Immunol. 2021, 12, 755856. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Assi, S.; Gershkovitz, M.; Sagiv, J.Y.; Polyansky, L.; Mishalian, I.; Fridlender, Z.G.; Granot, Z. Isolation and Characterization of Neutrophils with Anti-Tumor Properties. J. Vis. Exp. 2015, 100, e52933. [Google Scholar]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Perobelli, S.M.; Galvani, R.G.; Gonçalves-Silva, T.; Xavier, C.R.; Nóbrega, A.; Bonomo, A. Plasticity of neutrophils reveals modulatory capacity. Braz. J. Med. Biol. Res. 2015, 48, 665–675. [Google Scholar] [CrossRef]
- Tang, A.; Dadaglio, G.; Oberkampf, M.; Di Carlo, S.; Peduto, L.; Laubreton, D.; Desrues, B.; Sun, C.M.; Montagutelli, X.; Leclerc, C. B cells promote tumor progression in a mouse model of HPV-mediated cervical cancer. Int. J. Cancer 2016, 139, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Graef, S.; Mussai, F.; Thomas, A.; Wali, N.; Yenidunya, B.G.; Yuan, C.; Morrow, B.; Zhang, J.; Korangy, F.; et al. Tumor-Derived GM-CSF Promotes Granulocyte Immunosuppression in Mesothelioma Patients. Clin. Cancer Res. 2018, 24, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Hou, Y.; Yu, Y.; Li, Z.; Liu, H.; Liu, C.; He, J.; Miao, L. Circumventing Myeloid-Derived Suppressor Cell-Mediated Immunosuppression Using an Oxygen-Generated and -Economized Nanoplatform. ACS Appl. Mater. Interfaces 2020, 12, 55723–55736. [Google Scholar] [CrossRef]
- Umansky, V.; Blattner, C.; Fleming, V.; Hu, X.; Gebhardt, C.; Altevogt, P.; Utikal, J. Myeloid-derived suppressor cells and tumor escape from immune surveillance. Semin. Immunopathol. 2017, 39, 295–305. [Google Scholar] [CrossRef]
- Rostamian, H.; Khakpoor-Koosheh, M.; Jafarzadeh, L.; Masoumi, E.; Fallah-Mehrjardi, K.; Tavassolifar, M.J.; MPawelek, J.; Mirzaei, H.R.; Hadjati, J. Restricting tumor lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer 2022, 22, 39. [Google Scholar] [CrossRef]
- Murphy, D.A.; Cheng, H.; Yang, T.; Yan, X.; Adjei, I.M. Reversing Hypoxia with PLGA-Encapsulated Manganese Dioxide Nanoparticles Improves Natural Killer Cell Response to Tumor Spheroids. Mol. Pharm. 2021, 18, 2935–2946. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, M.; Deng, Z.; Zeng, S.; Hao, J. Low Dose Soft X-Ray Remotely Triggered Lanthanide Nanovaccine for Deep Tissue CO Gas Release and Activation of Systemic Anti-Tumor Immunoresponse. Adv. Sci. 2021, 8, e2004391. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Martner, A.; Wiktorin, H.G.; Lenox, B.; Ewald Sander, F.; Aydin, E.; Aurelius, J.; Thorén, F.B.; Ståhlberg, A.; Hermodsson, S.; Hellstrand, K. Histamine promotes the development of monocyte-derived dendritic cells and reduces tumor growth by targeting the myeloid NADPH oxidase. J. Immunol. 2015, 194, 5014–5021. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, M.A.; Çınar, Ö.; Holmdahl, R.; Mougiakakos, D.; Kiessling, R. Methylcholanthrene-Induced Sarcomas Develop Independently from NOX2-Derived ROS. PLoS ONE 2015, 10, e0129786. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lao, Y.; Teng, X.L.; Li, S.; Zhou, Y.; Wang, F.; Guo, X.; Deng, S.; Chang, Y.; Wu, X.; et al. SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat. Commun. 2018, 9, 3157. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef]
- Namdar, A.; Mirzaei, R.; Memarnejadian, A.; Boghosian, R.; Samadi, M.; Mirzaei, H.R.; Farajifard, H.; Zavar, M.; Azadmanesh, K.; Elahi, S.; et al. Prophylactic DNA vaccine targeting Foxp3+ regulatory T cells depletes myeloid-derived suppressor cells and improves anti-melanoma immune responses in a murine model. Cancer Immunol. Immunother. 2018, 67, 367–379. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, Z.; Wang, J.; Chen, M.; Wan, Y.; Huang, H.; Liu, Z.; Wang, J.; Hou, J. C-reactive protein impairs immune response of CD8+ T cells via FcγRIIb-p38MAPK-ROS axis in multiple myeloma. J. Immunother. Cancer 2023, 11, e007593, Erratum in J. Immunother. Cancer 2023, 11, e007593corr1. [Google Scholar] [CrossRef]
- Cribioli, E.; Giordano Attianese, G.M.P.; Ginefra, P.; Signorino-Gelo, A.; Vuillefroy de Silly, R.; Vannini, N.; Hess, C.; Irving, M.; Coukos, G. Enforcing GLUT3 expression in CD8+ T cells improves fitness and tumor control by promoting glucose uptake and energy storage. Front. Immunol. 2022, 13, 976628. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; von Delwig, A.A. Oxygen therapy in traditional and immunotherapeutic treatment protocols of cancer patients: Current reality and future prospects. Expert Rev. Anticancer Ther. 2022, 22, 575–581. [Google Scholar] [CrossRef]
- Balta, E.; Wabnitz, G.H.; Samstag, Y. Hijacked Immune Cells in the Tumor Microenvironment: Molecular Mechanisms of Immunosuppression and Cues to Improve T Cell-Based Immunotherapy of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5736. [Google Scholar] [CrossRef] [PubMed]
- Vedenko, A.; Panara, K.; Goldstein, G.; Ramasamy, R.; Arora, H. Tumor Microenvironment and Nitric Oxide: Concepts and Mechanisms. Adv. Exp. Med. Biol. 2020, 1277, 143–158. [Google Scholar] [PubMed]
- Hurst, K.E.; Lawrence, K.A.; Essman, M.T.; Walton, Z.J.; Leddy, L.R.; Thaxton, J.E. Endoplasmic Reticulum Stress Contributes to Mitochondrial Exhaustion of CD8+ T Cells. Cancer Immunol. Res. 2019, 7, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Cao, Y.; Rodriguez, P.C. Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: A promising opportunity for cancer immunotherapy. Cancer Immunol. Immunother. 2017, 66, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, W.; Zhang, H.; Ma, J.; Xu, P.; Yu, Y.; Fang, H.; Zhou, L.; Lv, J.; Xie, J.; et al. Macrophages reprogrammed by lung cancer microparticles promote tumor development via release of IL-1β. Cell Mol. Immunol. 2020, 17, 1233–1244. [Google Scholar] [CrossRef]
- Yuan, F.; Fu, X.; Shi, H.; Chen, G.; Dong, P.; Zhang, W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS ONE 2014, 9, e107063. [Google Scholar] [CrossRef]
- Gu, M.; Zhou, X.; Sohn, J.H.; Zhu, L.; Jie, Z.; Yang, J.Y.; Zheng, X.; Xie, X.; Yang, J.; Shi, Y.; et al. Author Correction: NF-κB-inducing kinase maintains T cell metabolic fitness in antitumor immunity. Nat. Immunol. 2021, 22, 530. [Google Scholar] [CrossRef]
- Oh, D.S.; Kim, H.; Oh, J.E.; Jung, H.E.; Lee, Y.S.; Park, J.H.; Lee, H.K. Intratumoral depletion of regulatory T cells using CD25-targeted photodynamic therapy in a mouse melanoma model induces antitumoral immune responses. Oncotarget 2017, 8, 47440–47453. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S.; Johnson, T.; Qi, R.; Zhang, S.; Zhang, J.; Yang, K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021, 35, 109235. [Google Scholar] [CrossRef]
- Hu, X.; Li, B.; Li, X.; Zhao, X.; Wan, L.; Lin, G.; Yu, M.; Wang, J.; Jiang, X.; Feng, W.; et al. Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J. Immunol. 2014, 192, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Aarts, C.E.M.; Hiemstra, I.H.; Béguin, E.P.; Hoogendijk, A.J.; Bouchmal, S.; van Houdt, M.; Tool, A.T.J.; Mul, E.; Jansen, M.H.; Janssen, H.; et al. Activated neutrophils exert myeloid-derived suppressor cell activity damaging T cells beyond repair. Blood Adv. 2019, 3, 3562–3574. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.M.; Liu, Z.G.; Zhou, X.; Song, S.H.; Weng, G.Y.; Wen, Y.; Liu, F.B.; Cao, D.L.; Liu, Y.F. Expansion of PMN-myeloid derived suppressor cells and their clinical relevance in patients with oral squamous cell carcinoma. Oral. Oncol. 2019, 95, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Speigl, L.; Burow, H.; Bailur, J.K.; Janssen, N.; Walter, C.B.; Pawelec, G.; Shipp, C. CD14+ HLA-DR-/low MDSCs are elevated in the periphery of early-stage breast cancer patients and suppress autologous T cell proliferation. Breast Cancer Res. Treat. 2018, 168, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Kang, K.; Cha, J.S.; Kim, J.W.; Lee, H.G.; Kim, Y.; Yang, Y.; Lee, M.S.; Lim, J.S. Interferon regulatory factor 4 (IRF4) controls myeloid-derived suppressor cell (MDSC) differentiation and function. J. Leukoc. Biol. 2016, 100, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Askman, S.; Pettersson, Å.; Wichert, S.; Hellmark, T.; Johansson, Å.C.M.; Hansson, M. Bone Marrow Neutrophils of Multiple Myeloma Patients Exhibit Myeloid-Derived Suppressor Cell Activity. J. Immunol. Res. 2021, 2021, 6344344. [Google Scholar] [CrossRef] [PubMed]
- Emmons, T.R.; Giridharan, T.; Singel, K.L.; Khan, A.N.H.; Ricciuti, J.; Howard, K.; Silva-Del Toro, S.L.; Debreceni, I.L.; Aarts, C.E.M.; Brouwer, M.C.; et al. Mechanisms Driving Neutrophil-Induced T-cell Immunoparalysis in Ovarian Cancer. Cancer Immunol. Res. 2021, 9, 790–810. [Google Scholar] [CrossRef]
- Khou, S.; Popa, A.; Luci, C.; Bihl, F.; Meghraoui-Kheddar, A.; Bourdely, P.; Salavagione, E.; Cosson, E.; Rubod, A.; Cazareth, J.; et al. Tumor-Associated Neutrophils Dampen Adaptive Immunity and Promote Cutaneous Squamous Cell Carcinoma Development. Cancers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Shi, C.; Liu, T.; Guo, Z.; Zhuang, R.; Zhang, X.; Chen, X. Reprogramming Tumor-Associated Macrophages by Nanoparticle-Based Reactive Oxygen Species Photogeneration. Nano Lett. 2018, 18, 7330–7342. [Google Scholar] [CrossRef]
- Liu, L.; He, H.; Liang, R.; Yi, H.; Meng, X.; Chen, Z.; Pan, H.; Ma, Y.; Cai, L. ROS-Inducing Micelles Sensitize Tumor-Associated Macrophages to TLR3 Stimulation for Potent Immunotherapy. Biomacromolecules 2018, 19, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Eckert, F.; Zwirner, K.; Boeke, S.; Thorwarth, D.; Zips, D.; Huber, S.M. Rationale for Combining Radiotherapy and Immune Checkpoint Inhibition for Patients with Hypoxic Tumors. Front. Immunol. 2019, 10, 407. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.L.; Ye, S.B.; Ouyang, L.Y.; Chen, Y.S.; He, J.; Huang, H.Q.; Zeng, Y.X.; Zhang, X.S.; Li, J. Myeloid-derived suppressor cells inhibit T cell proliferation in human extranodal NK/T cell lymphoma: A novel prognostic indicator. Cancer Immunol. Immunother. 2015, 64, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Poschke, I.; Kiessling, R. Tumour-induced immune suppression: Role of inflammatory mediators released by myelomonocytic cells. J. Intern. Med. 2014, 276, 154–170. [Google Scholar] [CrossRef]
- Kwak, Y.; Kim, H.E.; Park, S.G. Insights into Myeloid-Derived Suppressor Cells in Inflammatory Diseases. Arch. Immunol. Ther. Exp. 2015, 63, 269–285. [Google Scholar] [CrossRef]
- Lim, H.X.; Hong, H.J.; Cho, D.; Kim, T.S. IL-18 enhances immunosuppressive responses by promoting differentiation into monocytic myeloid-derived suppressor cells. J. Immunol. 2014, 193, 5453–5460. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Myeloid-derived suppressor cells (MDSC): An important partner in cellular/tissue senescence. Biogerontology 2018, 19, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ding, X.; Du, H.; Yan, C. Myeloid-derived suppressor cells are involved in lysosomal acid lipase deficiency-induced endothelial cell dysfunctions. J. Immunol. 2014, 193, 1942–1953. [Google Scholar] [CrossRef]
- Lv, W.; Chen, N.; Lin, Y.; Ma, H.; Ruan, Y.; Li, Z.; Li, X.; Pan, X.; Tian, X. Macrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/NF kappa B axis. Cancer Lett. 2016, 375, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Beury, D.W.; Carter, K.A.; Nelson, C.; Sinha, P.; Hanson, E.; Nyandjo, M.; Fitzgerald, P.J.; Majeed, A.; Wali, N.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cell Survival and Function Are Regulated by the Transcription Factor Nrf2. J. Immunol. 2016, 196, 3470–3478. [Google Scholar] [CrossRef]
- Kumagai, S.; Togashi, Y.; Kamada, T.; Sugiyama, E.; Nishinakamura, H.; Takeuchi, Y.; Vitaly, K.; Itahashi, K.; Maeda, Y.; Matsui, S.; et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020, 21, 1346–1358. [Google Scholar] [CrossRef]
- Huang, C.; Fan, X.; Shen, Y.; Shen, M.; Yang, L. Neutrophil subsets in noncancer liver diseases: Cellular crosstalk and therapeutic targets. Eur. J. Immunol. 2023, 53, e2250324. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Miyato, H.; Kanamaru, R.; Sadatomo, A.; Takahashi, K.; Ohzawa, H.; Koyanagi, T.; Saga, Y.; Takei, Y.; Fujiwara, H.; et al. Activated neutrophils inhibit chemotactic migration of activated T lymphocytes to CXCL11 by multiple mechanisms. Cell Immunol. 2023, 384, 104663. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, T.; Ma, X.; Yang, Q.C.; Yang, L.L.; Yang, S.C.; Liang, M.; Xu, Z.; Sun, Z.J. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv. Sci. 2021, 8, e2101840. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yang, F.; Kuang, J.; Xiong, Y.; Mi, B.B.; Zhou, Y.; Hu, J.J.; Song, S.J.; Wan, T.; Wan, Z.Z.; et al. A Versatile Nanoplatform for Broad-Spectrum Immunotherapy by Reversing the Tumor Microenvironment. ACS Appl. Mater. Interfaces 2021, 13, 45335–45345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Deng, H.; Yang, W.; Wang, Z.; Lin, L.; Munasinghe, J.; Jacobson, O.; Liu, Y.; Tang, L.; Ni, Q.; et al. Early stratification of radiotherapy response by activatable inflammation magnetic resonance imaging. Nat. Commun. 2020, 11, 3032. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, Y.; Zhang, J.; Chen, C.; Gao, L.; Shi, C.; Fu, Z.; Han, M.; Tang, C.; Sun, P.; et al. Reversing immune evasion using a DNA nano-orchestrator for pancreatic cancer immunotherapy. Acta Biomater. 2023, 166, 512–523. [Google Scholar] [CrossRef]
- Dan Lu Liu, L.; Sun, Y.; Song, J.; Yin, Q.; Zhang, G.; Qi, F.; Hu, Z.; Yang, Z.; Zhou, Z.; Hu, Y.; et al. The phosphatase PAC1 acts as a T cell suppressor and attenuates host antitumor immunity. Nat. Immunol. 2020, 21, 287–297. [Google Scholar] [CrossRef]
- Yan, Y.; Chang, L.; Tian, H.; Wang, L.; Zhang, Y.; Yang, T.; Li, G.; Hu, W.; Shah, K.; Chen, G.; et al. 1-Pyrroline-5-carboxylate released by prostate Cancer cell inhibit T cell proliferation and function by targeting SHP1/cytochrome c oxidoreductase/ROS Axis. J. Immunother. Cancer 2018, 6, 148. [Google Scholar] [CrossRef]
- Nath, P.R.; Pal-Nath, D.; Mandal, A.; Cam, M.C.; Schwartz, A.L.; Roberts, D.D. Natural Killer Cell Recruitment and Activation Are Regulated by CD47 Expression in the Tumor Microenvironment. Cancer Immunol. Res. 2019, 7, 1547–1561. [Google Scholar] [CrossRef]
- Yin, T.; Wang, G.; He, S.; Liu, Q.; Sun, J.; Wang, Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell Immunol. 2016, 300, 41–45. [Google Scholar] [CrossRef]
- Shams, M.; Owczarczak, B.; Manderscheid-Kern, P.; Bellnier, D.A.; Gollnick, S.O. Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease. Cancer Immunol. Immunother. 2015, 64, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, W.J.; Cao, Q.; Zhang, H.; Liu, B.; Ling, Y.; Zhou, X.; Mao, Z.W. A Carbonic Anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer Evokes Pyroptosis for Enhanced Anti-Tumor Immunity. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115800. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, Z.; Huang, H.; Xu, Z.; Chen, Z.; Shen, G.; Li, Z.; Ren, Y.; Li, G.; Hu, Y. VB12-Sericin-PBLG-IR780 Nanomicelles for Programming Cell Pyroptosis via Photothermal (PTT)/Photodynamic (PDT) Effect-Induced Mitochondrial DNA (mitoDNA) Oxidative Damage. ACS Appl. Mater. Interfaces 2022, 14, 17008–17021. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.W.C.; Liu, H.; Kah, J.C.Y. Innate immune activation by conditioned medium of cancer cells following combined phototherapy with photosensitizer-loaded gold nanorods. J. Mater. Chem. B 2020, 8, 10812–10824. [Google Scholar] [CrossRef] [PubMed]
- Huis In’t Veld, R.V.; Lara, P.; Jager, M.J.; Koning, R.I.; Ossendorp, F.; Cruz, L.J. M1-derived extracellular vesicles enhance photodynamic therapy and promote immunological memory in preclinical models of colon cancer. J. Nanobiotechnol. 2022, 20, 252. [Google Scholar] [CrossRef] [PubMed]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Chen, W.R.; Wang, X. DC vaccine generated by ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncoimmunology 2015, 5, e1072674. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Heusinkveld, L.E.; Cheng, C.E.; Lefatshe, L.; De Silva, P.; Hasan, T.; Maytin, E.V. Combination of 5-Fluorouracil with Photodynamic Therapy: Enhancement of Innate and Adaptive Immune Responses in a Murine Model of Actinic Keratosis. Photochem. Photobiol. 2023, 99, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Cherukula, K.; Bang, Y.J.; Vijayan, V.; Moon, M.J.; Thiruppathi, J.; Puth, S.; Jeong, Y.Y.; Park, I.K.; Lee, S.E.; et al. Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression. Cells 2020, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, J.; Lee, M.J.; Peng, C.; Luo, T.; Tillman, L.; Weichselbaum, R.R.; Lin, W. Nanoscale coordination polymer synergizes photodynamic therapy and toll-like receptor activation for enhanced antigen presentation and antitumor immunity. Biomaterials 2023, 302, 122334. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Chen, B.; Gao, J.; Zhou, F.; Saeed, M.; Hou, B.; Li, Y.; Yu, H. Sheddable Prodrug Vesicles Combating Adaptive Immune Resistance for Improved Photodynamic Immunotherapy of Cancer. Nano Lett. 2020, 20, 353–362. [Google Scholar] [CrossRef]
- Rocha, L.B.; Gomes-da-Silva, L.C.; Dąbrowski, J.M.; Arnaut, L.G. Elimination of primary tumours and control of metastasis with rationally designed bacteriochlorin photodynamic therapy regimens. Eur. J. Cancer 2015, 51, 1822–1830. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, G.L.; Fan, J.H.; Zheng, R.R.; Zhao, L.P.; Yuan, P.; Zhao, X.Y.; Yu, X.Y.; Li, S.Y. A Self-Delivery Chimeric Peptide for Photodynamic Therapy Amplified Immunotherapy. Macromol. Biosci. 2019, 19, e1800410. [Google Scholar] [CrossRef]

| Inclusion |
| Papers describing adaptive immune response induced by PDT were included |
| Both review articles and research articles were included |
| Papers written since 2014 were included |
| Both in vivo and in vitro studies were included |
| Papers describing reports on the immune efficacy of PDT were included |
| Papers describing the reasons for the failure of PDT to activate the adaptive immune response have been included |
| Exclusion |
| Papers focusing on the innate immune response induced by PDT were excluded |
| Articles in a language other than English or Polish |
| Papers written before 2014 were excluded |
| Papers describing adaptive immune response induced by PDT in the treatment of diseases other than cancer were excluded |
| Authors | Title | Year of Publication | Results |
|---|---|---|---|
| Lou J et al. [35] | Repeated photodynamic therapy mediates the abscopal effect through multiple innate and adaptive immune responses with and without immune checkpoint therapy | 2023 | The association of PDT with anti-PD1 monoclonal antibody has the ability to enhance the immune system response |
| Anand S et al. [121] | Combination of 5-Fluorouracil with Photodynamic Therapy: Enhancement of Innate and Adaptive Immune Responses in a Murine Model of Actinic Keratosis | 2023 | Combining 5FU and PDT with PS protoporphyrin IX may work synergistically, and provide better treatment for squamous cell carcinoma |
| Jiang X et al. [123] | Nanoscale coordination polymer synergizes photodynamic therapy and toll-like receptor activation for enhanced antigen presentation and antitumor immunity | 2023 | The association of PDT and toll-like receptor agonist in the form of chlorin e6/R848 polymer has the ability to induce immunogenic death of MC38 colon cancer cells achieving a 50% cure rate and 99.4% inhibition of tumor growth |
| Redkin TS et al. [21] | Dendritic Cells Pulsed with Tumor Lysates Induced by Tetracyanotetra(aryl)porphyrazines-Based Photodynamic Therapy Effectively Trigger Anti-Tumor Immunity in an Orthotopic Mouse Glioma Model | 2023 | Dendritic cell vaccines pulsed with the lysates of glioma GL261 cells pre-treated with pz-I-PDT or pz-III-PDT could act as effective inducers of adaptive anti-tumor immunity in an intracranial orthotopic glioma mouse model |
| Huis In ‘t Veld RV et al. [34] | M1-derived extracellular vesicles enhance photodynamic therapy and promote immunological memory in preclinical models of colon cancer | 2022 | PDT with PS zinc phthalocyanine mediated by extracellular vesicles derived from M1-like macrophages has the ability to induce a complete MC38 tumor response in mouse models |
| Guo W et al. [116] | VB12-Sericin-PBLG-IR780 Nanomicelles for Programming Cell Pyroptosis via Photothermal (PTT)/Photodynamic (PDT) Effect-Induced Mitochondrial DNA (mitoDNA) Oxidative Damage | 2022 | PDT with nanomicelles composed of modified sericin, tumor-targeting agent V12, and PS IR780 has the ability to induce tumor cell pyroptosis and generate anti-tumor immunity |
| Su X et al. [30] | A Carbonic Anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer Evokes Pyroptosis for Enhanced Anti-Tumor Immunity | 2022 | PDT with rhenium(I) photosensitizer anchored to carbonic anhydrase IX has the ability to induce pyropotic tumor cell death and stimulate tumor immunogenicity |
| Zhang et al. [32] | Enhancement of innate and adaptive anti-tumor immunity by serum obtained from vascular photodynamic therapy-cured BALB/c mouse | 2021 | Vascular PDT (VPDT) has the ability to induce varying degrees of resistance to attack other types of mouse tumor cells |
| Gao A et al. [124] | Sheddable Prodrug Vesicles Combating Adaptive Immune Resistance for Improved Photodynamic Immunotherapy of Cancer | 2020 | PDT with PEGylated PS and indoleamine 2,3-dioxygenase inhibitor 1 has the ability to suppress CT26 colon cancer recurrence |
| Hwang HS et al. [122] | Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression | 2020 | PDT in combination with peptide vaccination of a tumor-specific TLR5 agonist can be enhanced by association with a PD-1 checkpoint inhibitor |
| MWC et al. [117] | Innate immune activation by conditioned medium of cancer cells following combined phototherapy with photosensitizer-loaded gold nanorods | 2020 | Combination of PDT and photothermal therapy using gold nanorods with PS chlorin e6 on endogenously formed mouse serum protein coronas has the ability to enhance activation of dendritic cells and macrophages in breast cancer EMT6 |
| Im S et al. [47] | Hypoxia-Triggered Transforming Immunomodulator for Cancer Immunotherapy via Photodynamically Enhanced Antigen Presentation of Dendritic Cell | 2019 | PDT can be used in dendritic cell-based immunotherapy |
| Cheng et al. [126] | A Self-Delivery Chimeric Peptide for Photodynamic Therapy Amplified Immunotherapy | 2019 | PDT with the chimeric peptide PpIX-PEG8 -KVPRNQDWL has the ability to induce an anti-tumor immune response in the treatment of malignant melanoma |
| Zhang H et al. [32] | Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice | 2018 | PDT dendritic cell vaccination is an effective prophylactic therapy for squamous cell carcinoma |
| Oh DS. et al. [82] | Intratumoral depletion of regulatory T cells using CD25-targeted photodynamic therapy in a mouse melanoma model induces antitumoral immune responses | 2017 | PDT targeting tumor-associated regulatory T cells can specifically modulate the tumor microenvironment and may be used as a new technique for cancer immunotherapy |
| Duan X et al. [28] | Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer | 2016 | PDT of Zn-pyrophosphate nanoparticles with PS pyrolipid has the ability to sensitize tumors to checkpoint inhibition by PD-L1 antibodies and lead to complete eradication of primary tumor and metastasis |
| Shams et al. [114] | Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease | 2015 | PDT can be an effective adjuvant for therapies that do not stimulate the host’s anti-tumor immune response |
| Ji J et al. [120] | DC vaccine generated by ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma | 2015 | ALA-PDT-DC vaccine has ability to inhibit the growth of skin squamous cell carcinoma |
| Rocha LB et al. [125] | Elimination of primary tumors and control of metastasis with rationally designed bacteriochlorin photodynamic therapy regimens | 2015 | PDT can be an effective adjuvant for therapies that do not stimulate the host’s anti-tumor immune response |
| Wachowska et al. [7] | 5-Aza-2′-deoxycytidine potentiates antitumor immune response induced by photodynamic therapy | 2014 | Induction of expression of silenced tumor P1A antigen by 5-Aza-2′-deoxycytidine may enhance activation of PDT-induced adaptive immune response |
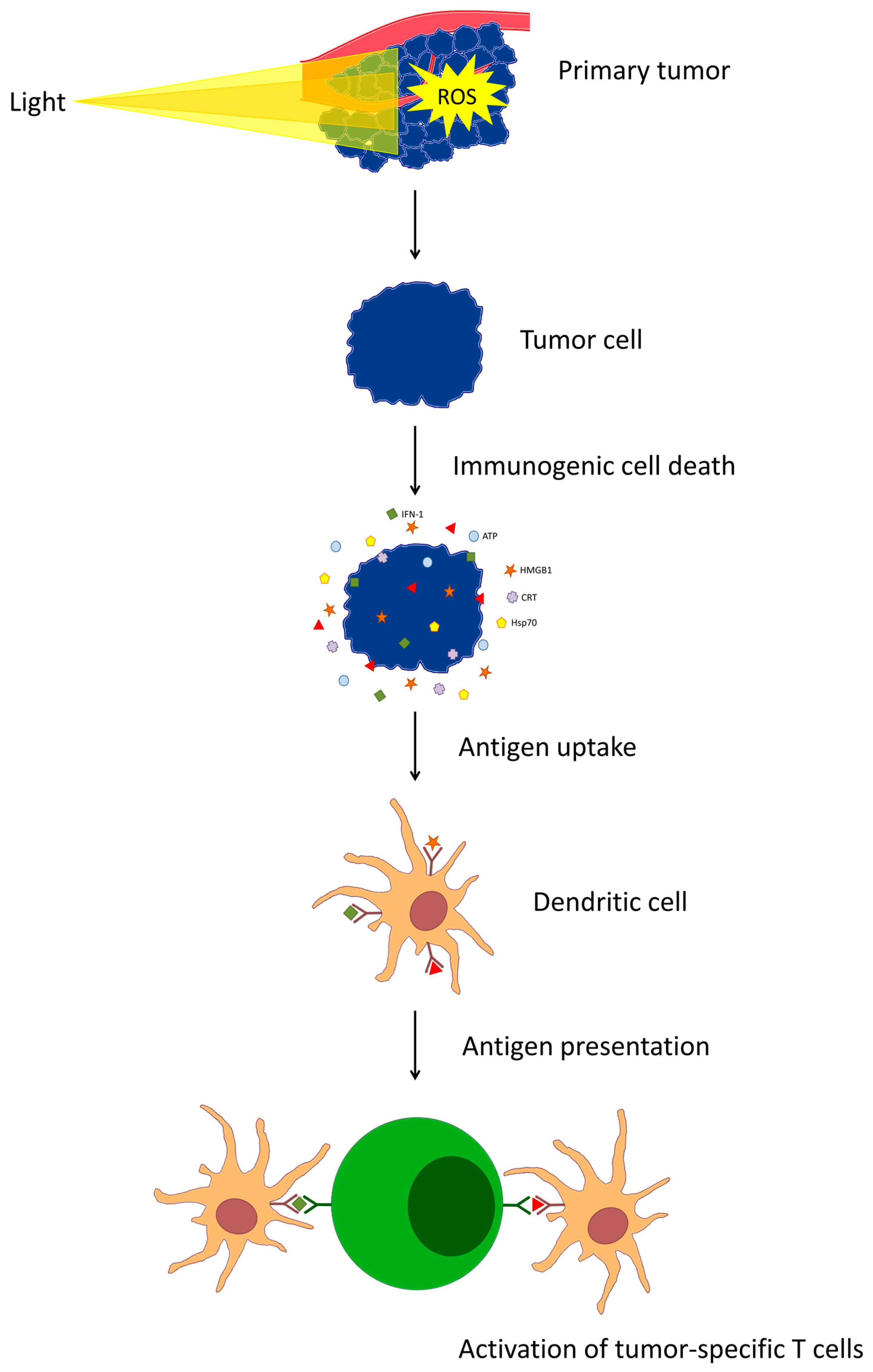
| Effect | Mechanism |
|---|---|
| DAMP release | Generation of damage and cell death by produced reactive oxygen species |
| Increase the ability of dendritic cells to present antigens | Stimulation of dendritic cell maturation by released DAMPs |
| Increased expression of MHC II on the surface of DCs | |
| Promotion of DC maturation by released tumor lysates | |
| Increased CD8+ T-cell response | Increase in activation of CD8+ T cells due to increased antigen cross-presentation |
| Increased incidence of CD8+ T cells in distal non-irradiated lymph nodes draining the tumor | |
| Induction of anti-tumor response of other cells | Production of tumor-specific antibodies that stimulate engulfment of cancer cells by macrophages and activate neutrophil antibody-dependent cytotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aebisher, D.; Woźnicki, P.; Bartusik-Aebisher, D. Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports. Cancers 2024, 16, 967. https://doi.org/10.3390/cancers16050967
Aebisher D, Woźnicki P, Bartusik-Aebisher D. Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports. Cancers. 2024; 16(5):967. https://doi.org/10.3390/cancers16050967
Chicago/Turabian StyleAebisher, David, Paweł Woźnicki, and Dorota Bartusik-Aebisher. 2024. "Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports" Cancers 16, no. 5: 967. https://doi.org/10.3390/cancers16050967
APA StyleAebisher, D., Woźnicki, P., & Bartusik-Aebisher, D. (2024). Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports. Cancers, 16(5), 967. https://doi.org/10.3390/cancers16050967









